Abstract
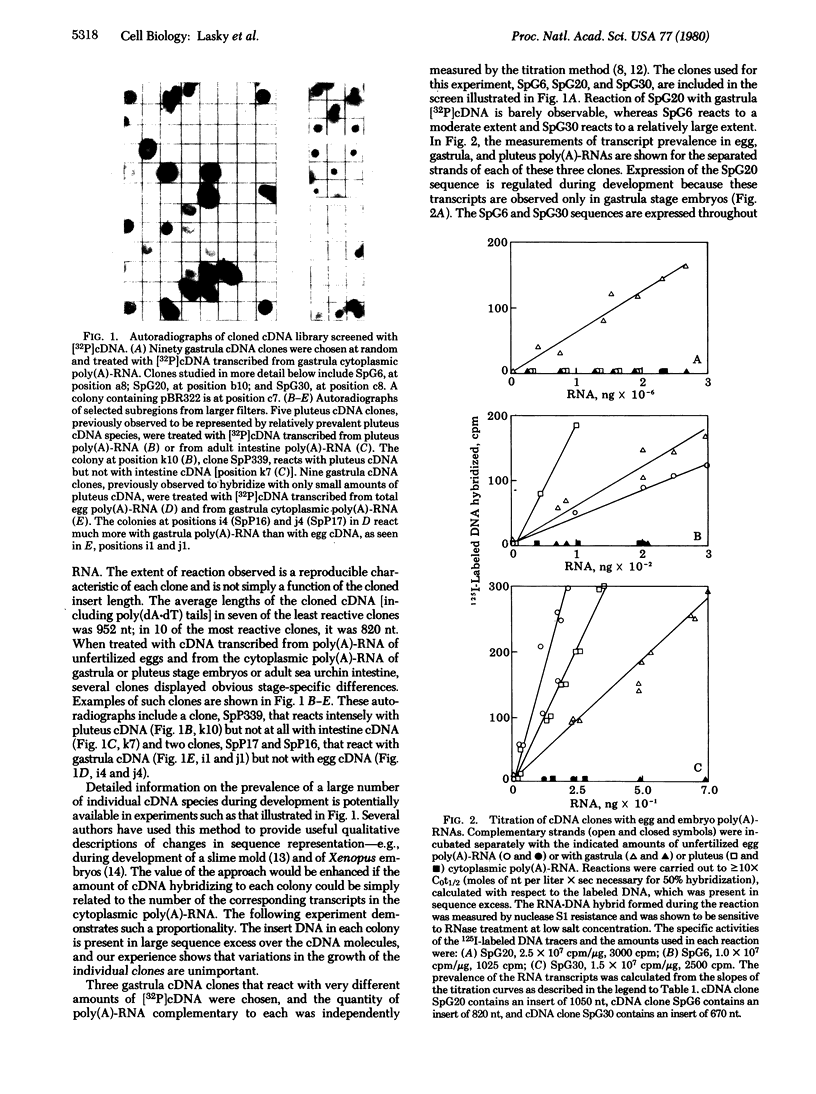
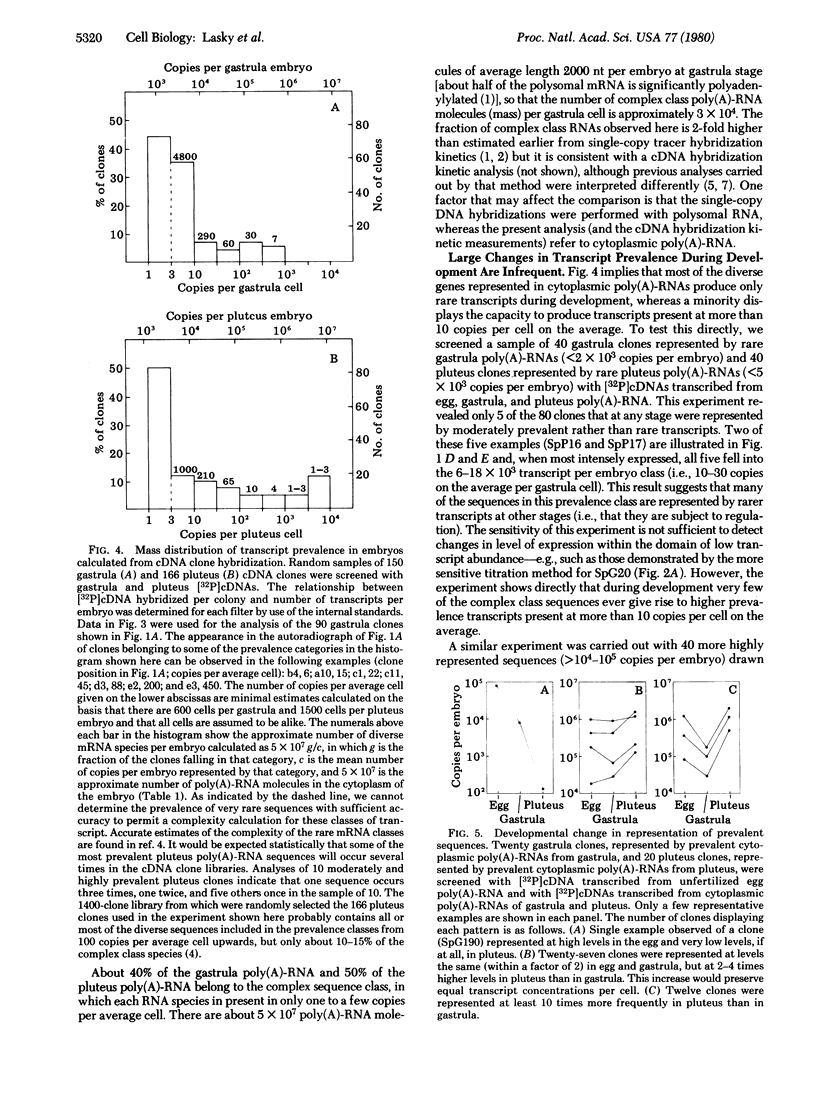
mRNA prevalence during sea urchin development was measured by treating cDNA clone colonies with labeled cDNAs transcribed from unfertilized egg and embryo poly(A)-RNAs. The number of cytoplasmic transcripts per embryo complementary to several clones was determined independently by titration with poly(A)-RNA in solution, and the amount of cDNA bound to these clones in colony hybridizations was shown to be proportional to the concentration of the respective poly(A)-RNAs in the embryo cytoplasm. At the gastrula stage, the most prevalent mRNA species occur in about 10(6) molecules per embryo. If all cells were equivalent, this would be a few hundred molecules per cell. By pluteus stage, the prevalence of some sequences has increased more than 10-fold. Most, though not all, sequences prevalent in later embryos are also present in the maternal RNA of the unfertilized egg. For most poly(A)-RNA sequences, the prevalence levels determined during oogenesis are maintained through the pluteus stage, whereas a minority of sequences display sharp stage-specific changes in representation during development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P., Verma D. P., Fromson D. Polyadenylated and nonpolyadenylated messenger RNA fractions from sea urchin embryos code for the same abundant proteins. Dev Biol. 1979 Jul;71(1):128–141. doi: 10.1016/0012-1606(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Dworkin M. B., Dawid I. B. Use of a cloned library for the study of abundant poly(A)+RNA during Xenopus laevis development. Dev Biol. 1980 May;76(2):449–464. doi: 10.1016/0012-1606(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Lipson E. D., Britten R. J., Davidson E. H. Synthesis and turnover of polysomal mRNAs in sea urchin embryos. Cell. 1977 Mar;10(3):415–432. doi: 10.1016/0092-8674(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Evans B. R., Wold B. J., Ernst S. G., Britten R. J., Davidson E. H. Appearance and persistence of maternal RNA sequences in sea urchin development. Dev Biol. 1977 Oct 1;60(1):258–277. doi: 10.1016/0012-1606(77)90123-3. [DOI] [PubMed] [Google Scholar]
- Lev Z., Thomas T. L., Lee A. S., Angerer R. C., Britten R. J., Davidson E. H. Developmental expression of two cloned sequences coding for rare sea urchin embryo messages. Dev Biol. 1980 May;76(2):322–340. doi: 10.1016/0012-1606(80)90382-6. [DOI] [PubMed] [Google Scholar]
- McColl R. S., Aronson A. I. Changes in transcription patterns during early development of the sea urchin. Dev Biol. 1978 Jul;65(1):126–138. doi: 10.1016/0012-1606(78)90185-9. [DOI] [PubMed] [Google Scholar]
- Nemer M., Graham M., Dubroff L. M. Co-existence of non-histone messenger RNA species lacking and containing polyadenylic acid in sea urchin embryos. J Mol Biol. 1974 Nov 5;89(3):435–454. doi: 10.1016/0022-2836(74)90474-4. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Costantini F. D., Kozlowski M. R., Britten R. J., Davidson E. H. Specific representation of cloned repetitive DNA sequences in sea urchin RNAs. Cell. 1978 Sep;15(1):189–203. doi: 10.1016/0092-8674(78)90094-6. [DOI] [PubMed] [Google Scholar]
- Tufaro F., Brandhorst B. P. Similarity of proteins synthesized by isolated blastomeres of early sea urchin embryos. Dev Biol. 1979 Oct;72(2):390–397. doi: 10.1016/0012-1606(79)90129-5. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M. Changes in the abundance of polyadenylated RNA during slime mould development measured using cloned molecular hybridization probes. J Mol Biol. 1979 Mar 25;129(1):19–35. doi: 10.1016/0022-2836(79)90056-1. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]



