Abstract
Aims
Mast cells participate importantly in abdominal aortic aneurysms (AAAs) by releasing inflammatory cytokines to promote vascular cell protease expression and arterial wall remodelling. Mast cells accumulate in AAA lesions during disease progression, but the exact chemokines by which mast cells migrate to the site of vascular inflammation remain unknown. This study tested the hypothesis that mast cells use chemokine (C-C motif) receptor 2 (CCR2) for their accumulation in experimental mouse AAA lesions.
Methods and results
We generated mast cell and apolipoprotein E double-deficient (Apoe–/–KitW-sh/W-sh) mice and found that they were protected from angiotensin II (Ang II) chronic infusion-induced AAAs compared with Apoe–/– littermates. Using bone-marrow derived mast cells (BMMC) from Apoe–/– mice and CCR2 double-deficient (Apoe–/–Ccr2–/–) mice, we demonstrated that Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice, but not those from Apoe–/– mice, remained protected from AAA formation. Adoptive transfer of BMMC from Apoe–/– mice into Apoe–/–KitW-sh/W-sh mice also increased lesion content of macrophages, T cells, and MHC class II-positive cells; there was also increased apoptosis, angiogenesis, cell proliferation, elastin fragmentation, and medial smooth muscle cell loss. In contrast, adoptive transfer of BMMC from Apoe–/–Ccr2–/– mice into Apoe–/–KitW-sh/W-sh mice did not affect these variables.
Conclusions
The increased AAA formation and associated lesion characteristics in Apoe–/–KitW–sh/W–sh mice after receiving BMMC from Apoe–/– mice, but not from Apoe–/–Ccr2–/– mice, suggests that mast cells use CCR2 as the chemokine receptor for their recruitment in Ang II-induced mouse AAA lesions.
Keywords: Mast cell, Abdominal aortic aneurysm, Ang II, MCP-1, CCR2
1. Introduction
The development of abdominal aortic aneurysms (AAAs) involves extensive infiltration of inflammatory cells—such as macrophages, neutrophils, T cells, and mast cells—that are recruited from the lumen or the vasa vasorum. Mast cells are abundant in both the adventitia and the media in human AAA lesions1,2 and correlate positively and significantly with AAA maximal diameter.3,4 In ruptured human AAAs, mast cells dwell in the adventitia and media and often colocalize with macrophages and T cells,5–7 suggesting their interplay in AAA lesions. AAA lesions also contain more activated mast cells than do normal aortas or atherosclerotic lesions.2 After activation, mast cells release cytokines, chemokines, and proteases to mediate consequent inflammatory cell recruitment, neighbouring cell protease expression and activation, angiogenesis, and apoptosis.
Angiogenesis is a central process of AAA pathogenesis,8 and associates closely with AAA rupture.9 In human AAA lesions, many mast cells in the media and adventitia correlate with densities of neovascularization.3 Inflammatory responses in human AAAs also associate with arterial cell apoptosis.6,10 Mast cells release chymase to inactivate the focal adhesion kinase-mediated smooth-muscle cell (SMC) survival signalling or to release tumour necrosis factor-α (TNF-α), which binds to the ‘death receptors’ on the surface of endothelial cells (ECs) and induces caspase-dependent EC apoptosis.11,12 Mast cells also release chymase to degrade SMC fibronectin, inhibit SMC collagen synthesis, and activate caspases; they also release transforming growth factor-β1 from extracellular matrix to disrupt SMC adhesion and inhibit SMC proliferation and growth, thereby promoting SMC apoptosis.13,14
Several experimental AAA models recently have examined the direct participation of mast cells in AAA pathogenesis. In aortic elastase perfusion-induced and peri-aortic CaCl2 injury-induced AAA, all C57BL/6 wild-type (WT) mice developed AAA lesions, but mast cell-deficient KitW-sh/W-sh mice under the same genetic background were fully protected at any tested time point.15 Reduced AAA in KitW-sh/W-sh mice accompanied impaired internal elastic lamina degradation, decreased numbers of macrophages, CD3+ T lymphocytes, SMCs, apoptotic cells, and CD31+ microvessels, and decreased levels of aortic tissue interleukin-6 (IL-6) and interferon-γ (IFN-γ). Activation of mast cells in WT mice with compound 48/80 enhanced AAA growth, while mast cell stabilization with cromolyn diminished AAA formation, suggesting the importance of mast cell granule release to AAA pathogenesis. Mechanistically, we demonstrated that mast cells release pro-inflammatory IL-6 and IFN-γ to stimulate vascular wall elastinolytic cathepsin and matrix metalloproteinase (MMP) expression.15,16 In peri-aortic CaCl2-induced AAA in rats, total mast cells and activated mast cells were increased over time (3, 7, and 14 days post-CaCl2). Mast cell-deficient Ws/Ws rats also failed to develop AAA.2 Although lesion macrophage contents were not affected, lesion T-cell accumulation was fully blocked in Ws/Ws rats. Ws/Ws rats also showed reduced MMP-2 and MMP-9 activities and capillary density.2 In the same experimental AAA, mast cell inactivation with Tranilast completely blocked AAA formation and reduced lesion mast cell and T-cell numbers, capillary density, and MMP-2 and MMP-9 activities.2
Leucocyte migration to the site of inflammation in the arterial wall is essential to AAA pathogenesis. Deficiency of the chemokine leukotriene B4 receptor BLT1 in apolipoprotein E-deficient (Apoe–/–) mice reduced angiotensin-II (Ang II) infusion-induced AAAs in mice. AAA lesions from these mice contained significantly lower numbers of Mac-3+ macrophages and CD4+ T cells than did AAA lesions from Apoe–/– control mice.17 In the same Apoe–/– mice, transplantation of bone-marrow cells from chemokine (C-C motif) receptor 2 (CCR2)-deficient mice reduced Ang II-induced AAA and atherosclerosis and impaired Mac-3+ macrophage accumulation and inflammatory cytokine production in these lesions.18 These observations suggest that BLT1, CCR2, and possibly other chemokine receptors on macrophages are required for their migration into AAA and atherosclerotic lesions. For example, expression of eotaxin and its receptor CCR3 were increased in human atherosclerotic lesions.19 Absence of chemokine monocyte chemoattractant protein-1 (MCP-1)20 or its receptor, CCR2,21 reduced atherosclerosis in mice, supporting the importance of CCR2 and CCR3 in leucocyte accumulation during atherogenesis. We do not know exactly which chemokine(s) mast cells use for their migration into AAA lesions, but many direct and indirect studies suggest that mast cell accumulation in AAA lesions may enhance recruitment of subsequent inflammatory cells. We showed that mast cells release TNF-α to stimulate the expression of adhesion molecules, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1, P-selectin, and E-selectin from mouse aortic ECs, and enhance neutrophil adhesion.22 Mast cell tryptase treatment of human coronary artery ECs increased adhesion and transendothelium migration of human umbilical cord blood CD133+ mast cell precursors.23 Mast cells also may enhance leucocyte–EC interaction indirectly by generating Ang II.24 Superfusion of Ang II into rat mesenteric postcapillary venules induces leucocyte rolling, adhesion, and migration.25 Systemic activation of mast cells with dinitrophenyl-albumin challenge induces leucocyte migration to the central atheroma near the elastic laminae.26 Therefore, mast cell appearance in early human atherosclerotic lesions,5 and possibly in early AAA lesions, can be essential to the pathogenesis of the lesions. Mast cells also might use BLT1, CCR3, and CCR2 for their migration to human AAA lesions. The current study used Ang II chronic infusion-induced AAA in Apoe–/– mice to examine whether the absence of mast cells affects AAA formation, whether mast cells use chemokine MCP-1 receptor CCR2 when they infiltrate to AAA lesions, and whether we can control AAA formation by regulating mast cell migration.
2. Methods
2.1. Mouse AAA production and lesion characterization
Apoe–/– mice (C57BL/6, N12) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). KitW-sh/W-sh mice (C57BL/6, N20)15,16 were crossbred with Apoe–/– mice to generate Apoe+/-KitW-sh/+ mice as breeding pairs to produce Apoe–/–KitW-sh/W-sh mice and littermate Apoe–/– control mice. To induce AAAs, anaesthetized (200 mg/kg ketamine, 10 mg/kg xylazine, i.p.) 3-month-old male mice were infused with 1000 ng kg−1 min−1 Ang II (Sigma, St Louis, MO, USA) subcutaneously delivered by Alzet model 2004 osmotic minipumps (DURECT Corp., Cupertino, CA, USA) for 28 days while on a high-fat diet (C12108; Research Diets, Inc., New Brunswick, NJ, USA). Post-operative analgesia (buprenophine, 0.05 mg/kg/12 h, i.p.) was administered every 12 h for 48 h. Mice were sacrificed with carbon dioxide narcosis, followed by cardiac puncture blood collection and aortic tissue harvest. Experimental aneurysms were quantified using the methods of Daugherty et al.27 and those of our earlier studies.28 The percentage of incidence per group was determined, and aortas were graded. The maximal diameter of each aneurysm was measured after the peri-adventitial tissue was carefully removed from the aortic wall. All animal procedures conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Harvard Medical School Standing Committee on Animals (protocol # 03759).
2.2. BMMC culture and BMMC reconstitution
Ccr2–/– mice (C57BL/6, N9) were purchased from The Jackson Laboratory, and crossbred with Apoe–/– mice to generate Apoe+/–Ccr2+/– mice as breeding pairs to produce Apoe–/–Ccr2–/– and Apoe–/– littermate control mice. To prepare BMMC, bone-marrow cells from Apoe–/– and Apoe–/–Ccr2–/– mice were cultured for 5 weeks in the presence of mouse IL-3 (PeproTech, Inc., Rocky Hill, NJ, USA) and stem cell factor (PeproTech), as reported previously.15,16,29,30 To reconstitute BMMC in vivo, 5-week-old male Apoe–/–KitW-sh/W-sh mice were given BMMC by tail vein injection (1 × 107 cells per mouse). Unlike other methods of bone-marrow-cell transplantation, irradiating the Apoe–/–KitW-sh/W-sh recipient mice before receiving BMMC intravenous transfer is not necessary, and donor cells migrate to most organs and injured aortas.15,16,29,30 Recipient mice were introduced to the Ang II infusion-induced experimental AAA 7 weeks after BMMC injection.
2.3. Immunohistochemical analysis
All aortas were embedded with OCT for immunohistochemical analysis as described previously.15,16 Aorta segments for immunohistochemistry were cut from the maximal external aneurysm diameter. Slides of each sample from identical levels were used for staining with each antibody. Serial cryostat cross-sections (6 µm) were used for immunostaining for macrophages (Mac-3, BD Bioscience, San Jose, CA, USA, 1:1000), T cells (CD4, BD Bioscience, 1:90; and CD8, Abcam, Cambridge, MA, USA, 1:300), mast cells (CD117, eBioscience, San Diego, CA, USA, 1:50), chemokine (MCP-1, BD Bioscience, 1:50), major histocompatibility complex class II (MHC-II, BD Bioscience, 1:250), SMC (α-actin, Sigma, 1:750), elastin (Verhoeff–van Gieson, Sigma), proliferation (Ki67, Vector Laboratories, Inc., Burlingame, CA, USA, 1:500), and angiogenesis (CD31, BD Bioscience, 1:1500, and laminin-5γ2, 1:250).28 Lesion apoptotic cells were determined with the in situ apoptosis detection kit, according to the manufacturer's instructions (Millipore, Billerica, MA, USA). Elastin degradation and lesion SMC content were graded according to the grading keys. T cells, apoptotic cells, mast cells, MCP-1-positive cells, and CD31-positive microvessels were counted blindly. Macrophage-positive, MHC-II-positive, and laminin-5 γ2-positive areas were measured using computer-assisted image analysis software (Image-Pro Plus; Media Cybernetics, Bethesda, MD, USA).
2.4. Real-time polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from BMMC from Apoe–/– and Apoe–/–Ccr2–/– mice using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA samples were then treated with an RNase-free DNase (Ambion, Carlsbad, CA, USA) to remove genomic DNA contaminants. Equal amounts of RNA were reverse transcribed, and quantitative PCR was performed in a single-colour real-time polymerase chain reaction (RT-PCR) detection system (Stratagene, La Jolla, CA, USA). The mRNA levels of CCR2, CCR3, BLT1, and BLT2 were normalized to those of β-actin.
2.5. Statistical analysis
Because of relatively small sample sizes and data distribution abnormality, we selected the non-parametric Mann–Whitney U-test for non-paired data sets and the Wilcoxon signed-rank test for paired data sets to examine statistical significance throughout this study. P < 0.05 was considered statistically significant.
3. Results
3.1. Mast cell deficiency reduces Ang II infusion-induced AAA formation in mice
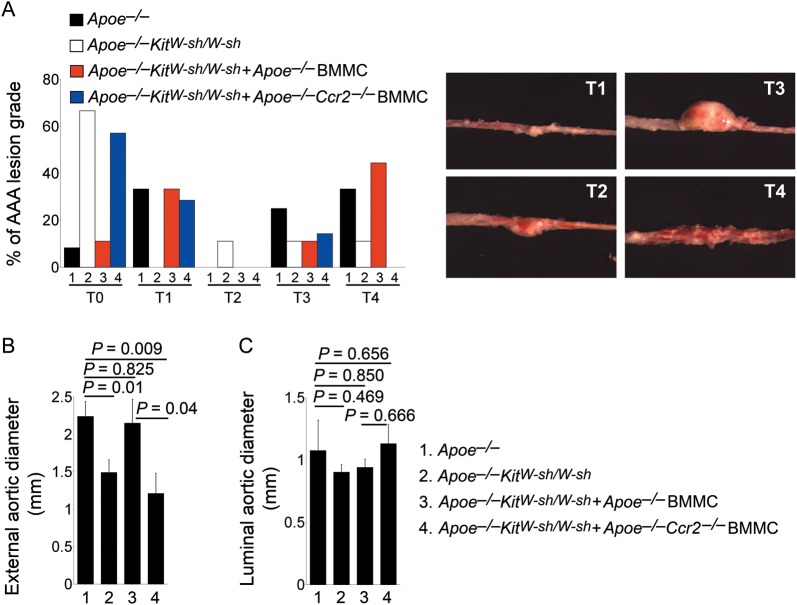
We previously showed that the absence of mast cells protected mice from the formation of both aortic elastase perfusion-induced and peri-aortic CaCl2 injury-induced AAAs.15 Using Apoe–/– and Apoe–/–KitW-sh/W-sh littermate mice, we demonstrated that mast cell deficiency also reduced Ang II chronic infusion-induced experimental AAAs. According to a prior grading system used broadly in this experimental model,27,28 we found that the majority of Apoe–/–KitW-sh/W-sh mice had T0 lesions, whereas a much higher percentage of the Apoe–/– mice developed T3 and T4 lesions (Figure 1A). External aortic diameters at the site of maximal-dilated suprarenal AAA were also significantly smaller in Apoe–/–KitW-sh/W-sh mice than in Apoe–/– mice (Figure 1B), although mast cell deficiency did not affect luminal aortic diameters (Figure 1C). These observations agree with our prior conclusions from aortic elastase perfusion-induced and peri-aortic CaCl2 injury-induced AAAs that mast cells participate directly in experimental AAA formation.
Figure 1.
Deficiency of mast cells or mast cell CCR2 reduced Ang II infusion-induced AAA. Percentages of mice with different AAA lesion grades (from T0 to T4) (A), maximal external aortic diameters (B), and luminal aortic diameters (C) were determined from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Data are mean ± SEM. Lesion grading keys in (A) are indicated to the right.
To examine whether mast cells migrate to AAAs using CCR2, we adoptively transferred Apoe–/–KitW-sh/W-sh recipient mice with BMMC from Apoe–/– mice and Apoe–/–Ccr2–/– mice. Similar to our earlier findings,15 adoptive transfer of BMMC from Apoe–/– mice increased AAA formation in Apoe–/–KitW-sh/W-sh recipient mice to the levels in Apoe–/– control mice. High percentages of these Apoe–/– mouse BMMC-reconstituted recipients had AAA lesions at the T3 and T4 levels (Figure 1A). The mean external aortic diameter sizes also reached those of Apoe–/– control mice, supporting the importance of mast cells in Ang II infusion-induced AAAs. In contrast, when Apoe–/–KitW-sh/W-sh recipient mice received BMMC from Apoe–/–Ccr2–/– mice, these recipients behaved like non-reconstituted Apoe–/–KitW-sh/W-sh mice in both semiquantitative lesion grades and external aortic diameters (Figure 1A and B). BMMC reconstitution did not affect luminal aortic diameters (Figure 1C). These results suggest that mast cell migration to AAA lesions is important to AAA formation and that CCR2 mediates mast cell migration.
3.2. Mast cell migration to experimental AAA lesions requires CCR2 activities
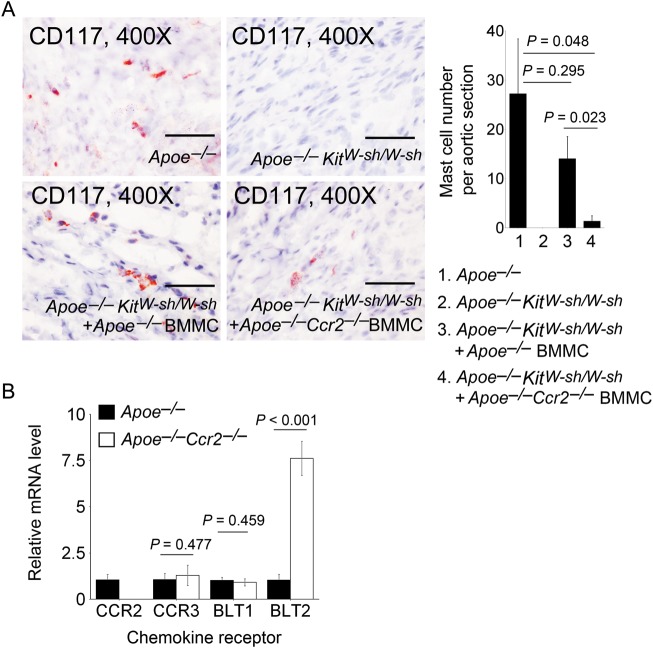
We examined our hypothesis that mast cell migration to AAA lesions required MCP-1 activities. By immunostaining frozen AAA lesion sections with anti-CD117, we found that the AAA lesion mast cell numbers were not significantly different (P = 0.295) between Apoe–/– mice and Apoe–/–KitW-sh/W-sh mice that received Apoe–/– mouse BMMC. In contrast, mast cell numbers in AAA lesions from Apoe–/–KitW-sh/W-sh mice that received Apoe–/–Ccr2–/– mouse BMMC were significantly lower than those in Apoe–/– mice (P = 0.048) or in Apoe–/–KitW-sh/W-sh mice that received Apoe–/– mouse BMMC (P = 0.023) (Figure 2A).
Figure 2.
Mast cells in AAA lesions. (A) Anti-mouse CD117 monoclonal antibody immunostaining determined mast cell numbers in AAA lesions from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Representative images are shown. Scale bar: 50 µm. (B) RT-PCR determined mRNA levels of chemokine receptors CCR2, CCR3, BLT1, and BLT2 in BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice. Data are mean ± SEM.
CCR2 deficiency in BMMC may affect the expression of other chemokine receptors. Impaired mast cell infiltration (Figure 2A) and reduced AAA formation (Figure 1A and B) in Apoe–/–KitW-sh/W-sh mice receiving Apoe–/–Ccr2–/– mouse BMMC may be confounded by altered expressions of other untested chemokine receptors in BMMC from Apoe–/–Ccr2–/– mice. Using RT-PCR, we did not find any significant differences between the expression of eotaxin and RANTES receptor CCR331,32 and the leukotriene B4 receptor BLT1.33 Indeed, expression of the leukotriene B4 receptor BLT233 was significantly higher in BMMC from Apoe–/–Ccr2–/– mice than those from Apoe–/– mice (Figure 2B). These observations indirectly support the hypothesis that CCR2 mediates mast cell migration to AAA lesions in Ang II-induced experimental AAA.
3.3. Mast cell deficiency attenuates AAA lesion inflammation
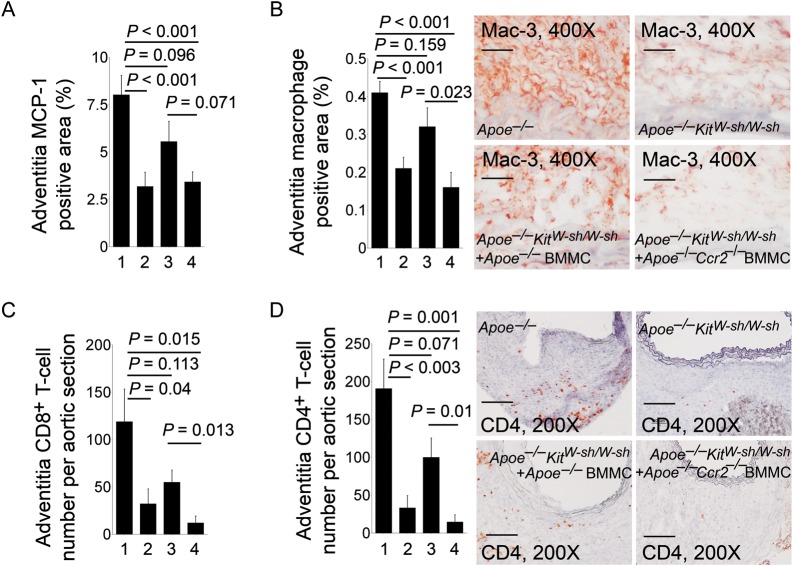
Mast cells release TNF-α and tryptase to stimulate EC adhesion molecule expression and leucocyte adhesion and migration.22,23 The absence of mast cells therefore should reduce AAA lesion leucocyte accumulation and inflammation—important mechanisms leading to impaired AAA growth. AAA lesion section immunostaining demonstrated significant reduction in lesion chemokine MCP-1 levels (Figure 3A), adventitia Mac-3+ macrophage-positive areas (Figure 3B), CD8+ T-cell numbers (Figure 3C), CD4+ T-cell numbers (Figure 3D), and MHC class II-positive areas (Supplementary material online, Figure S1). These observations suggest that mast cell accumulation in AAA lesions is essential to the recruitment of other inflammatory cells (e.g. macrophages, T cells). Similar to AAA lesion sizes (Figure 1A and B), adoptive transfer of BMMC from Apoe–/– mice into Apoe–/–KitW-sh/W-sh recipient mice increased AAA lesion MCP-1-positive areas, Mac-3+ macrophages, CD8+ T cells, CD4+ T cells, and MHC class II-positive areas in recipient mice. In contrast, when BMMC from Apoe–/–Ccr2–/– mice were used for the reconstitution experiment, significantly reduced lesion mast cell contents from Apoe–/–KitW-sh/W-sh recipient mice (Figure 2A) led to negligible changes in lesion MCP-1-positive areas, Mac-3+ macrophages, CD8+ T cells, CD4+ T cells, and MHC class II-positive areas, compared with those from Apoe–/–KitW-sh/W-sh mice that received no adoptive transfer (Figure 3A–D and Supplementary material online, Figure S1). Lesion leucocyte recruitment therefore may require CCR2-mediated mast cell accumulation in AAA lesions.
Figure 3.
Inflammatory cells in AAA lesions. MCP-1-positive areas (A), Mac-3+ macrophage-positive areas (B), CD8+ T-cell numbers (C), and CD4+ T-cell numbers (D) in adventitia from AAA lesions from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Data are mean ± SEM. Representative micrographs for (B) (scale bar: 50 µm) and (D) (scale bar: 100 µm) are indicated to the right. Experimental group denotations are the same as in Figure 2.
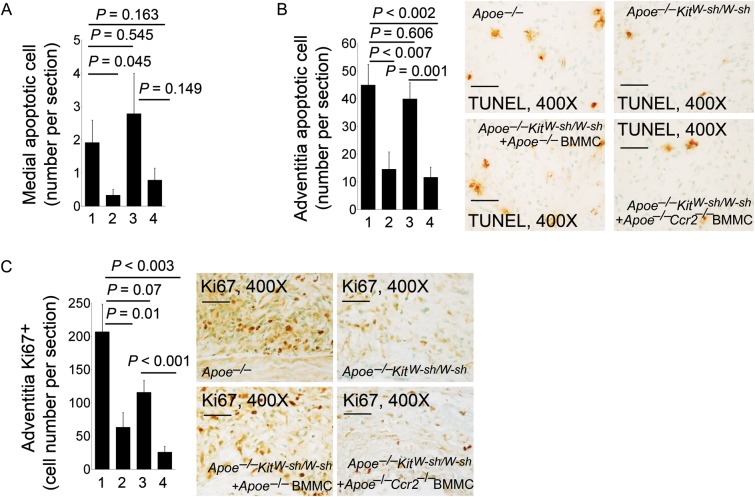
3.4. Mast cell deficiency impaired AAA lesion angiogenesis, lesion cell apoptosis, and proliferation
We have previously shown that mast cells use chymase to promote angiogenesis.15,34
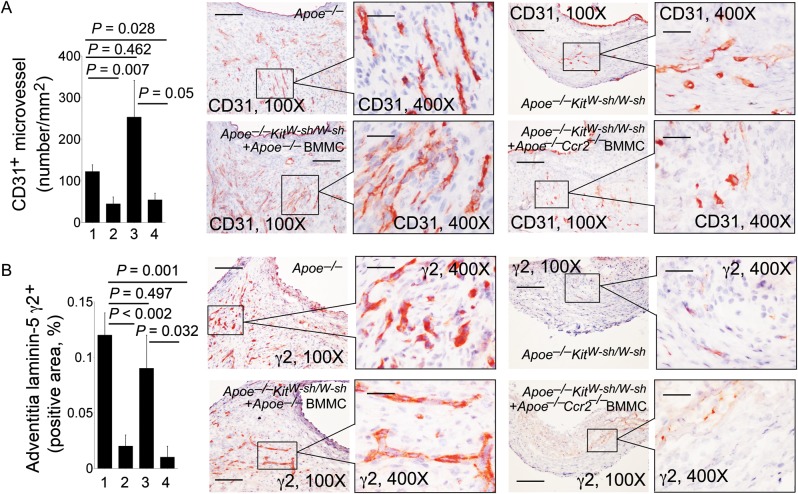
These cells also produce IL-6, IFN-γ, chymase, and tryptase to induce aortic SMC apoptosis.15,34,35 The absence of mast cells in AAA lesions from Apoe–/–KitW-sh/W-sh mice or low numbers of mast cells in AAA lesions from Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice significantly reduced AAA lesion CD31+ microvessel numbers compared with those in AAA lesions from Apoe–/– control mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–mice (Figure 4A). Pro-angiogenic matrix protein laminin-5 fragment γ2+ areas in AAA lesions from Apoe–/–KitW-sh/W-sh mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice were also significantly smaller than those in Apoe–/– control mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–mice (Figure 4B). Laminin-5 γ2 fragments are 80 kDa and 100 kDa fragments from matrix protein laminin-5 proteolysis, and are potent pro-angiogenic pepetides,36 indicating that mast cells participate indirectly in laminin-5 fragmentation during AAA formation. The absence of mast cells in AAA lesions from Apoe–/–KitW-sh/W-sh mice, or low numbers of mast cells in AAA lesions from Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice, also had significantly reduced TUNEL-positive apoptotic cells in the media (Figure 5A) and adventitia (Figure 5B), compared with those in AAA lesions from Apoe–/– control mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–mice. Although not tested in this study, mast cells may use both inflammatory cytokines and proteases to promote vascular cell apoptosis.15,34,35
Figure 4.
Angiogenesis in AAA lesions. Adventitia CD31-positive microvessel numbers per mm2 (A) and laminin-5 γ2-positive areas (B) in AAA lesions from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Data are mean ± SEM. Representative images (scale bar: 200 µm) are shown to the right. Inset scale bar: 50 µm. Experimental group denotations are the same as in Figure 2.
Figure 5.
Cell apoptosis and proliferation in AAA lesions. Medial TUNEL-positive apoptotic cell numbers (A), adventitia TUNEL-positive cell numbers (B), and adventitia Ki67-positive cell numbers (C) in AAA lesions from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Data are mean ± SEM. Representative images for (B) and (C) are shown. Scale bar: 50 µm. Experimental groups denotation are the same as in Figure 2.
Reduced AAA growth in Apoe–/–KitW-sh/W-sh mice or those receiving BMMC from Apoe–/–Ccr2–/– mice is also accompanied by significantly reduced AAA lesion cell proliferation (numbers of Ki67+ cells), compared with those in AAA lesions from Apoe–/– control mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice (Figure 5C). Mast cell-derived chemokines such as MCP-1 also promote the proliferation of arterial leucocytes and SMCs.37,38 In a femoral artery injury model, CCR2 deficiency decreased intima hyperplasia, establishing an important role of CCR2 in SMC proliferation.39 Mast cells also mediate inflammatory cell activation and proliferation through direct cell–cell interaction.40 Reduced cell proliferation (Figure 5C) in AAA lesions from mast cell-deficient mice, or those with low numbers of mast cells, therefore may result in part from low chemokine levels in these lesions (Figure 3A).
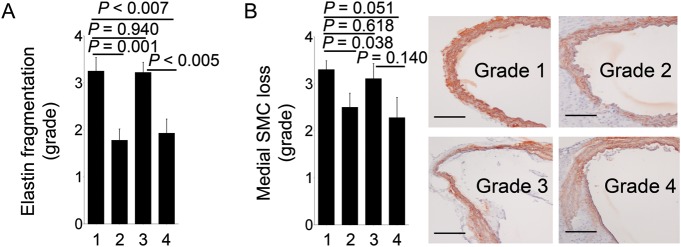
3.5. Mast cell deficiency impairs AAA lesion elastin fragmentation and medial SMC loss
One of the most important features of human and animal AAA is medial elastin degradation, mediated by elastases, including MMPs and cathepsins.41–43 Mast cells are rich reservoirs of these proteases15 in addition to their unique proteases, such as chymase, which also has elastinolytic activities.44 Lack of mast cells in Apoe–/–KitW-sh/W-sh mice, or reduction in mast cells in Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice, decreased AAA lesion medial elastin fragmentation significantly, compared with those in Apoe–/–mice or in Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice (Figure 6A). Preserved elastin filaments in AAA lesions from Apoe–/–KitW-sh/W-sh mice or Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/–Ccr2–/– mice protected medial SMC loss significantly (Figure 6B).
Figure 6.
Elastin fragmentation grade (A) and medial SMC loss grade (B) in AAA lesions from Apoe–/– mice, Apoe–/–KitW-sh/W-sh mice, and Apoe–/–KitW-sh/W-sh mice receiving BMMC from Apoe–/– mice or Apoe–/–Ccr2–/– mice, n = 9–11 per group. Data are mean ± SEM. Grading keys for SMC loss in (B) are indicated to the right. Scale bar: 200 µm. Experimental group denotations are the same as in Figure 2.
4. Discussion
This study provided evidence from experimental AAAs that mast cells participate importantly in AAA formation in mice, and that mast cell migration to AAA lesions relies on the expression of the mast cell surface chemokine MCP-1 receptor CCR2. The absence of mast cells protected Apoe–/– mice from Ang II infusion-induced AAAs, and deficiency of CCR2 on mast cells reduced mast cell recruitment into AAA lesions; blocked consequent accumulation of lesion Mac-3+ macrophages, CD4+ and CD8+ T cells; and failed to restore reduced AAA formation in mast cell-deficient mice.
Leucocyte migration and accumulation in atherosclerotic plaques,16 AAA lesions,15 and white adipose tissue in obese subjects44 are important in disease pathogenesis. We have been interested in which inflammatory cells appear first in these inflammatory lesions, and whether and how they recruit subsequent inflammatory cells. Although we do not know whether mast cells are the first inflammatory cells at the site of inflammation, they seem to appear before macrophages and T cells. In white adipose tissue, deficiency or pharmacological stabilization of mast cells prohibited macrophage accumulation.44 In this study, the absence of mast cells also reduced macrophage and CD4+ and CD8+ T-cell accumulation in AAA lesions. Once mast cells failed to infiltrate into AAA lesions due to the lack of MCP-1 receptor CCR2, infiltration of macrophages and T cells was also blocked, suggesting that mast cells are essential in recruiting other inflammatory cells.
This study left at least two fundamental questions unresolved. First, we showed that CCR2 deficiency in mast cells did not affect the expression of CCR3 or BLT1, and even increased the expression of BLT2. These observations support the hypothesis that mast cell migration uses CCR2 in this experimental AAA model. Mast cells lacking CCR3, BLT1, or BLT2, however, also may exhibit delayed infiltration. Mast cell infiltration into AAA lesions therefore may also use other chemokines, such as eotaxin or leukotriene; our study did not exclude these possibilities. Second, this study, and earlier studies by us and by others,2,15,16,44 suggested that mast cell accumulation to the site of inflammation in AAAs, atherosclerosis, and adipose tissue is essential to consequent cell infiltration. Depletion of T cells45–47 or neutrophils48 reduced macrophage and T-cell infiltration into AAA lesions, atherosclerotic lesions, and white adipose tissue, thereby protecting experimental animals from AAA, atherosclerosis, and obesity. It is possible that depletion of T cells or neutrophils also affects mast cell infiltration to AAA lesions. None of these studies, however, examined this possibility. Like many other chemokine receptors, CCR2 is a heterotrimeric G protein-coupled receptor that mediates MCP-1-induced leucocyte signalling. In macrophages, MCP-1 binding on CCR2 leads to immediate phosphorylation of p42/p44 (mitogen-activated protein kinases) MAPK, c-Jun N-terminal kinases, Lyn, Janus kinase 2 (JAK2), and cytoskeleton-binding protein paxillin followed by association of CCR2 with Lyn and paxillin and activation of downstream transcription factors c-Jun, signal transducer and activator of transcription (STAT)-3, and STAT-5.49,50 Tyrosine-phosphorylated proteins localize together with actin filament at the macrophage focal adhesion points.49 The same signalling pathways were detected in monocytes and CCR2-transfected human embryonic kidney 293 cells.51 Although not tested in this study, mast cells may also use these signalling pathways for their migration and accumulation in AAA lesions. This hypothesis is consistent with earlier observations that bone-marrow-derived macrophages from Apoe–/–Ccr2–/– mice showed significantly delayed accumulation in Ang II-induced AAA lesions compared with those from Apoe–/–mice.18 Both macrophages and mast cells use CCR2 for their migration and accumulation in Ang II-induced AAA lesions.
In this study, we proposed that the absence of mast cells or defective mast cell infiltration reduced AAA lesion angiogenesis (Figure 4), lesion cell apoptosis and proliferation (Figure 5), and medial elastin degradation and SMC loss (Figure 6). Although mast cells help promote angiogenesis, cell apoptosis, protease expression and secretion, the phenotypes presented in Figures 4–6 also can be secondary to reduced inflammatory cell infiltration and impaired AAA lesion formation. T cells and macrophages are rich in inflammatory cytokines, growth factors, and proteases that act similarly to mast cells. Reduced lesion angiogenesis, lesion cell apoptosis and cell proliferation, and AAA medial elastin degradation and SMC loss, therefore, can result from the combined loss of mast cells and reduction in macrophages, T cells, and other untested inflammatory cells. Regardless of whether the reduced AAA in Apoe–/–KitW-sh/W-sh mice receiving BMMCs from Apoe–/–Ccr2–/– mice was due to reduced accumulation of mast cells or consequently inflammatory macrophages and T cells (Figure 3), pharmacological blockade of CCR2 with its antibodies or antagonists may affect infiltration of mast cells and other inflammatory cells into human AAA lesions, thereby preventing or reducing human AAA formation or progression. This possibility has been tested in several other experimental inflammatory diseases and in human clinical trials. In mouse experimental collagen-induced rheumatoid arthritis, CCR2-neutralizing antibody MC-21 reduced disease severity significantly.52 Similarly, human CCR2 blocking antibody (MLN1202) has been used to treat patients with rheumatoid arthritis. Although MLN1202 did not ameliorate synovial inflammation in active rheumatoid arthritis, this antibody greatly reduced free CCR2 on the surface of peripheral blood monocytes.53 In an experimental mouse model of Porphyromonas gingivalis infection-induced periodontitis, CCR2 antagonist (JNJ-17166864) treatment significantly reduced the area of alveolar bone loss.54 Johnson & Johnson recently completed a randomized, blinded, placebo-controlled, parallel-group, two-centre outpatient study to test the efficacy of this CCR2 antagonist on patients with symptoms of seasonal allergic rhinitis, which was improved by 16% or greater (ClinicalTrials.gov Identifier: NCT00604123). JAK2 phosphorylation of CCR2 associates with Ca2+ mobilization.51 MCP-1 sensitized neurons or non-neuronal cells from rat spinal cords showed increased MCP-1 concentration-dependent Ca2+, which was abolished by AZ889—a CCR2 antagonist that diminishes chronic constriction injury-induced neuropathic pain in rats.55 This AstraZeneca compound has also been used in clinical trials for rheumatoid arthritis and chronic obstructive pulmonary disease. The CCR2 antagonist MK-0812 can efficiently block monocyte migration to the skin of rhesus monkeys in a delayed-type hypersensitivity reaction model.56 This Merck compound has been assessed in a clinical trial for patients with multiple sclerosis (ClinicalTrials.gov Identifier: NCT00239655). Indeed, there are many other CCR2 antibodies or antagonists undergoing clinical trials for human diseases, including rheumatoid arthritis, multiple sclerosis, chronic pain, lupus, restenosis, and atherosclerosis.57 These CCR2 antagonists or antibodies may be effective in blocking mast cell infiltration in human AAA lesions. This study nevertheless establishes a role of mast cells in AAA formation and the involvement of mast cell CCR2 in their infiltration to AAA lesions, and likely in other arterial or non-arterial lesions.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study is supported by grants from the National Institutes of Health (HL60942, HL81090, HL88547, to G.P.S.), and by an EIA award (0840118N) from the American Heart Association (to G.P.S.).
Supplementary Material
Acknowledgements
The authors thank Mrs Eugenia Shvartz for technical assistance, and Ms Sara Karwacki for editorial assistance.
Conflict of interest: none declared.
References
- 1.Tsunemi K, Takai S, Nishimoto M, Yuda A, Hasegawa S, Sawada Y, et al. Possible roles of angiotensin II-forming enzymes, angiotensin converting enzyme and chymase-like enzyme, in the human aneurysmal aorta. Hypertens Res. 2002;25:817–822. doi: 10.1291/hypres.25.817. doi:10.1291/hypres.25.817. [DOI] [PubMed] [Google Scholar]
- 2.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, et al. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. doi:10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 3.Mäyränpää MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, et al. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. doi: 10.1016/j.jvs.2009.03.055. discussion 395–396 doi:10.1016/j.jvs.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto S, Tsuruda T, Hatakeyama K, Sekita Y, Kato J, Imamura T, et al. Mast cell density and distribution in human abdominal aortic aneurysm. In. Grundmann R, ed. Etiology, Pathogenesis and Pathophysiology of Aortic Aneurysms and Aneurysm Rupture. Rijeka, Croatia: InTech, 2011. p55–66. [Google Scholar]
- 5.Xu J, Shi GP. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev. 2012;33:71–108. doi: 10.1210/er.2011-0013. doi:10.1210/er.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. doi:10.1016/S0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 7.Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20:466–469. doi: 10.1053/ejvs.2000.1217. doi:10.1053/ejvs.2000.1217. [DOI] [PubMed] [Google Scholar]
- 8.Thompson MM, Jones L, Nasim A, Sayers RD, Bell PR. Angiogenesis in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1996;11:464–469. doi: 10.1016/s1078-5884(96)80183-3. doi:10.1016/S1078-5884(96)80183-3. [DOI] [PubMed] [Google Scholar]
- 9.Choke E, Thompson MM, Dawson J, Wilson WR, Sayed S, Loftus IM, et al. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol. 2006;26:2077–2082. doi: 10.1161/01.ATV.0000234944.22509.f9. doi:10.1161/01.ATV.0000234944.22509.f9. [DOI] [PubMed] [Google Scholar]
- 10.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. doi:10.1161/01.CIR.99.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Heikkilä HM, Lätti S, Leskinen MJ, Hakala JK, Kovanen PT, Lindstedt KA. Activated mast cells induce endothelial cell apoptosis by a combined action of chymase and tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2008;28:309–314. doi: 10.1161/ATVBAHA.107.151340. doi:10.1161/ATVBAHA.107.151340. [DOI] [PubMed] [Google Scholar]
- 12.Kovanen PT. Mast cells: multipotent local effector cells in atherothrombosis. Immunol Rev. 2007;217:105–122. doi: 10.1111/j.1600-065X.2007.00515.x. doi:10.1111/j.1600-065X.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 13.Leskinen MJ, Heikkilä HM, Speer MY, Hakala JK, Laine M, Kovanen PT, et al. Mast cell chymase induces smooth muscle cell apoptosis by disrupting NF-kappaB-mediated survival signaling. Exp Cell Res. 2006;312:1289–1298. doi: 10.1016/j.yexcr.2005.12.033. doi:10.1016/j.yexcr.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Shiota N, Leskinen MJ, Lindstedt KA, Kovanen PT. Mast cell chymase inhibits smooth muscle cell growth and collagen expression in vitro: transforming growth factor-beta1-dependent and -independent effects. Arterioscler Thromb Vasc Biol. 2001;21:1928–1933. doi: 10.1161/hq1201.100227. doi:10.1161/hq1201.100227. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. doi:10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. doi:10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 17.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, et al. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol. 2007;179:691–697. doi: 10.4049/jimmunol.179.1.691. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, et al. Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174–e178. doi: 10.1161/01.ATV.0000143384.69170.2d. doi:10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- 19.Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. doi:10.1161/01.CIR.102.18.2185. [DOI] [PubMed] [Google Scholar]
- 20.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. doi:10.1016/S1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 21.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. doi:10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. doi:10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rastogi P, White MC, Rickard A, McHowat J. Potential mechanism for recruitment and migration of CD133 positive cells to areas of vascular inflammation. Thromb Res. 2008;123:258–266. doi: 10.1016/j.thromres.2008.03.020. doi:10.1016/j.thromres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. doi: 10.1016/j.pharmthera.2006.05.008. doi:10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Piqueras L, Kubes P, Alvarez A, O'Connor E, Issekutz AC, Esplugues JV, et al. Angiotensin II induces leukocyte-endothelial cell interactions in vivo via AT(1) and AT(2) receptor-mediated P-selectin upregulation. Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. doi:10.1161/01.CIR.102.17.2118. [DOI] [PubMed] [Google Scholar]
- 26.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. doi:10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty A, Manning MW, Cassis LA. Antagonism of AT2 receptors augments angiotensin II-induced abdominal aortic aneurysms and atherosclerosis. Br J Pharmacol. 2001;134:865–870. doi: 10.1038/sj.bjp.0704331. doi:10.1038/sj.bjp.0704331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulte S, Sun J, Libby P, Macfarlane L, Sun C, Lopez-Ilasaca M, et al. Cystatin C deficiency promotes inflammation in angiotensin II-induced abdominal aortic aneurisms in atherosclerotic mice. Am J Pathol. 2010;177:456–463. doi: 10.2353/ajpath.2010.090381. doi:10.2353/ajpath.2010.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. doi:10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. doi:10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. doi:10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struyf S, Menten P, Lenaerts JP, Put W, D'Haese A, De Clercq E, et al. Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol. 2001;31:2170–2178. doi: 10.1002/1521-4141(200107)31:7<2170::aid-immu2170>3.0.co;2-d. doi:10.1002/1521-4141(200107)31:7<2170::AID-IMMU2170>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. doi:10.1016/S0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. doi:10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327. doi: 10.1161/CIRCRESAHA.111.243758. doi:10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006;281:6020–6029. doi: 10.1074/jbc.M509134200. doi:10.1074/jbc.M509134200. [DOI] [PubMed] [Google Scholar]
- 37.Rizas KD, Ippagunta N, Tilson MD., 3rd Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. doi:10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 38.Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kübler W, et al. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler Thromb Vasc Biol. 2002;22:914–920. doi: 10.1161/01.atv.0000019009.73586.7f. doi:10.1161/01.ATV.0000019009.73586.7F. [DOI] [PubMed] [Google Scholar]
- 39.Roque M, Kim WJ, Gazdoin M, Malik A, Reis ED, Fallon JT, et al. CCR2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554–559. doi: 10.1161/hq0402.105720. doi:10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 40.Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. doi:10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004;110:3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. doi:10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–2508. doi: 10.1161/ATVBAHA.111.230201. doi:10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin k deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2012;32:15–23. doi: 10.1161/ATVBAHA.111.235002. doi:10.1161/ATVBAHA.111.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Divoux A, Sun J, Zhang J, Clément K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. doi:10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong W, Zhao Y, Prall A, Greiner TC, Baxter BT. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 46.Daugherty A, Puré E, Delfel-Butteiger D, Chen S, Leferovich J, Roselaar SE, et al. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E-/- mice. J Clin Invest. 1997;100:1575–1580. doi: 10.1172/JCI119681. doi:10.1172/JCI119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. doi:10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 48.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. doi:10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 49.Biswas SK, Sodhi A. Tyrosine phosphorylation-mediated signal transduction in MCP-1-induced macrophage activation: role for receptor dimerization, focal adhesion protein complex and JAK/STAT pathway. Int Immunopharmacol. 2002;2:1095–1107. doi: 10.1016/s1567-5769(02)00055-3. doi:10.1016/S1567-5769(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 50.Sodhi A, Biswas SK. Monocyte chemoattractant protein-1-induced activation of p42/44 MAPK and c-Jun in murine peritoneal macrophages: a potential pathway for macrophage activation. J Interferon Cytokine Res. 2002;22:517–526. doi: 10.1089/10799900252981990. doi:10.1089/10799900252981990. [DOI] [PubMed] [Google Scholar]
- 51.Mellado M, Rodríguez-Frade JM, Aragay A, del Real G, Martín AM, Vila-Coro AJ, et al. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161:805–813. [PubMed] [Google Scholar]
- 52.Brühl H, Cihak J, Schneider MA, Plachý J, Rupp T, Wenzel I, et al. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+ T cells. J Immunol. 2004;172:890–898. doi: 10.4049/jimmunol.172.2.890. [DOI] [PubMed] [Google Scholar]
- 53.Vergunst CE, Gerlag DM, Lopatinskaya L, Klareskog L, Smith MD, van den Bosch F, et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2008;58:1931–1939. doi: 10.1002/art.23591. doi:10.1002/art.23591. [DOI] [PubMed] [Google Scholar]
- 54.Barros SP, Arce RM, Galloway P, Lawter R, Offenbacher S. Therapeutic effect of a topical CCR2 antagonist on induced alveolar bone loss in mice. J Periodontal Res. 2011;46:246–251. doi: 10.1111/j.1600-0765.2010.01340.x. doi:10.1111/j.1600-0765.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 55.Serrano A, Paré M, McIntosh F, Elmes SJ, Martino G, Jomphe C, et al. Blocking spinal CCR2 with AZ889 reversed hyperalgesia in a model of neuropathic pain. Mol Pain. 2010;6:90. doi: 10.1186/1744-8069-6-90. doi:10.1186/1744-8069-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wisniewski T, Bayne E, Flanagan J, Shao Q, Wnek R, Matheravidathu S, et al. Assessment of chemokine receptor function on monocytes in whole blood: In vitro and ex vivo evaluations of a CCR2 antagonist. J Immunol Methods. 2010;352:101–110. doi: 10.1016/j.jim.2009.10.010. doi:10.1016/j.jim.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. doi:10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.