Summary
Upon aging, the number of hematopoietic stem cells (HSCs) in the bone marrow increases while their repopulation potential declines. Moreover, aged HSCs exhibit lineage bias in reconstitution experiments with an inclination towards myeloid at the expense of lymphoid potential. The adaptor protein Lnk is an important negative regulator of HSC homeostasis, as Lnk deficiency is associated with a 10-fold increase in HSC numbers in young mice. However, the age-related increase in functional HSC numbers found in wild type (WT) HSCs was not observed in Lnk-deficient animals. Importantly, HSCs from aged Lnk null mice possess greatly enhanced self-renewal capacity and diminished exhaustion, as evidenced by serial transplant experiments. In addition, Lnk deficiency ameliorates the aging-associated lineage bias. Transcriptome analysis revealed that WT and Lnk-deficient HSCs share many aging-related changes in gene expression patterns. Nonetheless, Lnk null HSCs displayed altered expression of components in select signaling pathways with potential involvement in HSC self-renewal and aging. Taken together, these results suggest that loss of Lnk partially mitigates age-related HSC alterations.
Keywords: hematopoietic stem cells, hematopoiesis, cytokine, cell proliferation, self-renewal
Introduction
Blood cells are continually produced from the hematopoietic stem cells (HSCs) that reside in the bone marrow (BM). Throughout the lifespan of the organism, this stem cell reservoir sustains life. A major property of stem cells is their ability to self-renewal, which is important to maintain the HSC pool for the lifespan of the organism. However with aging, the HSC compartment undergoes distinct changes in terms of clonal composition and repopulating ability. The proportions of the myeloid-biased HSC subtype expands during aging while the HSC subtype producing balanced reconstitution remains unchanged, therefore the output of aging HSCs show myeloid skewing and diminished lymphoid differentiation potential. Age associated declines in immune function are multifactoral and involve many factors related and unrelated to HSCs (Myers et al. 2011). Notably, aged-related changes in the HSC compartment correlate with organismal aging, which manifests as anemia, increased propensity for myeloproliferative neoplasms (MPNs), decreased immune function, and increased cancer incidence (Morrison et al. 1996; Sudo et al. 2000; Rossi et al. 2005; Chambers et al. 2007).
Although HSCs are endowed with self-renewal capability in vivo (Harrison et al. 1978), they do have a limited life-span and exhaust after regenerative stress such as serial bone marrow transplantation (BMT) (Harrison & Astle 1982). Paradoxically, in both mice and humans, the number of HSCs increase during aging, while their repopulating and self-renewal abilities are reduced (Janzen et al. 2006). Accumulating evidence indicates that the unique aging properties of HSCs are genetically regulated, and both cell intrinsic and non-intrinsic factors contribute to HSC aging (Liang et al. 2005). However, the mechanisms for HSC alterations during aging are not well understood.
Multiple pathways are involved in regulating aging. A link between accumulated DNA damage and HSC aging has been demonstrated by the age-related decline of HSC function observed in mice deficient in several genomic maintenance pathways including DNA damage repair, telomere maintenance and FOXO transcription factors that regulates the oxidative stress response (Rossi et al. 2007; Tothova & Gilliland 2007). Proto-oncogenes and tumor suppressors that control cancer cell proliferation also regulate stem cell self-renewal and stem cell aging. For example, the polycomb family proto-oncogene, Bmi-1, is required for the self-renewal of diverse adult stem cells, as well as for the proliferation of cancer cells (Park et al. 2003). Bmi-1 functions in part by repressing the expression of p16Ink4a and p19Arf tumor suppressor genes (Pardal et al. 2005). p16Ink4a expression increases with age, and reduces stem cell frequency and function (Janzen et al. 2006). Additionally, caloric restriction and particularly inhibition of the mTOR pathway has been shown to increase lifespan and restore HSC function during aging in mice (Chen et al. 2009; Harrison et al. 2009). Therefore, networks of proteins have evolved to coordinately regulate stem cell function throughout life. Imbalances within such networks cause cancer or premature decline in stem cell activity that resemble accelerated aging. Despite these observations, there are few genetic systems that display reduced HSC aging. The development of an in vivo model that mitigates age related decline in HSC would provide an invaluable tool to understand the molecular basis of HSC aging.
We and other have previously reported that the intracellular adaptor protein Lnk negatively regulates cytokine signaling in multiple hematopoietic lineages, including HSCs. Mice deficient in Lnk harbor an expanded HSC pool during postnatal development. (Ema et al. 2005; Buza-Vidas et al. 2006). Young adult Lnk−/− mice have a 10-fold increase in HSC numbers and show superior multi-lineage repopulation after BMT (Ema et al. 2005; Buza-Vidas et al. 2006; Bersenev et al. 2008). Lnk controls HSC homeostasis and self-renewal, in part through restricting TPO/MPL/JAK2 activity (Buza-Vidas et al. 2006; Seita et al. 2007; Bersenev et al. 2008). Activating mutations in JAK2 as well as loss of function mutations in Lnk have been found in MPNs (Bersenev et al. 2010; Abdel-Wahab 2011), which are clonal stem cell diseases that arise mostly in older individuals. These observations suggest that JAK2 regulation is a pivotal determinant of HSC function and MPNs.
This study describes the role of Lnk in HSC aging by examining HSC frequency, lineage repopulating activity, self-renewal, and exhaustion upon regenerative stress. Transcriptome analysis comparing aged WT and Lnk−/− HSCs was performed to further reveal molecular mechanisms underlying Lnk regulation of HSC aging. Collectively, these analyses uncover novel aspects of HSC aging by Lnk.
Results
Young Lnk−/− HSCs exhibit enhanced self-renewal without premature exhaustion
The BM from young adult Lnk−/− mice harbor a 10-fold increase in HSC numbers (Ema et al. 2005; Buza-Vidas et al. 2006). On the other hand, we recently demonstrated that Lnk−/− HSCs show decelerated cell cycle and are more quiescent than their WT counterparts (Bersenev et al. 2008), raising the possibility that Lnk deficiency may protect HSCs from premature exhaustion despite superior self-renewal capability. Thus, we determined whether this vast expansion in the HSC compartment of young mice would lead to early HSC exhaustion upon regenerative stress (Ema et al. 2005; Buza-Vidas et al. 2006).
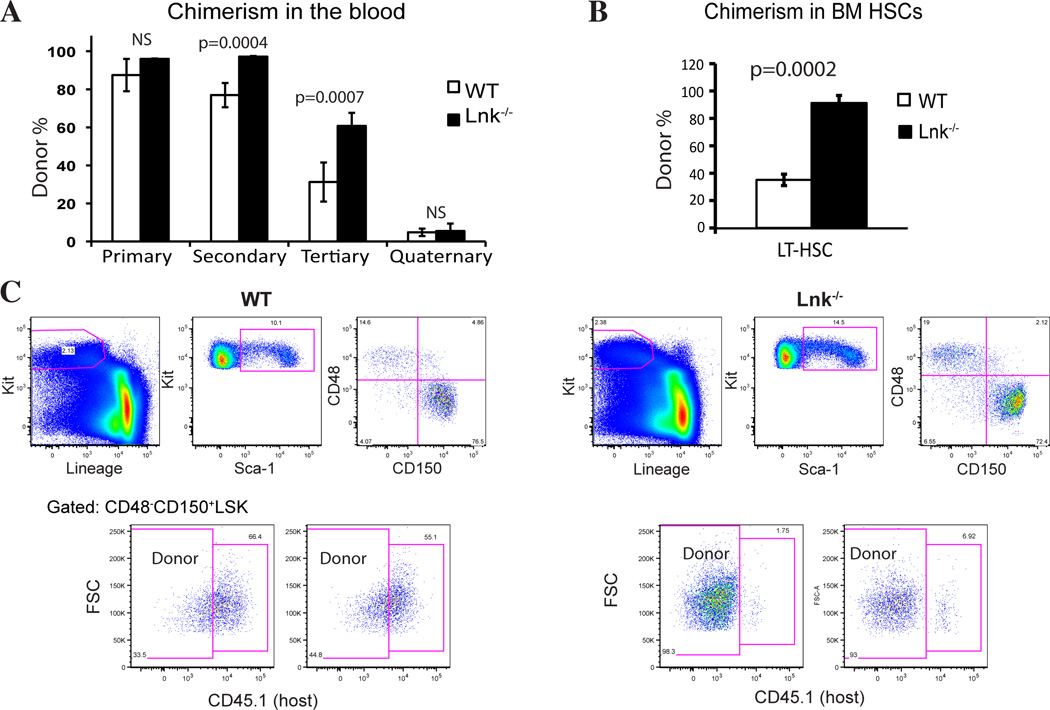
To experimentally determine the role of Lnk in HSC self-renewal and exhaustion, we first studied young Lnk−/− HSCs using serial BMT assays (Fig. 1). Since there is a 10-fold increase in HSC frequency in young Lnk−/− BM, we used 10-fold fewer unfractionated Lnk−/− BM cells (1×105) than WT cells (1×106) in the primary transplant to ensure equivalent HSC input. In each round of serial transplants, we found that Lnk−/− BM cells gave rise to significantly higher repopulation in the recipient peripheral blood (PB) (Fig. 1). This is due to increased HSC self-renewal in the BM, as evidenced by higher chimerism in the donor-derived HSC fractions in the BM measured using flow cytometry (FACS) analysis (Fig. 1). Therefore, young Lnk−/− HSCs have superior repopulating capability without premature exhaustion.
Figure 1. Young Lnk−/− HSCs exhibit enhanced self-renewal without premature exhaustion.
(A) 1 million BM cells from 2-month-old WT or 1×105 cells from 2-month-old Lnk−/− mice were transplanted into irradiated recipient mice (Primary BMT). Primary recipients were sacrificed at 8 months and 2 million BM cells were transplanted into secondary recipient mice (Secondary BMT). Similarly tertiary and quaternary BMTs were performed to functionally assay HSC self-renewal and exhaustion. Chimerisms of the donor-derived cells in peripheral blood (mean ± SE) from each transplant are shown. n=5–10. (B) The percentage of donor-derived LT-HSCs of tertiary transplanted mice was determined by flow cytometry using the SLAM marker (CD150+CD48−LSK). Donor-derived cells are judged by CD45.1− (host−). n=4. (C) Representative FACS plots showing the percentage of donor-derived HSCs are shown (left) and quantified (right).
Aged Lnk−/− mice show expanded phenotypical and functional HSCs compared to aged WT mice
In mice, various age-related changes in the HSC compartment have been reported [2]. Interestingly, while the number of HSCs in C57BL/6 (B6) mice increases with age, the regenerative ability is reduced and accompanied by an increase in myeloid-biased HSCs and decrease in lymphoid progenitors (Rossi et al. 2005; Janzen et al. 2006; Beerman et al. 2010; Benz et al. 2012).
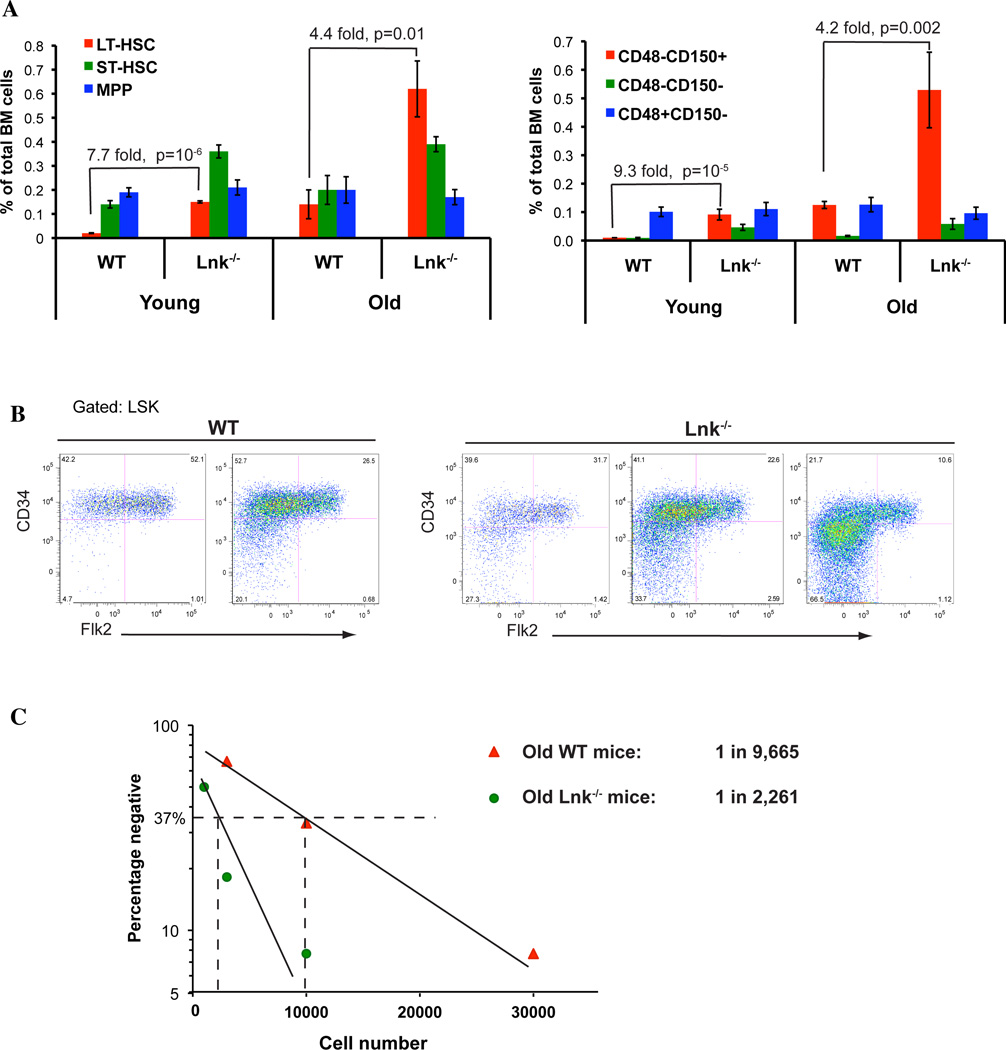
To study the role of Lnk in HSC aging, we examined various hematopoietic stem and progenitor (HSPC) subsets in aged Lnk−/− mice. Using multi-color FACS to examine cell surface markers for different HSPC subsets, we quantified phenotypic HSPC frequencies in young and old Lnk−/− mice in comparison to their young counterparts using two sets of well-established markers (Fig. 2A). In the first set, long-term (LT-) HSCs are defined as CD34−Flk2−Lineage−Sca-1+Kit+ (or CD34−Flk2−LSK), short-term (ST-) HSCs as CD34+Flk2−LSK, and multi-potential progenitors (MPP) as CD34+Flk2+LSK (Osawa et al. 1996). In the second set, LT-HSCs are defined as SLAM LSKs (or CD150+CD48−LSKs) (Kiel et al. 2005). Consistently, we found that Lnk−/− mice exhibit an expansion of phenotypic HSCs in the BM during aging (Fig. 2A and B). This expansion appears to be most pronounced in the LT-HSC compartment, less so in the ST-HSC or MPP compartments (Fig. 2A and B). Both sets of established HSC markers showed a consistent result that the phenotypic HSCs were 8–9 fold greater in young Lnk−/− mice while 4 fold greater in old Lnk−/− mice, when compared to their WT counterparts.
Figure 2. Aged Lnk−/− mice exhibit increased frequency of phenotypic and functional HSCs in comparison to aged WT mice.
(A) Using multi-color flow cytometry analysis to examine cell surface markers for different HSPC subsets, we quantified phenotypic HSPC frequencies in young and old Lnk−/− mice in comparison to WT mice. Left panel: LT-HSC: long-term HSC defined as CD34−Flk2−LSK; ST-HSC: short-term HSC defined as CD34+Flk2−LSK; MPP: multi-potential progenitor defined as CD34+Flk2+LSK. n=5–7. Right panel: HSPC compartments were quantified by their phenotypic expression of CD150+CD48−LSK, CD150−CD48−LSK, and CD150−CD48+LSK. n=7–15. (B) Representative FACS plots showing different HSPC subsets as determined by CD34 and Flk2 staining in the LSK population. (C) HSC frequencies of old WT and Lnk−/− mice were quantified using limiting dilution BMT assay. BM cells were serially diluted at the indicated numbers and mixed with 2 × 105 isogenic B6.SJL (CD45.1) competitors, and subsequently transplanted into lethally irradiated-recipient mice. Plotted is the percentage of recipient mice containing less than 1% donor-descendent cells in the peripheral blood 4–6 months post transplant. HSC frequencies are calculated as 37% negative repopulation using L-Cal software (StemCell Technology). 11–13 mice were used in each cell concentration.
We next quantified functional HSC numbers in old WT and Lnk−/− mice compared to their young counterparts, by limiting dilution BMT assays using unfractionated BM cells (Bersenev et al. 2008). BM cells from aged mice were serially diluted and mixed with 2×105 CD45.1 competitors, and subsequently transplanted into lethally irradiated recipient mice. Recipient mice containing over 1% donor-descended cells in peripheral blood 4–6 months post transplant were scored as positive repopulations (Szilvassy et al. 1990) (Fig. 2C). We previously showed that young Lnk−/− mice have a 10-fold increase in HSC frequency compared with WT mice (1 in 1,848 in Lnk−/− mice versus 1 in 18,570 in WT mice) (Bersenev et al. 2008). Here we found that aged Lnk−/− mice exhibited 4-fold greater HSC number than aged WT mice (1 in 2,261 in Lnk−/− mice versus 1 in 9,665 in WT mice) (Fig. 2C). Therefore, both young and aged Lnk−/− mice indeed exhibit an expanded HSC pool, phenotypically and functionally, when compared to age appropriate WT controls. Interestingly the difference between aged Lnk−/− and aged WT mice is smaller than that between young Lnk−/− and young WT mice. It is important to note that with age, WT HSCs as determined by limiting dilution BMTs were increased 2-fold in number, while Lnk−/− HSCs remained the same. Therefore, we conclude that aged-associated increase in functional HSCs was not observed in Lnk−/− mice.
Aged Lnk−/− HSCs exhibit enhanced self-renewal without premature exhaustion
In spite of an age-related increase in the number of HSCs in old mice, the repopulating and self-renewal ability are reduced. Since we found that young Lnk−/− HSCs have superior repopulating capability without premature exhaustion (Fig. 1), we set out to investigate whether aged Lnk−/− HSCs also have enhanced self-renewal ability and whether they are spared from early exhaustion when regenerative stress is encountered. To this end, we performed serial BMTs using both unfractionated BM cells (Fig. 3) as well as purified HSCs (Fig. 4).
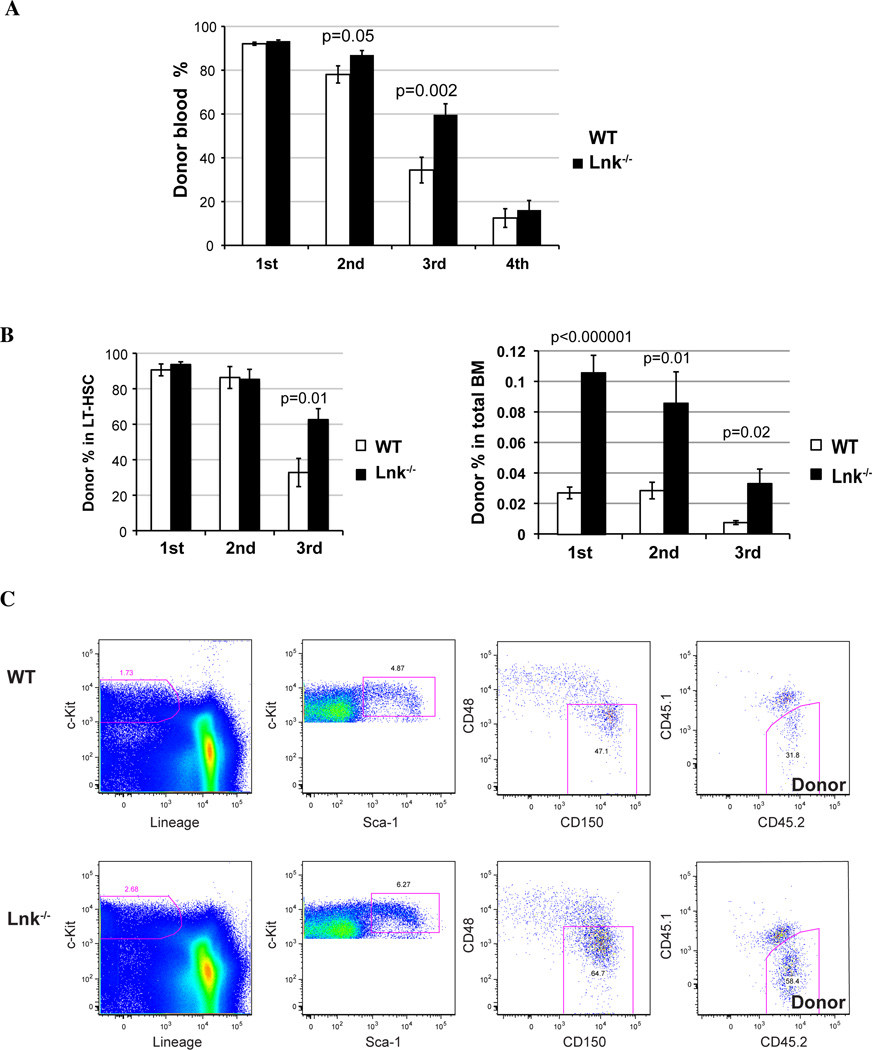
Figure 3. Aged Lnk−/− HSCs exhibit enhanced self-renewal without premature exhaustion.
(A) 1 million BM cells from 18-month-old WT or 2×105 cells from Lnk−/− mice were transplanted into irradiated recipient mice (Primary BMT). Serial BMTs were performed as describe in Fig. 1 to functionally assay HSC self-renewal and exhaustion. Chimerisms of the donor-derived cells in peripheral blood (mean ± SE) from each transplant are shown. Two-tailed t-tests were performed and p values are labeled on top of each comparison between WT and Lnk−/− mice. The results are pooled from 3 independent experiments. n=13–15. (B) The percentages of donor cells in LT-HSC compartment (left) and the percentages of donor LT-HSCs in total BM cells (right) from each transplant were determined and quantified by flow cytometry using the SLAM marker (mean ± SE). p values indicate comparisons between WT and Lnk−/− in each transplant. The results are pooled from 3 independent experiments. n=13–20. (C) Representative FACS plots showing the percentage of donor-derived HSCs are shown. Donor-derived cells are judged by CD45.2− while the host cells CD45.1+/CD45.2+ (F1).
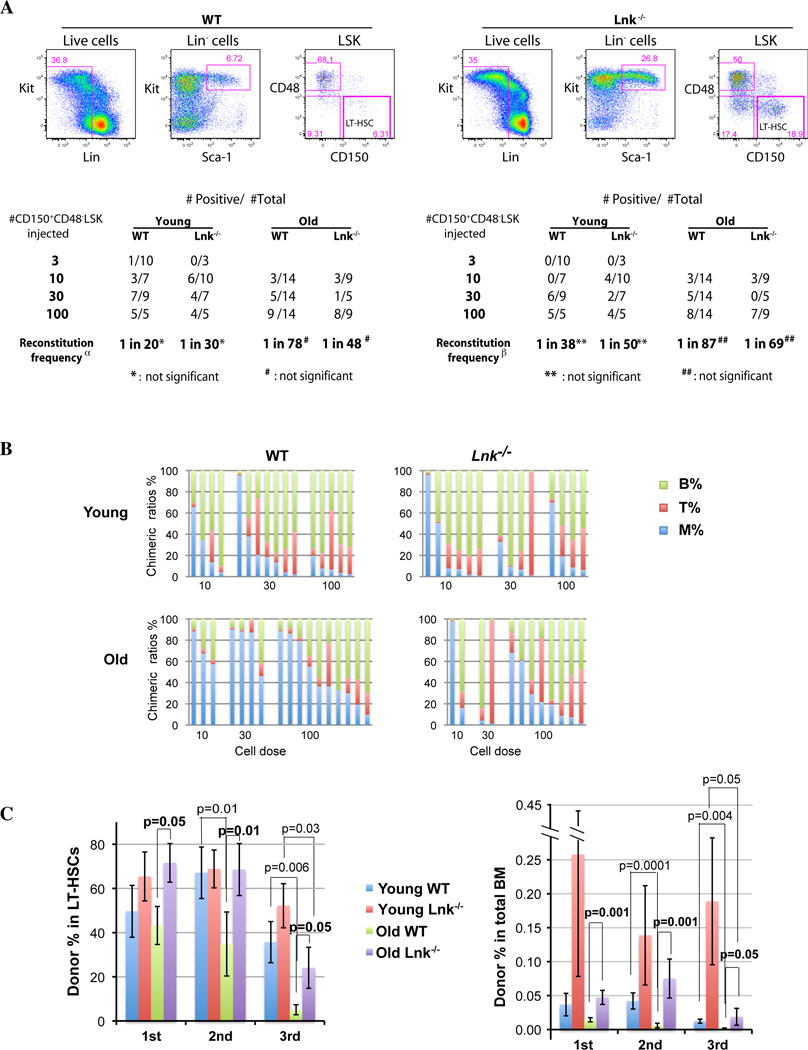
Figure 4. Purified HSCs from aged Lnk−/− mice show balanced reconstitution, enhanced self-renewal and delayed exhaustion.
(A) HSCs were double sorted for CD150+CD48−LSK (CD45.2) surface markers, and a representative plot is shown on the top. 3, 10, 30, or 100 purified HSCs were transplanted to lethally irradiated F1 mice along with 2×105 competitor cells. Peripheral blood chimerism was analyzed 4 months after reconstitution. Positively-engrafted mice versus total mice injected are shown. Reconstitution frequency was calculated using L-Calc software. Positive engraftment was defined as either >1% donor derived cells in the total blood (left α) or >1% donor cells in the myeloid lineage and secondary transplantability (right β). (B) Bars show lineage chimeric ratios of individual recipients of the primary transplanted mice with more than 1% donor-derived cells in the blood. Two-tailed t-test is used to compare aged and young WT HSCs (M%: 0.004; T%: p=0.04; B%: p=0.0003;), as well aged WT versus Lnk−/− HSCs (M%: 0.01; T%: p=0.03). The results are pooled from 3 independent experiments. n=10– 20. (C) All the positively repopulated mice in Fig. 4A (or 4B) were used for secondary and successive transplantations. The percentages (AVG±SE) of donor-derived LT-HSCs (left) and the percentages of donor LT-HSCs in total BM cells (right) from each transplant were determined and quantified by flow cytometry using the SLAM marker (CD150+CD48−LSK). Donor-derived cells are judged by CD45.2+ while the host cells as CD45.1+/CD45.2+. Two-tailed t-tests were used and p values are indicated on top of the bars.
We first performed serial BMTs using unfractionated total BM cells. 1 × 106 BM cells from old WT and 2 × 105 cells from Lnk−/− mice were transplanted into primary recipients to ensure equivalent HSC input. There was no difference observed in the first transplants due to saturated reconstitution (>90%) in both WT and Lnk−/− transplanted mice (Fig. 3A). However, in the second and tertiary transplants, Lnk−/− BM cells gave rise to significantly higher repopulation in the recipient peripheral blood (Fig. 3A). After three rounds of serial transplants, both WT and Lnk−/− transplanted cells dropped in repopulating capacity at the fourth transplant.
Importantly, the chimeric ratios of B-, T-, and myeloid- reconstitution in the blood were altered in the aged Lnk−/− transplants but not in young Lnk−/− transplants, when compared to their respective WT counterparts (Supplemental Fig. 1S). While aged WT HSCs showed significantly increased myeloid but decreased lymphoid reconstitution when compared to young WT HSCs as previously reported (Muller-Sieburg et al. 2004; Rossi et al. 2005), aged Lnk−/− HSCs exhibited a more balanced reconstitution in both myeloid and lymphoid compartments (Supplemental Fig. 1S). Interestingly, we also found that myeloid potential accrues and myeloid-biased HSCs become dominate upon serial transplants, at both young and old ages and in both genotypes.
Furthermore, we measured donor-HSC percentages in the BM after each transplant by their surface SLAM markers using FACS, to directly interrogate HSC self-renewal capability after each round of regenerative stress. Indeed, changes in the peripheral blood chimerism were preceded by HSC changes in the previous transplants, indicating we are examining HSC self-renewal properties rather than compensational expansion of progenitor cells. When the donor percentages in the HSC compartment were calculated, aged Lnk−/− HSCs were much greater than that of WT HSCs at the end of the third transplant, probably due to saturated reconstitutions in the first 2 rounds of transplants (Fig. 3B, left panel). Importantly, when we took into account HSC numbers in transplanted mice by calculating donor percentages of HSCs in total BM cells, we found that Lnk−/− cells gave rise to markedly increased HSC numbers in all three rounds of transplants (Fig. 3C, right panel). Of note, Lnk−/− HSCs declined at the third transplant (Fig. 3C) and were exhausted at the fourth (Fig. 3A), similarly to their WT counterparts. Taken together, HSCs from aged Lnk−/− mice exhibit superior repopulating capability and enhanced self-renewal without premature exhaustion.
Purified HSCs from aged Lnk−/− mice show balanced reconstitution, enhanced self-renewal, and delayed exhaustion
Unfractionated BM cells contain lineage specific progenitors as well as mature cells, some of which are long lived. Therefore, lineage reconstitution using unfractionated BM may be confounded by these factors. In addition, large numbers of progenitors and mature cells lessen the need for the stem cells to proliferate extensively. Thus, to investigate the intrinsic ability of HSCs as well as functionally quantify HSC self-renewal capability, we serially transplanted purified HSCs from both young and aged mice (Fig. 4). Specifically, we sorted for LT-HSCs using the SLAM markers (CD150+CD48−LSK) and injected a graded numbers of purified HSCs (3, 10, 30, or 100) into each irradiated recipient along with competitors. We compared the LT-repopulating ability of young and aged WT and Lnk−/− HSCs by analyzing peripheral blood reconstitution of donor-descended cells in all lineages (Fig. 4A and B). Since HSCs deficient in myeloid reconstitution are defective in secondary transplantation assays (ref (Dykstra et al. 2007) and our data not shown), we defined positive engraftment as >1% donor derived cells in the myeloid lineage as well as >1% donor derived cells in total blood (Fig. 4A). WT and Lnk−/− HSCs showed similar repopulating frequency at both young and old age (Fig. 4A), suggesting that HSC surface markers remain unperturbed in Lnk−/− mice and the frequencies of Lnk−/− HSC are indeed expanded in both young and aged mice. Importantly, the chimeric ratios of B-, T-, and myeloid- reconstitution in the blood were improved in the aged Lnk−/− transplants (Fig. 4B). While aged WT HSCs showed significantly increased myeloid but decreased lymphoid reconstitution when compared to young WT HSCs as previously reported (Muller-Sieburg et al. 2004; Rossi et al. 2005), aged Lnk−/− HSCs exhibited a more balanced reconstitution in both myeloid and lymphoid compartments (Fig. 4B).
We next examined the self-renewal and exhaustion of purified HSCs from WT and Lnk−/− mice at both young and old ages. We directly measured the donor-derived HSC population in the BM after each transplant using FACS analysis (Fig. 4C and Supplemental Fig. 2S). When the donor percentages in the HSC compartment were calculated, superior Lnk−/− HSC self-renewal in the aged mice was apparent even at the first transplant when compared to aged WT mice, and remained greater than that of WT HSCs in all subsequent transplants (Fig. 4C, left panel). Aged WT HSCs declined rapidly in regenerative ability when compared to young WT HSCs. In contrast, aged Lnk−/− HSCs did not decline significantly until the third transplant. Consequently, the differences between aged WT and Lnk−/− HSCs were more pronounced in all rounds of transplants, than those between young mice (Fig. 4C, left panel).
Importantly, by calculating donor HSCs in total BM cells, we found that Lnk−/− cells gave rise to markedly increased HSC numbers in all three rounds of transplants at both young and old ages (Fig. 4C, right panel). WT HSCs from aged mice declined faster (1st versus 2nd, p<0.03) and exhausted earlier than that from young mice (2nd versus 3rd, p=0.05), suggesting that HSCs lose regenerative ability during aging. In contrast, aged Lnk−/− HSCs were indistinguishable from young WT HSCs, and both groups did not decline until the third transplant. Strikingly, young Lnk−/− HSCs remained high in all transplants (Fig. 4C, right panel). Aged Lnk−/− HSCs did show inferior self-renewal ability than young Lnk−/− HSCs. It suggests that although aged Lnk−/− HSCs display enhanced self-renewal and delayed exhaustion compared to aged WT HSCs, Lnk−/− HSCs do decline during aging.
Microarray analysis of WT and Lnk−/− HSCs during aging
To investigate the molecular mechanisms by which Lnk regulates HSCs during aging, we performed genome-wide gene profiling using purified LT-HSCs from WT and Lnk−/− mice at both young (n=3 in each genotype) and old (n=4 in each genotype) ages.
We interrogated our data with two different unbiased approaches. The first is an over-representation test that evaluates whether the genes of a GeneOntology (GO) term are over-represented by a list of selected genes having differential expression, using Functional Annotation Bioinformatics Microarray Analysis (DAVID) analysis (Huang da et al. 2009). The other approach is an enrichment test that evaluate whether the genes of a GO term have unevenly distributed rankings given that all genes in the data set had been ranked by their differential expression, using Gene Set Enrichment analysis (GSEA) (Subramanian et al. 2005). We further categorized the data by comparing the significantly changed gene sets from both comparisons by genotype or by age, to identify common and unique processes.
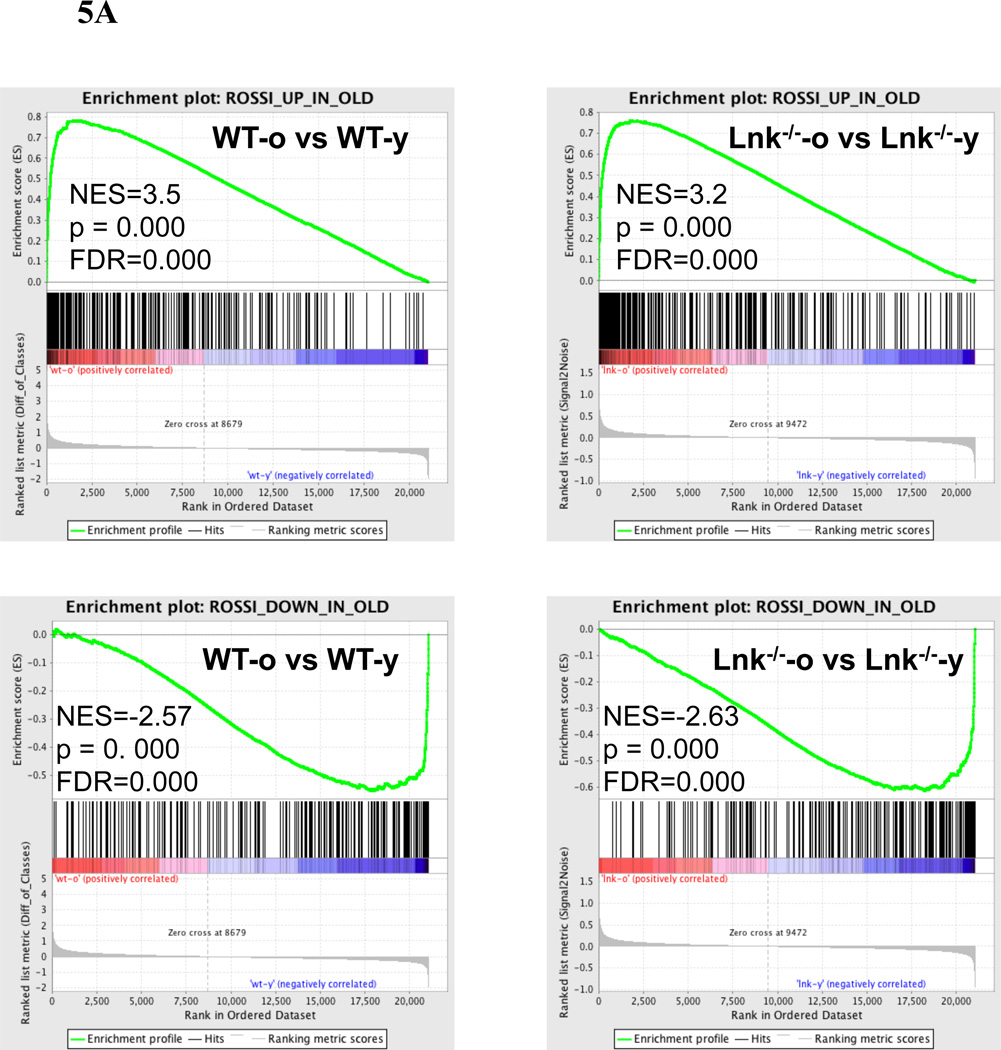
GSEA analysis of old versus young Lnk−/− HSCs and old versus young WT HSCs were both aligned remarkably well to the “aging signature” that is derived from the published dataset from the Weissman group (Rossi et al. 2005) (Fig. 5A). Our data is also in good agreement with the dataset from the Goodell group (Chambers et al. 2007), in particular the upregulated genes (NES=3.2, p<0.0001; FDR<0.0001) and to a lesser extent, the downregulated genes (NES=−1.2, p=0.012; FDR=0.055) (data not shown). WT and Lnk−/− HSCs shared numerous similar changes during aging (Supplemental Table 1). Among the common changes in both genotypes were a decrease in cell cycle/ replication and DNA repair pathways and an increase in cytokine/ chemokine signaling and cell adhesion pathways, which is consistent with previous reports (Rossi et al. 2005; Chambers et al. 2007) (Supplemental Table 1).
Figure 5. Microarray analysis of WT and Lnk−/− HSCs.
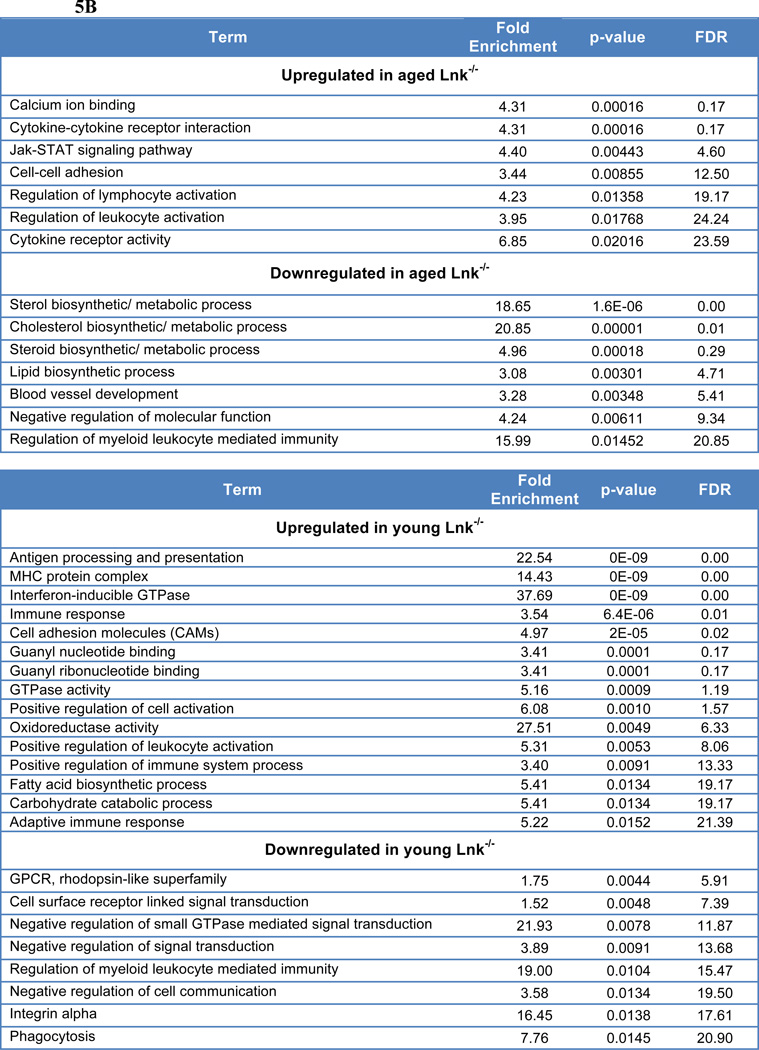
(A) Enrichment plots of GSEA analysis using both aged Lnk−/− versus young Lnk−/− HSC, and aged WT versus young WT HSC expression data against a list of aging-related gene changes reported previously (Rossi et al. 2005). NES: normalized enrichment score; NOM: nominal p-value; FDR: false discovery rate. (B) DAVID analysis using GO categories of 250 most up-regulated or down-regulated genes when compared aged Lnk−/− to aged WT HSCs (Top) or young Lnk−/− to young WT HSCs (Bottom).
Importantly, the immune signaling and coagulation pathways showed a more pronounced increase in representations in the aging WT HSCs (Supplemental Table 1). Remarkably, directly comparing aged Lnk−/− versus aged WT HSCs, we found no significant enrichment of any canonical pathways in aged Lnk−/− HSCs using the cut-off criteria NES ≥ 1.7 or ≤ −1.7, p val< 0.05 and FDR ≤ 0.2 (Supplemental Table 2). It suggests that the aging process is partially mitigated by Lnk deficiency.
In addition, GSEA revealed that aged WT HSCs exhibited enrichments in cell cycle and DNA replication (Supplemental Table 2), which is consistent with our previous findings that Lnk−/− HSCs display decelerated cell cycle kinetics and more quiescence (JCI 2008). When directly comparing aged Lnk−/− versus aged WT HSCs using DAVID analysis, which was done with the top 250 differentially expressed genes, we found that JAK/STAT and cytokine receptor activity were elevated in aged Lnk−/− HSCs (Fig. 5B). This is also consistent with the previous findings that Lnk−/− HSCs exhibit an enhanced response to Tpo in activating JAK2/Stat5 (Buza-Vidas et al. 2006; Seita et al. 2007; Bersenev et al. 2008).
When directly compared with young WT HSCs, both DAVID (Fig. 5B) and GSEA analysis (Supplemental Table 3) revealed a significant upregulation of metabolic pathways in young Lnk−/− HSCs. Furthermore, young Lnk−/− HSCs showed an upregulation in RNA/protein processing, immune signaling, as well as DNA repair and damage response by GSEA (Supplemental Table 3). Among downregulated gene in Lnk−/− HSCs were GPCR/ olfactory pathways, adhesion, as well as DNA replication (Fig. 5B and Supplemental Table 3).
Notably, regulation of myeloid leukocyte mediated immunity was down in both young and old Lnk−/− HSCs when compared to their WT counterparts, while regulation of lymphocyte activation or positive regulation of immune system process was upregulated in young and aged Lnk−/− HSCs (Fig. 5B). This is consistent with our finding of a more balanced HSC composition in Lnk−/− HSCs upon aging, in comparison to a myeloid-biased HSC composition in aged WT HSCs.
Discussion
A critical question in stem cell biology is how HSCs age, in other words what accounts for their increases in numbers, their functional decline, and their lineage bias. Our studies attempt to address these questions with the remarkable findings that the loss of the signaling molecule Lnk partially ameliorates these phenotypes of aged HSCs. Our results showed that both young and old Lnk−/− mice harbor an expanded HSC pool in the BM. Both young and old Lnk−/− HSCs are endowed with superior self-renewal when compared to their WT counterparts. Aged Lnk−/− HSCs showed a more balanced lineage reconstitution than aged WT HSCs in the primary transplant. Lnk deficiency partially reversed the aging-related decline in HSC self-renewal upon serial transplants and slowed the rate of exhaustion. However, they do decline after extensive regenerative stress. Taken together, we demonstrate that the loss of the signaling molecule Lnk partially ameliorates functional phenotypes related to HSC aging.
By surface markers, we found that aged WT HSCs increase 6-fold that is consistent with previous reports (Morrison et al. 1996; Sudo et al. 2000; Rossi et al. 2005). However, by repopulation activity using unfractionated BM cells, we found that aged HSCs increase 2-fold in number, similarly to a previous report (Sudo et al. 2000; Janzen et al. 2006). One reason that may account for this inconsistency is that the HSC surface markers in old mice do not equivalently represent functional HSCs to that in young mice. Another non-mutually exclusive possibility is that although aged HSCs retain similar surface markers to that in young mice, they decline in repopulating ability. Our results revealed an increase in phenotypic HSCs upon aging, similarly to WT mice. However, it is important to note that with age, while functional HSC numbers were increased 2-fold in WT mice, Lnk−/− HSCs remained the same. Therefore, aged-assocaited increase in functional HSCs was not observed in Lnk−/− mice. We obtained a consistent result in terms of relative differences between Lnk−/− and WT mice at both young or old ages, when we used either functional or phenotypic criteria. Lnk−/− mice display increased functional and phenotypic HSC numbers both at the young and old ages, when compared to age appropriate WT controls. Interestingly, the difference in frequency between aged Lnk−/− and WT HSCs is smaller than that at the young age. It is puzzling that both human and murine HSCs increase in number upon aging (Rossi et al. 2005; Pang et al. 2011). Is it a mechanism to compensate for the loss of the regenerative ability? Or is it an intrinsic HSC property, or merely a consequence of altered systemic or micro- environment upon aging? These questions remain to be addressed in the future. In sum, our data suggest that Lnk deficiency mitigates the age-related increase in HSC frequency.
Accumulating evidence suggest that an alteration in the composition of the HSC compartment occurs during aging. It is now known that the myeloid-biased HSC (or α-HSC) subset with robust self-renewal potential but diminished lymphopoietic potential expands, and becomes the dominant HSC subtype during aging. This is accompanied by a proportional decrease in the balanced HSC (or β-HSC) subtype (Cho et al. 2008; Beerman et al. 2010; Dykstra et al. 2011; Benz et al. 2012). Our WT HSC data also support these conclusions. In contrast, aged Lnk−/− HSCs exhibit a more balanced HSC lineage reconstitution than aged WT HSCs. A similar lineage reconstitution is noted in young WT and Lnk−/− HSCs. The expansion of myeloid-biased HSCs upon aging in WT mice could be due to their intrinsic robust self-renewal capability, and to compensate for the overall decline in HSC repopulating ability during aging. We speculate that Lnk−/− HSCs with superior self-renewal ability lessens the need for expansion of myeloid-biased HSCs. Alternatively, myeloid-biased HSCs in Lnk−/− mice do not accrue an age-related decline in lymphopoietic potential. Our microarry data support this notion in that Lnk−/− HSCs show increased lymphopoietic and immunity-related changes but down regulation of myeloid leukocyte mediated immunity when compared to their WT counterparts. Future investigation is warranted to directly interrogate the differentiation potential of HSC subtypes as well as lineage-committed progenitors in Lnk−/− mice.
In spite of an age-related increase in the number of HSCs in old mice, the repopulating and self-renewal ability are reduced. Both young and old Lnk−/− mice exhibit a remarkable superior self-renewal capability than their WT counterparts. Aged Lnk−/− HSCs are indistinguishable from young WT HSCs in their regenerative ability. However, Lnk−/− HSCs do decline and exhaust after extensive replication stress. Therefore Lnk deficiency alone is not enough to reverse the aging clock although it can unleash a remarkable self-renewal potential. Our data raise an intriguing possibility that HSC self-renewal and exhaustion are distinctly regulated. Exhaustion may not simply reflect a loss of self-renewal potential. It perhaps is an actively- regulated process that is subjected to both cell intrinsic and non-intrinsic mechanisms. Accumulating evidence points to DNA damage, oxidative stress and mitochondria-related dysfunction affecting stem cell aging (Norddahl et al. 2011; Yahata et al. 2011). Telomerase activity/ telomere length and senescence are potential mechanisms that dictate lifespan and extinction (Ju et al. 2007; Campisi et al. 2011). The physiological, functional and mechanistic relationships between self-renewal and exhaustion therefore warrant future investigations. Lnk−/− mice will be an important model system to address this topic.
We and others previously showed that Lnk constrains self-renewal in part through limiting TPO/MPL/JAK2 activity (Buza-Vidas et al. 2006; Seita et al. 2007; Bersenev et al. 2008). Our finding that Lnk deficiency partially reverses HSC aging phenotypes, suggests that the TPO/JAK2 pathway is an important regulator of HSC aging. The repopulating potential of human HSCs progressively deteriorates as they undergo extensive repopulation. This correlates with their reduced intrinsic proliferative capacity, determined as S-phase entry in response to cytokines in vitro (Yahata et al. 2011). Lnk−/− HSCs have potentiated cytokine responses and JAK/STAT signaling, possibly account for their diminished exhaustion under regenerative stress. It is tempting to speculate that cytokine therapy during bone marrow transplantation will therefore improve clinical outcomes. Cytokine therapy may not only enhance the differentiation and production of mature hematopoietic cells, but also improve the engraftment and expansion of HSCs.
To probe the molecular mechanisms underlying HSC aging, we performed transcriptional profiling of young and aged mice. Our microarray data are consistent with functional studies showing that aged WT and Lnk−/− HSCs share similar aging-related changes. Increased inflammatory response is hypothesized to be an intrinsic process associated with aging (Chambers et al. 2007), and pro-inflammatory cytokines activate oxidative DNA damage checkpoint mechanisms in HSCs to limit HSC function (Zhang et al. 2007). Notably, WT HSCs show a more pronounced upregulation of immune signaling and coagulation genes during aging, than Lnk−/− HSCs. The top genes upregulated in immune signaling pathways include Fos, Jun, and Leukocyte antigens (HLAs), while in the coagulation pathways this involves platelet-selectin (selp), Clusterin (clu), integrin beta3 (itgb3), and Von Willebrand factor (vWF). Although there is overlap with inflammatory cytokine response genes, these are not part of the classical NF-κB pathway. Thus, our data suggest that Lnk deficiency mitigates the difference in gene expression between young and old HSCs; however, the precise mechanism for its partial reversal of HSC aging remains to be established. Furthermore, we found that genes relate to oxidative activity and mitochondria-related metabolic pathways were upregulated in young Lnk−/− HSCs. Accumulative reactive oxidative species (ROS)-induced DNA damage during transplantation restricts the self-renewal capacity of HSCs (Yahata et al. 2011). Therefore, our results warrant future investigation into a potential new mechanism by which Lnk controls HSC self-renewal through regulating ROS production and the consequent DNA damage.
Taken together, we demonstrate that the loss of the signaling molecule Lnk partially ameliorates functional phenotypes related to HSC aging. These studies could provide new insights into stem cell aging and facilitate clinical applications to stem cell therapy in the treatment of aging-related diseases.
Experimental Procedures
Mice
Lnk−/− mice were generously provided by Drs. Tony Pawson (Samuel Lunenfeld Research Institute) (Velazquez et al. 2002), and backcrossed onto the C57/BL6 (CD45.2) background for over 9 generations. Young (2–4 months old) and old (15– 24 months old) mice were used in this study. This work is under an approved protocol from the Institutional Animal Care and Use committee (IACUC) of Children’s Hospital of Philadelphia (CHOP).
Limiting dilution competitive BMT assay
Unfractionated BM cells were serially diluted, mixed with 2 × 105 CD45.1 competitors, and subsequently transplanted into lethally irradiated (CD45.2/CD45.1) recipient mice (a split dose of 10 Gy of othovoltage X-ray, Precision X-Ray Inc.). Four to six months post transplant, the percentage of chimerism in the peripheral blood (PB) was analyzed as the fraction of donor-descended CD45.2 cells using flow cytometry as previously described (Tong et al. 2007). Mice with over 1% donor-descent cells in the peripheral blood were counted as “positive reconstitution” (Szilvassy et al. 1990). Results from 3 independent experiments were pooled. Calculation of competitive repopulation units (CRUs) was conducted using L-Calc software (StemCell Technologies).
Total BM transplants
For young mice, 1 million total BM cells from WT and 0.1 million cells from Lnk−/− mice were injected into lethally-irradiated hosts without competitors. For old mice, 1 million total BM cells from WT and 0.2 million cells from Lnk−/− mice were injected into lethally-irradiated hosts without competitors. The donor (CD45.2+) and the remaining host (CD45.1+) populations were used to quantify donor % in the peripheral blood and BM HSCs (Tong et al. 2007; Schindler et al. 2009).
Flow cytometric analysis of HSPC subsets and HSC purification for transplants
The staining procedure is performed according previously established protocol (Ema et al. 2006; Bersenev et al. 2008; Jiang et al. 2012). Briefly, for HSPC subset BM cells were first stained with biotinylated-lineage cocktail antibodies that contains anti-CD4, CD8, CD5, Ter119, Gr-1, Mac-1, B220, CD19 and IL-7R antibodies (eBiosciences), followed by streptavidin-PETexasRed secondary antibodies (Caltag, Invitrogen) and PE-Cy5.5-conjugated anti-Sca-1, APC-Alexa750- Kit, APC-CD34, and PE-Flk2 (eBiosciences) antibodies. For SLAM markers, APC-Cy7- CD150 and FITC-CD48 antibodies were obtained from BioLegend. Cells were subsequently analyzed on LSRII flow cytometer (BD Bioscences).
HSC purification for BMT was performed as previously described (Kiel et al. 2005). Briefly, Lin- BM cells were first enriched with Dynabeads (Invitrogen), followed by FACS staining as described above. CD150+CD48−LSK HSCs were double-sorted using FACS Aria (BD Biosciences) high-speed sorters. HSCs were sorted into 96-well plates as 3, 10, 30, or 100 cells per well, and subsequently transplanted with 2×105 competitors.
Serial BMTs
Primary transplanted mice were sacrificed at 4 – 6 months, and 2 million total BM cells were transplanted into each lethally- irradiated secondary recipient mouse. Tertiary and quaternary transplants were performed similarly. The day of each transplant, a fraction of the cells were analyzed for donor-derived HSCs as CD45.2+CD45.1− CD150+CD48−LSKs.
Microarray analysis of purified HSCs
CD150+CD48−LSK HSCs were double sorted from WT and Lnk−/− mice at both young and old ages (2 months and 20 months, respectively). RNA was isolated using miRNeasy kit from QIAGEN and the microarray analysis was performed at the Penn Molecular Profiling/Genomics Facility using GeneChip Mouse Gene 1.0ST array (Affymetrix). Resulting expression data was normalized using robust multichip analysis (RMA) directly from the CELL files. Significant differential expression between the two groups was analyzed and genes with SAM p values less than 0.05 were selected. Upon the acceptance of this manuscript, the microarray data will be deposited at the Gene Expression Omnibus. The top 250 differentially expressed genes were subjected to DAVID analysis using Gene Ontology (GO) terms. Microarray data were also tested for gene set enrichment analysis (GSEA) MSigDb c2.cp v3.0 (Subramanian et al, 2005) (http://www.broad.mit.edu/gsea/).
Supplementary Material
Acknowledgements
WT was supported by NIH grants R01HL095675, R01HL110806, and R21HL102688. WT received a New Investigator Award from the Myeloproliferative Neoplasm Research Foundation and Gabrielle’s Angel Foundation for Cancer Research. AB was supported by NIH training grants T32HL007971. JJ and KR were supported by postdoctoral fellowships from the American Heart Association (AHA). We would like to thank the Human Hematopoietic Stem Cell Center of Excellence P30 DK090969 for its support of microarray analysis. We are grateful to Drs. Gerd Blobel and Roger Greenberg for critical review of the manuscript.
Non-standard abbreviations
- BMT
bone marrow transplant
- HSCs
hematopoietic stem cells
Footnotes
The authors have declared that no conflict of interest exists.
Author contributions
WT designed experiments, analyzed the data, and wrote the manuscript. JB, KR, AB, JJ, CW performed the experiments, analyzed the data, and helped write the manuscript. The authors declare no competing financial interests.
References
- Abdel-Wahab O. Genetics of the myeloproliferative neoplasms. Curr Opin Hematol. 2011;18:117–123. doi: 10.1097/MOH.0b013e328343998e. [DOI] [PubMed] [Google Scholar]
- Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C, Copley MR, Kent DG, Wohrer S, Cortes A, Aghaeepour N, Ma E, Mader H, Rowe K, Day C, Treloar D, Brinkman RR, Eaves CJ. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell. 2012;10:273–283. doi: 10.1016/j.stem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Bersenev A, Wu C, Balcerek J, Jing J, Kundu M, Blobel GA, Chikwava KR, Tong W. Lnk constrains myeloproliferative diseases in mice. J Clin Invest. 2010;120:2058–2069. doi: 10.1172/JCI42032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza-Vidas N, Antonchuk J, Qian H, Mansson R, Luc S, Zandi S, Anderson K, Takaki S, Nygren JM, Jensen CT, Jacobsen SE. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Semin Cancer Biol. 2011;21:354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Olthof S, Schreuder J, Ritsema M, de Haan G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. J Exp Med. 2011;208:2691–2703. doi: 10.1084/jem.20111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Morita Y, Yamazaki S, Matsubara A, Seita J, Tadokoro Y, Kondo H, Takano H, Nakauchi H. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nat Protoc. 2006;1:2979–2987. doi: 10.1038/nprot.2006.447. [DOI] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age cell number, and transplantation procedure. J Exp Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang daW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jiang J, Balcerek J, Rozenova K, Cheng Y, Bersenev A, Wu C, Song Y, Tong W. 14-3-3 regulates the LNK/JAK2 pathway in mouse hematopoietic stem and progenitor cells. J Clin Invest. 2012;122:2079–2091. doi: 10.1172/JCI59719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–4118. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- Myers CE, Mirza NN, Lustgarten J. Immunity, cancer and aging: lessons from mouse models. Aging Dis. 2011;2:512–523. [PMC free article] [PubMed] [Google Scholar]
- Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Molofsky AV, He S, Morrison SJ. Stem cell self-renewal and cancer cell proliferation are regulated by common networks that balance the activation of protooncogenes and tumor suppressors. Cold Spring Harb Symp Quant Biol. 2005;70:177–185. doi: 10.1101/sqb.2005.70.057. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler JW, Van Buren D, Foudi A, Krejci O, Qin J, Orkin SH, Hock H. TEL-AML1 corrupts hematopoietic stem cells to persist in the bone marrow and initiate leukemia. Cell Stem Cell. 2009;5:43–53. doi: 10.1016/j.stem.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Seita J, Ema H, Ooehara J, Yamazaki S, Tadokoro Y, Yamasaki A, Eto K, Takaki S, Takatsu K, Nakauchi H. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci U S A. 2007;104:2349–2354. doi: 10.1073/pnas.0606238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci U S A. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Ibarra YM, Lodish HF. Signals emanating from the membrane proximal region of the thrombopoietin receptor (mpl) support hematopoietic stem cell self-renewal. Exp Hematol. 2007;35:1447–1455. doi: 10.1016/j.exphem.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Velazquez L, Cheng AM, Fleming HE, Furlonger C, Vesely S, Bernstein A, Paige CJ, Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnkdeficient mice. J Exp Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, Ando K. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sejas DP, Qiu Y, Williams DA, Pang Q. Inflammatory ROS promote and cooperate with the Fanconi anemia mutation for hematopoietic senescence. J Cell Sci. 2007;120:1572–1583. doi: 10.1242/jcs.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.