Abstract
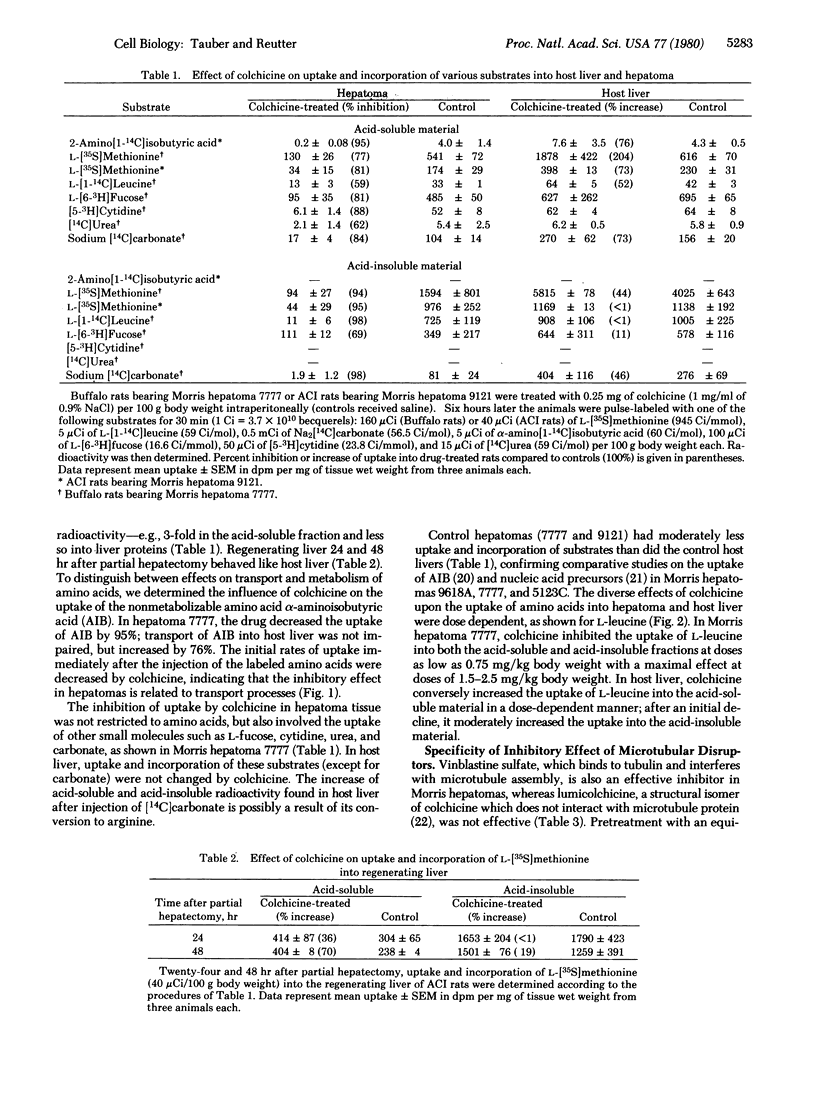
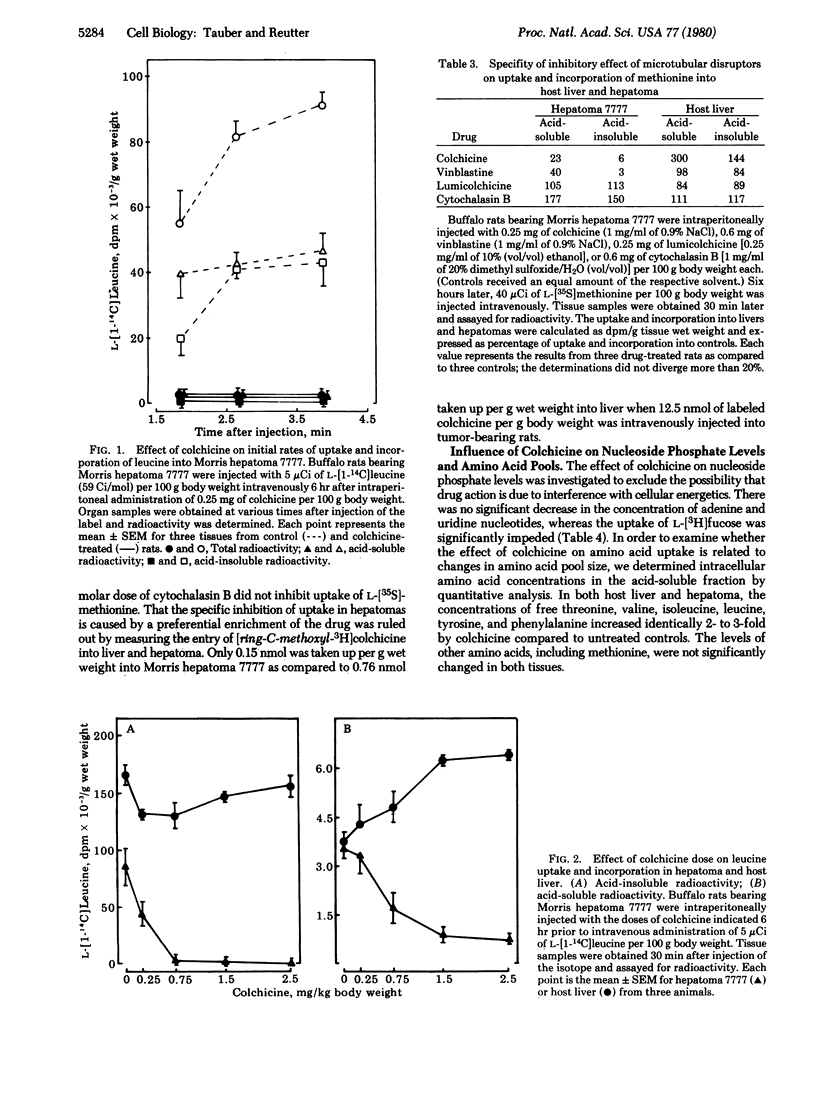
The interference of microtubular disruptors with the uptake of amino acids and other low molecular weight substrates has been studied in Morris hepatomas, host liver, and regenerating liver. Colchicine inhibits amino acid transport (alpha-aminoisobutyric acid, L-methionine, and L-leucine) in hepatomas by 59-98% whereas transport in host and regenerating liver is not impeded but increased. In hepatomas, treatment urea, and carbonate. Vinblastine, but not lumicolchicine or cytochalasin B, is an effective inhibitor. The inhibition of uptake is not linked to a decrease of cellular ATP and UTP. The data suggest that the transport of low molecular weight substrates in hepatomas is related to microtubules or other colchicine-binding structures, e.g., of the plasma membrane. This colchicine-sensitive uptake system in hepatomas may be due to the malignant transformation of hepatocytes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Borisy G. G., Shelanski M. L., Weisenberg R. C., Taylor E. W. Cytoplasmic filaments and tubules. Fed Proc. 1968 Sep-Oct;27(5):1186–1193. [PubMed] [Google Scholar]
- Baril E. F., Potter V. R., Morris H. P. Amino acid transport in rat liver and Morris hepatomas: effect of protein diet and hormones on the uptake of alpha-aminoisobutyric acid-14C. Cancer Res. 1969 Nov;29(11):2101–2115. [PubMed] [Google Scholar]
- Berlin R. D., Fera J. P. Changes in membrane microviscosity associated with phagocytosis: effects of colchicine. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1072–1076. doi: 10.1073/pnas.74.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya B., Volff J. Membrane-bound tubulin in brain and thyroid tissue. J Biol Chem. 1975 Oct 10;250(19):7639–7646. [PubMed] [Google Scholar]
- Dorling P. R., Quinn P. S., Judah J. D. Evidence for the coupling of biosynthesis and secretion of serum albumin in the rat. The effect of colchicine on albumin production. Biochem J. 1975 Nov;152(2):341–348. doi: 10.1042/bj1520341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe M. J., Loftfield S., Goldman I. D. A reduction in energy-dependent amino acid transport by microtubular inhibitors in Ehrlich ascites tumor cells. J Cell Physiol. 1975 Oct;86(2 Pt 1):201–211. doi: 10.1002/jcp.1040860203. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Parker C. M., Parker C. W. Colchicine-sensitive structures and lymphocyte activation. J Immunol. 1976 Sep;117(3):1015–1022. [PubMed] [Google Scholar]
- Holley R. W. A unifying hypothesis concerning the nature of malignant growth. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2840–2841. doi: 10.1073/pnas.69.10.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isselbacher K. J. Increased uptake of amino acids and 2-deoxy-D-glucose by virus-transformed cells in culture. Proc Natl Acad Sci U S A. 1972 Mar;69(3):585–589. doi: 10.1073/pnas.69.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Rich A., Potts J. T., Jr Microtubules and the intracellular conversion of proparathyroid hormone to parathyroid hormone. Endocrinology. 1975 Apr;96(4):903–912. doi: 10.1210/endo-96-4-903. [DOI] [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Lea M. A., Khalil F. L., Morris H. P., Bullock J. Incorporation of precursors and inhibitors of nucleic acid synthesis into hepatomas and liver of the rat. Cancer Res. 1974 Dec;34(12):3414–3420. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Manning C., Huang C. Y., Green K. In vivo effect of colchicine on hepatic protein synthesis and on the conversion of proalbumin to serum albumin. J Cell Biol. 1978 May;77(2):400–416. doi: 10.1083/jcb.77.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol B., Herman G., Keryer G. Inhibition by colchicine of carbamylcholine induced glycoprotein secretion by the submaxillary gland. A possible mechanism of cholinergic induced protein secretion. FEBS Lett. 1972 Mar 15;21(2):189–194. doi: 10.1016/0014-5793(72)80134-0. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Boutwell R. K., Potter V. R., Morris H. P. Lack of secretion of serum protein by transplanted rat hepatomas. Cancer Res. 1966 Nov;26(11):2357–2361. [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Stadler J., Franke W. W. Characterization of the colchicine binding of membrane fractions from rat and mouse liver. J Cell Biol. 1974 Jan;60(1):297–303. doi: 10.1083/jcb.60.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein O., Stein Y. Colchicine-induced inhibition of very low density lipoprotein release by rat liver in vivo. Biochim Biophys Acta. 1973 Apr 13;306(1):142–147. doi: 10.1016/0005-2760(73)90219-1. [DOI] [PubMed] [Google Scholar]
- Tauber R., Reutter W. Protein degradation in the plasma membrane of regenerating liver and Morris hepatomas. Eur J Biochem. 1978 Feb 1;83(1):37–45. doi: 10.1111/j.1432-1033.1978.tb12065.x. [DOI] [PubMed] [Google Scholar]
- Vischer P., Reutter W. Specific alterations of fucoprotein biosynthesis in the plasma membrane of Morris hepatoma 7777. Eur J Biochem. 1978 Mar 15;84(2):363–368. doi: 10.1111/j.1432-1033.1978.tb12176.x. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Whitfield J. F. Inhibition by colchicine of changes in amino acid transport and initiation of DNA synthesis in regenerating rat liver. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1394–1398. doi: 10.1073/pnas.75.3.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. II. The in vivo and in vitro binding of colchicine in grasshopper embryos and its possible relation to inhibition of mitosis. Biochemistry. 1967 Oct;6(10):3126–3135. doi: 10.1021/bi00862a021. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Müller R., Speth V. Direct evidence for a colchicine-induced impairment in the mobility of membrane components. Science. 1973 Dec 14;182(4117):1136–1138. doi: 10.1126/science.182.4117.1136. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J Cell Biol. 1973 Jul;58(1):27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


