Abstract
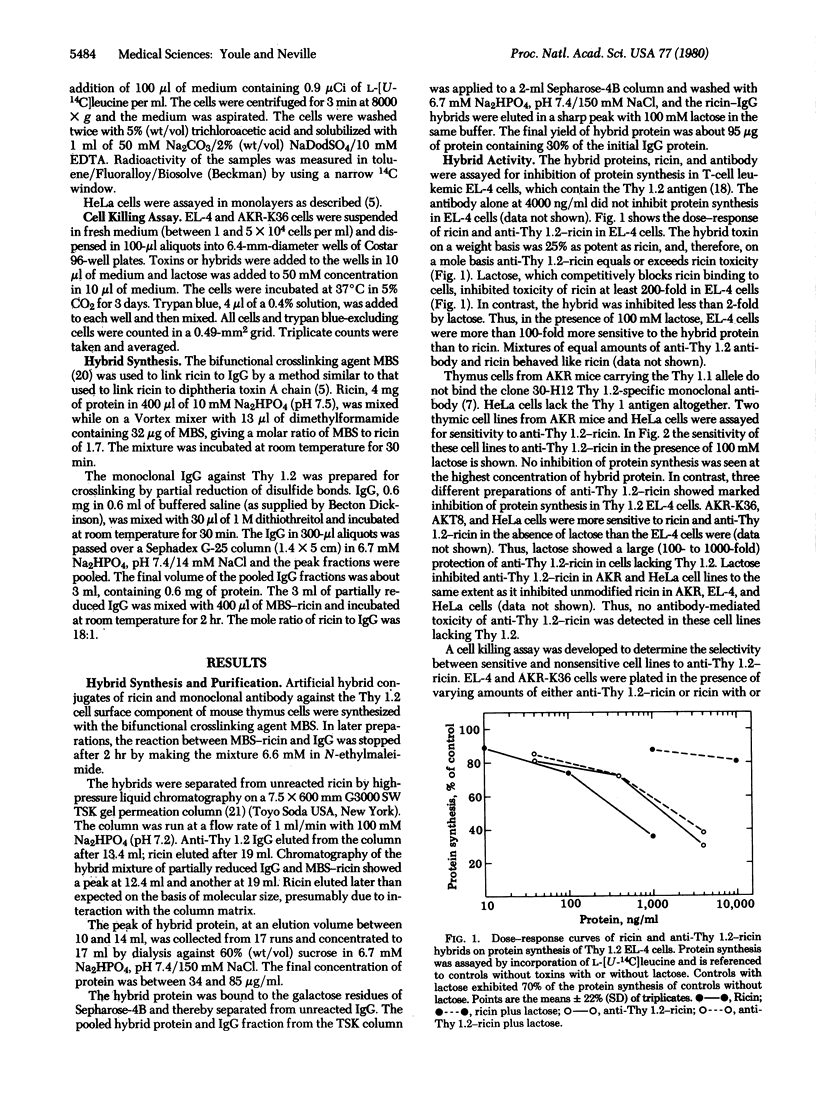
The cell-type specificity of the toxin ricin, which ordinarily binds, enters, and kills cells through receptors containing galactose, has been altered by covalently binding it to a monoclonal antibody and by reversibly binding it to lactose. The antibody, a monoclonal rat IgG2b directed against the Thy 1.2 antigen, provides ricin with a new binding site for the murine thymus cell surface. Addition of lactose saturates the galactose-binding site on ricin and inhibits ricin from binding and killing cells via the galactose-containing receptors. The antibody-ricin hybrid protein, anti-Thy 1.2-ricin, formed with a thioether linkage, has been purified by size exclusion and affinity chromatography. When assayed by inhibition of protein synthesis of EL-4 cells, which express the Thy 1.2 antigen anti-Thy 1.2-ricin is equally as toxic as ricin on a molar basis. The hybrid protein toxicity is unchanged in the presence of 100 mM lactose, whereas unmodified ricin toxicity is reduced to 1% of its toxicity in the absence of lactose. This demonstrates the altered receptor specificity of the ricin hybrid. The cell-type specificity of the anti-Thy 1.2-ricin inhibition of protein synthesis correlates with the presence of the Thy 1.2 antigen. Anti-Thy 1.2-ricin at 4 microgram/ml in the presence of lactose inhibits protein synthesis within 3.5 hr by 60-80% in EL-4 cells but does not affect Thy 1.1 alloantigen and HeLa cells that lack the Thy 1 antigen. Anti-Thy 1.2-ricin in the presence of lactose selectively kills EL-4 cells at concentrations that do not kill AKR-K36 cells. This selectivity, expressed as the ratio of anti-Thy 1.2-ricin concentrations required to kill 40% of both cell types, is 700. Ricin-monoclonal antibody hybrids of this type combine a high degree of cell-type selectivity and toxicity and may have pharmacologic utility as antitumor reagents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang T. M., Dazord A., Neville D. M., Jr Artificial hybrid protein containing a toxic protein fragment and a cell membrane receptor-binding moiety in a disulfide conjugate. II. Biochemical and biologic properties of diphtheria toxin fragment A-S-S-human placental lactogen. J Biol Chem. 1977 Feb 25;252(4):1515–1522. [PubMed] [Google Scholar]
- Chang T. M., Neville D. M., Jr Artificial hybrid protein containing a toxic protein fragment and a cell membrane receptor-binding moiety in a disulfide conjugate. I. Synthesis of diphtheria toxin fragment A-S-S-human placental lactogen with methyl-5-bromovalerimidate. J Biol Chem. 1977 Feb 25;252(4):1505–1514. [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABLIKS J., SOLOTOROVSKY M. Cell culture reactivity to diphtheria, Staphylococcus, tetanus and Escherichia coli toxins. J Immunol. 1962 Apr;88:505–512. [PubMed] [Google Scholar]
- Gilliland D. G., Collier R. J., Moehring J. M., Moehring T. J. Chimeric toxins: toxic, disulfide-linked conjugate of concanavalin A with fragment A from diphtheria toxin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5319–5323. doi: 10.1073/pnas.75.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Aoki T., Nunn M., Lavrin D. H., Soares N., Gazdar A., Holden H., Chang K. S. Specificity of 51Cr-release cytotoxicity of lymphocytes immune to murine sarcoma virus. J Natl Cancer Inst. 1974 Oct;53(4):1103–1111. doi: 10.1093/jnci/53.4.1103. [DOI] [PubMed] [Google Scholar]
- Herlyn M., Steplewski Z., Herlyn D., Koprowski H. Colorectal carcinoma-specific antigen: detection by means of monoclonal antibodies. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1438–1442. doi: 10.1073/pnas.76.3.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Gilbert F. Hybrid myelomas producing antibodies against a human neuroblastoma antigen present on fetal brain. Science. 1979 Mar 16;203(4385):1120–1121. doi: 10.1126/science.424740. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Aikawa T. Enzyme coupled immunoassay of insulin using a novel coupling reagent. J Biochem. 1976 Jan;79(1):233–236. doi: 10.1093/oxfordjournals.jbchem.a131053. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Herlyn D., Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Masuho Y., Hara T., Noguchi T. Preparation of a hybrid of fragment Fab' of antibody and fragment A of diphtheria toxin and its cytotoxicity. Biochem Biophys Res Commun. 1979 Sep 12;90(1):320–326. doi: 10.1016/0006-291x(79)91627-9. [DOI] [PubMed] [Google Scholar]
- Moolten F. L., Capparell N. J., Zajdel S. H., Cooperband S. R. Antitumor effects of antibody-diphtheria toxin conjugates. II. Immunotherapy with conjugates directed against tumor antigens induced by simian virus 40. J Natl Cancer Inst. 1975 Aug;55(2):473–477. [PubMed] [Google Scholar]
- Moolten F. L., Cooperband S. R. Selective destruction of target cells by diphtheria toxin conjugated to antibody directed against antigens on the cells. Science. 1970 Jul 3;169(3940):68–70. doi: 10.1126/science.169.3940.68. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Oeltmann T. N., Heath E. C. A hybrid protein containing the toxic subunit of ricin and the cell-specific subunit of human chorionic gonadotropin. I. Synthesis and characterization. J Biol Chem. 1979 Feb 25;254(4):1022–1027. [PubMed] [Google Scholar]
- Oeltmann T. N., Heath E. C. A hybrid protein containing the toxic subunit of ricin and the cell-specific subunit of human chorionic gonadotropin. II. Biologic properties. J Biol Chem. 1979 Feb 25;254(4):1028–1032. [PubMed] [Google Scholar]
- Olsnes S., Eiklid K. Isolation and characterization of Shigella shigae cytotoxin. J Biol Chem. 1980 Jan 10;255(1):284–289. [PubMed] [Google Scholar]
- Refsnes K., Munthe-Kaas A. C. Introduction of B-chain-inactivated ricin into mouse macrophages and rat Kupffer cells via their membrane Fc receptors. J Exp Med. 1976 Jun 1;143(6):1464–1474. doi: 10.1084/jem.143.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Stern P. L., Willison K. R., Lennox E., Galfrè G., Milstein C., Secher D., Ziegler A. Monoclonal antibodies as probes for differentiation and tumor-associated antigens: a Forssman specificity on teratocarcinoma stem cells. Cell. 1978 Aug;14(4):775–783. doi: 10.1016/0092-8674(78)90333-1. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C., Cumber A. J., Hinson C. A., Edwards D. C., Davies A. J. Toxicity of diphtheria toxin for lymphoblastoid cells is increased by conjugation to antilymphocytic globulin. Nature. 1978 Feb 23;271(5647):752–755. doi: 10.1038/271752a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Yamaizumi M., Mekada E., Okada Y., Tsuda M., Kurokawa T., Sugino Y. Reconstitution of hybrid toxin from Fragment A of diphtheria toxin and a subunit of Wistaria floribunda lectin. J Biol Chem. 1978 Sep 25;253(18):6307–6310. [PubMed] [Google Scholar]
- Yeh M. Y., Hellström I., Brown J. P., Warner G. A., Hansen J. A., Hellström K. E. Cell surface antigens of human melanoma identified by monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2927–2931. doi: 10.1073/pnas.76.6.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Murray G. J., Neville D. M., Jr Ricin linked to monophosphopentamannose binds to fibroblast lysosomal hydrolase receptors, resulting in a cell-type-specific toxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5559–5562. doi: 10.1073/pnas.76.11.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Neville D. M., Jr Receptor-mediated transport of the hybrid protein ricin-diphtheria toxin fragment A with subsequent ADP-ribosylation of intracellular elongation factor II. J Biol Chem. 1979 Nov 10;254(21):11089–11096. [PubMed] [Google Scholar]


