Abstract
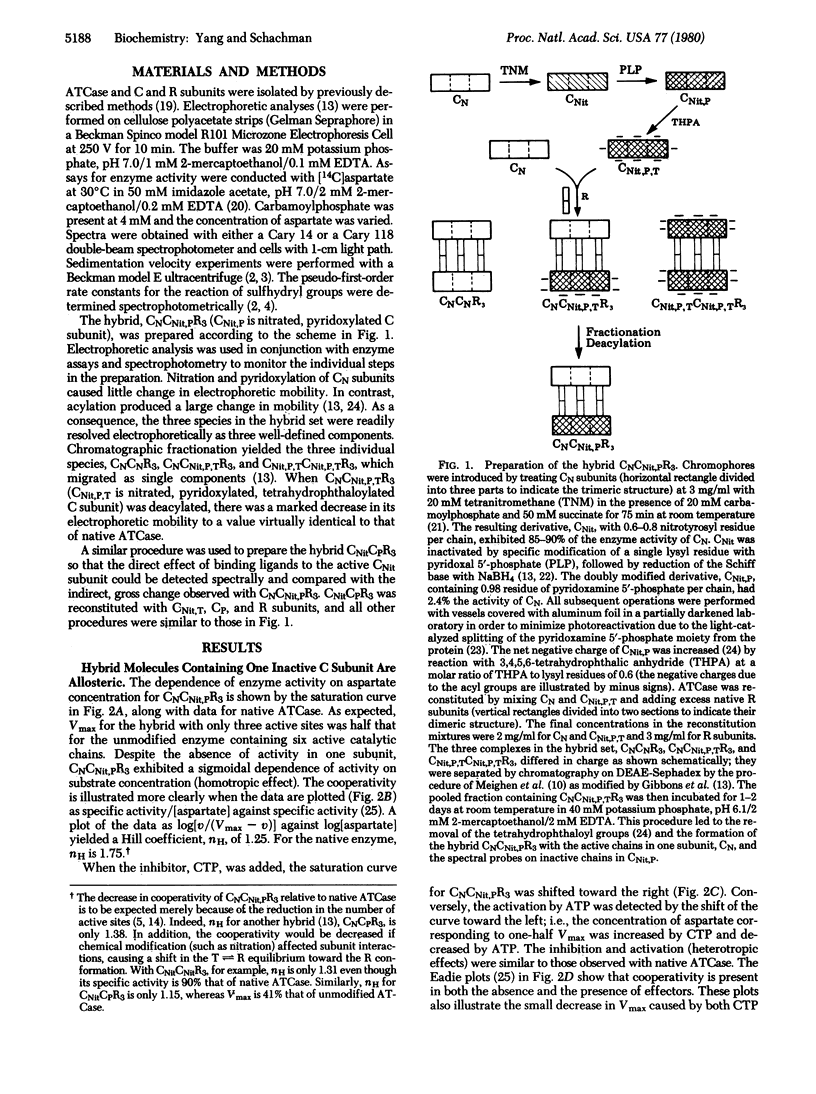
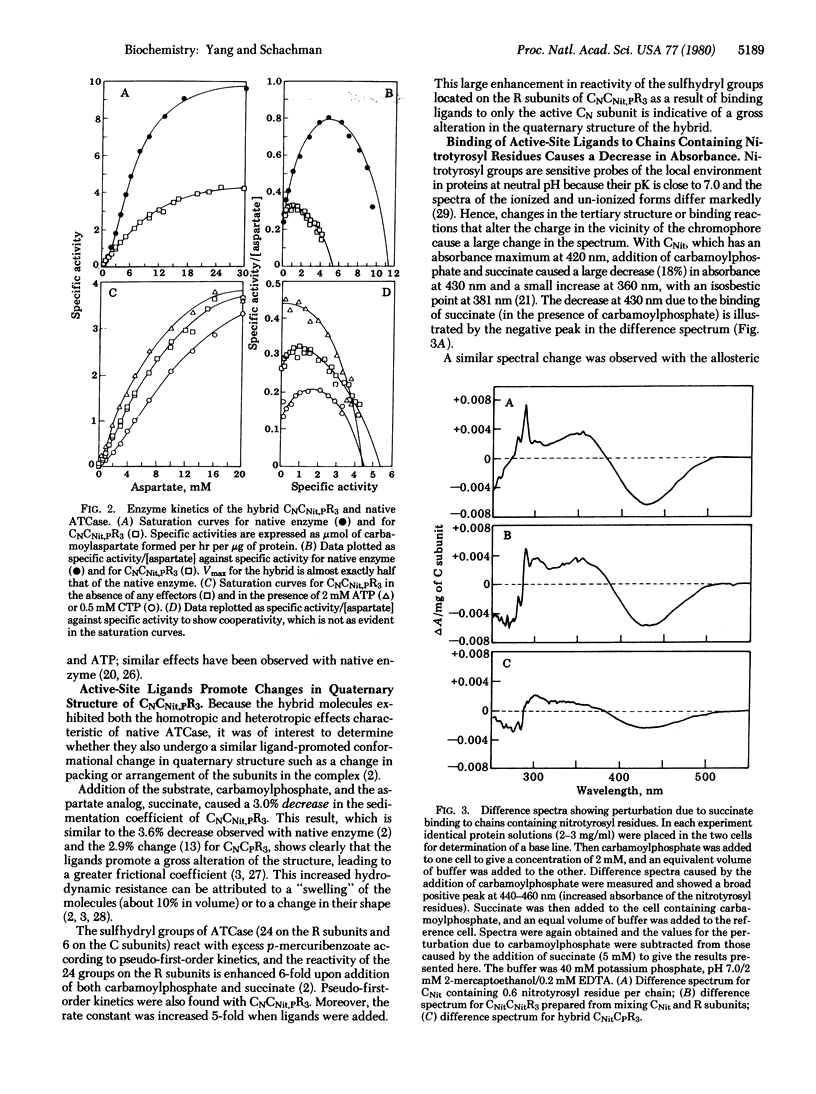
In the regulatory enzyme aspartate transcarbamoylase (aspartate carbamoyltransferase; carbamoylphosphate:L-aspartate carbamoyltransferase, EC 2.1.3.2) of Escherichia coli, the six catalytic polypeptide chains are arranged as two distinct catalytic trimers "crosslinked" by three regulatory dimers. Because in allosteric proteins it is assumed that the binding of a ligand to one site promotes a conformational change that affects the subsequent binding to other sites in the oligomeric protein, it was of interest to determine directly whether the effects of ligand binding to chains in one catalytic subunit are "communicated" to unliganded chains in the other subunit. Accordingly, hybrid enzyme molecules were constructed containing sensitive chromophores on the three inactive catalytic chains in one subunit along with an active catalytic subunit and three native regulatory subunits. The derivative exhibited the allosteric properties characteristic of the native enzyme. Communication between the two catalytic subunits was demonstrated by spectral measurements showing that the effects of ligand binding to the three active chains are propagated to the chromophores on the unliganded, inactive chains in the other subunit. Moreover, the change in the tertiary structure of the unliganded catalytic chains is tightly linked to the alteration in the quaternary structure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Blackburn M. N., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Effect of active site ligands on the reactivity of sulfhydryl groups of the regulatory subunits. Biochemistry. 1977 Nov 15;16(23):5084–5091. doi: 10.1021/bi00642a022. [DOI] [PubMed] [Google Scholar]
- Blackburn M. N., Schachman H. K. Alteration of the allosteric properties of aspartate transcarbamoylase by pyridoxylation of the catalytic and regulatory subunits. Biochemistry. 1976 Mar 23;15(6):1316–1323. doi: 10.1021/bi00651a023. [DOI] [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Dubin S. B., Cannell D. S. The effect of succinate on the translational diffusion coefficient of aspartate transcarbamylase. Biochemistry. 1975 Jan 14;14(1):192–195. doi: 10.1021/bi00672a032. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Ritchey J. M., Schachman H. K. Concerted allosteric transition in hybrids of aspartate transcarbamoylase containing different arrangements of active and inactive sites. Biochemistry. 1976 Mar 23;15(6):1324–1330. doi: 10.1021/bi00651a024. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Schachman H. K. A method for the separation of hybrids of chromatographically identical oligomeric proteins. Use of 3,4,5,6-tetrahydrophthaloyl groups as a reversible "chromatographic handle". Biochemistry. 1976 Jan 13;15(1):52–60. doi: 10.1021/bi00646a009. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Yang Y. R., Schachman H. K. Cooperative interactions in aspartate transcarbamoylase. 1. Hybrids composed of native and chemically inactivated catalytic polypeptide chains. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4452–4456. doi: 10.1073/pnas.71.11.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell P., Jewett S. L., Stark G. R. Aspartate transcarbamylase from Escherichia coli. The use of pyridoxal 5'-phosphate as a probe in the active site. J Biol Chem. 1973 Sep 10;248(17):5994–6001. [PubMed] [Google Scholar]
- Hensley P., Schachman H. K. Communication between dissimilar subunits in aspartate transcarbamoylase: effect of inhibitor and activator on the conformation of the catalytic polypeptide chains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3732–3736. doi: 10.1073/pnas.76.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Changes in the sedimentation coefficient promoted by the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Biochemistry. 1977 Nov 15;16(23):5077–5083. doi: 10.1021/bi00642a021. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Schachman H. K. Propagation of conformational changes in Ni(II)-substituted aspartate transcarbamoylase: effect of active-site ligands on the regulatory chains. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1995–1999. doi: 10.1073/pnas.77.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W., Schachman H. K. Local and gross conformational changes in aspartate transcarbamylase. Biochemistry. 1973 Jul 31;12(16):2997–3004. doi: 10.1021/bi00740a008. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Pigiet V., Schachman H. K. Hybridization of native and chemically modified enzymes. 3. The catalytic subunits of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1970 Jan;65(1):234–241. doi: 10.1073/pnas.65.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. F., Vachette P., Foote A. M. Changes in the x-ray solution scattering of aspartate transcarbamylase following the allosteric transition. J Mol Biol. 1979 Oct 9;133(4):517–532. doi: 10.1016/0022-2836(79)90405-4. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Riordan J. F., Sokolovsky M., Vallee B. L. Environmentally sensitive tyrosyl residues. Nitration with tetranitromethane. Biochemistry. 1967 Jan;6(1):358–361. doi: 10.1021/bi00853a053. [DOI] [PubMed] [Google Scholar]
- Ritchey J. M., Gibbons I., Schachman H. K. Reactivation of enzymes by light-stimulated cleavage of reduced pyridoxal 5'-phosphate-enzyme complexes. Biochemistry. 1977 Oct 18;16(21):4584–4590. doi: 10.1021/bi00640a008. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Subramani S., Bothwell M. A., Gibbons I., Yang Y. R., Schachman H. K. Ligand-promoted weakening of intersubunit bonding domains in aspartate transcarbamolylase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3777–3781. doi: 10.1073/pnas.74.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. G., Edwards B. F., Evans D. R., Wiley D. C., Lipscomb W. N. Aspartate transcarbamoylase from Escherichia coli: electron density at 5.5 A resolution. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1117–1121. doi: 10.1073/pnas.70.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Kirschner M. W., Schachman H. K. Aspartate transcarbamoylase (Escherichia coli): preparation of subunits. Methods Enzymol. 1978;51:35–41. doi: 10.1016/s0076-6879(78)51007-0. [DOI] [PubMed] [Google Scholar]


