Abstract
Nitric oxide binds reversibly to the Fe(III) complex of a well-developed tetra-amido macrocyclic ligand. Reaction with NO results in formation of a species consistent with an S=1 {Fe-NO}6 ground state as characterized by UV-vis, IR, EPR, and Mössbauer spectroscopy. The resultant nitrosyl is labile and dissociates readily upon purging with N2, thus providing a rare example of reversible NO binding to non-heme iron.
Nitric oxide plays an important role in biological signaling, with numerous pathways involving interactions with transition metal centers.1 Accordingly, bioinorganic chemists have been inspired to investigate the interactions of NO with a variety of transition metal centers.2 Our interest in transition metal-based nitrosyl chemistry, which includes the design and implementation of intra- and extracellular sensors of NO, prompted our investigation of non-heme iron(III) complexes as a platform for binding NO reversibly. Current small-molecule-based methods for biological NO detection rely on irreversible NO reactions, thereby only allowing for observation of cumulative NO production, rather than real-time concentration changes. A system that could react with NO reversibly would allow for direct detection of both increases and decreases in intracellular NO concentrations; a currently unmet need in NO sensing.
Transition metals offer an attractive platform for reversible NO binding. For this study, we focused on non-heme {M-NO}6 centers, where n in {M-NO}n refers to the total the total number electrons from the metal-d and NO-π* orbitals,3 particularly Fe(III) and Ru(III) nitrosyls stabilized by poly-anionic ligands.4 Although many of these compounds release NO upon photolysis,5 release of NO without an external stimulus remains difficult. A major challenge in developing systems that bind NO reversibly is to ensure that NO is released as ·NO rather than NO− or NO+, for the latter events are accompanied by metal oxidation or reduction, respectively. We envisioned that an Fe(III) center housed in a ligand that stabilizes this oxidation state might promote NO binding and release without formal reduction of iron. We therefore explored the reaction of NO with an Fe(III) complex of the well-studied tetra-amido macrocycle (TAML) scaffold6 depicted in Scheme 1. Although this and related systems have previously supported chemistry of high-valent metal complexes,7 the reactivity with NO was previously unexplored.
Scheme 5.
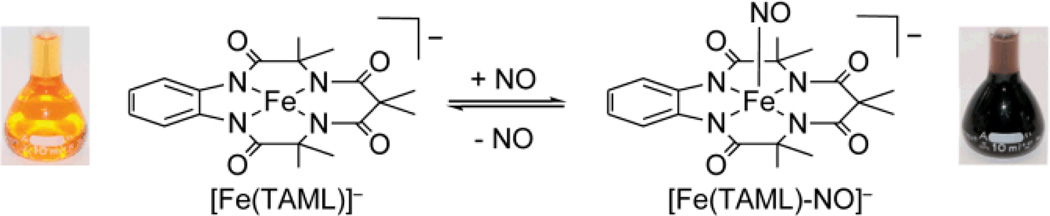
Reversible binding of NO to Fe(III) complexes.
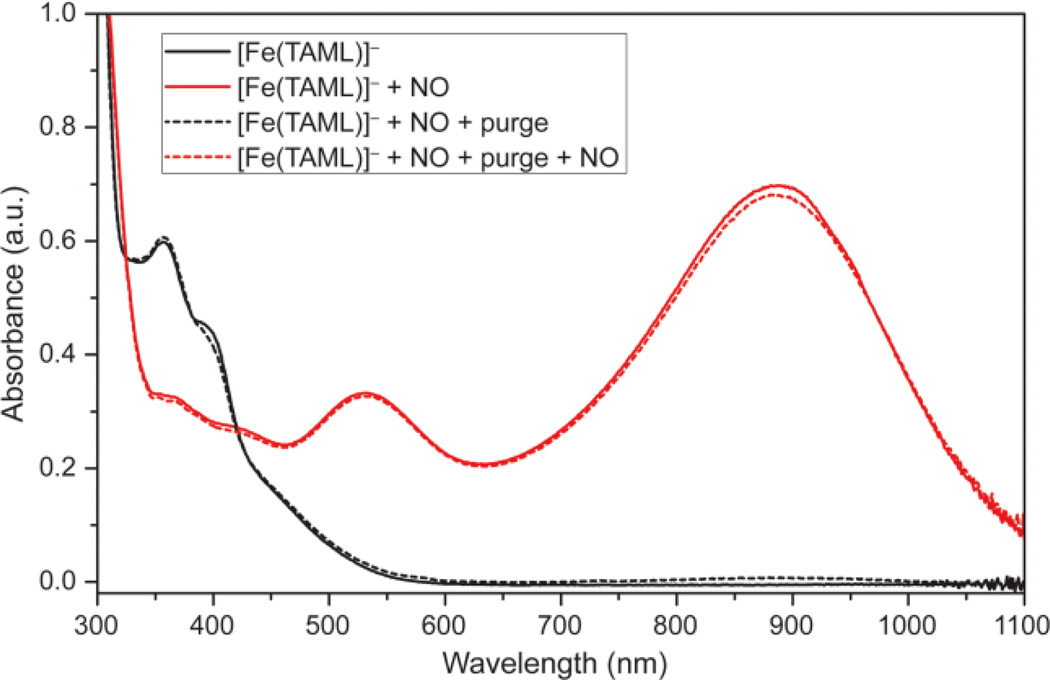
Anaerobic treatment of [Fe(TAML)]− with NO in a 1:100 MeOH:MeCN solution8 resulted in immediate conversion of the initial orange solution to deep purple with concomitant appearance of new bands at 533 (ε = 1600 M−1 cm−1) and 888 (ε = 3500 M−1 cm−1) nm in the UV-vis spectrum (Figure 1). Purging the solution with dry nitrogen either under ambient light or in the dark regenerated the optical spectrum of [Fe(TAML)]−. Further addition of NO restored the purple solution. These results suggest that bound NO is in equilibrium with free NO in solution, and that purging promotes complete release of the gas, subsequent reintroduction of which restores the NO adduct. This process was repeated numerous times without apparent decomposition of [Fe(TAML)]−.
Figure 1.
UV-vis spectra of [Fe(TAML)]− before (black) and after (red) addition of NO. Purging the sample 60 s with N2 (dotted black) regenerates the starting spectrum. Further addition of NO (dotted red) regenerates the NO adduct.
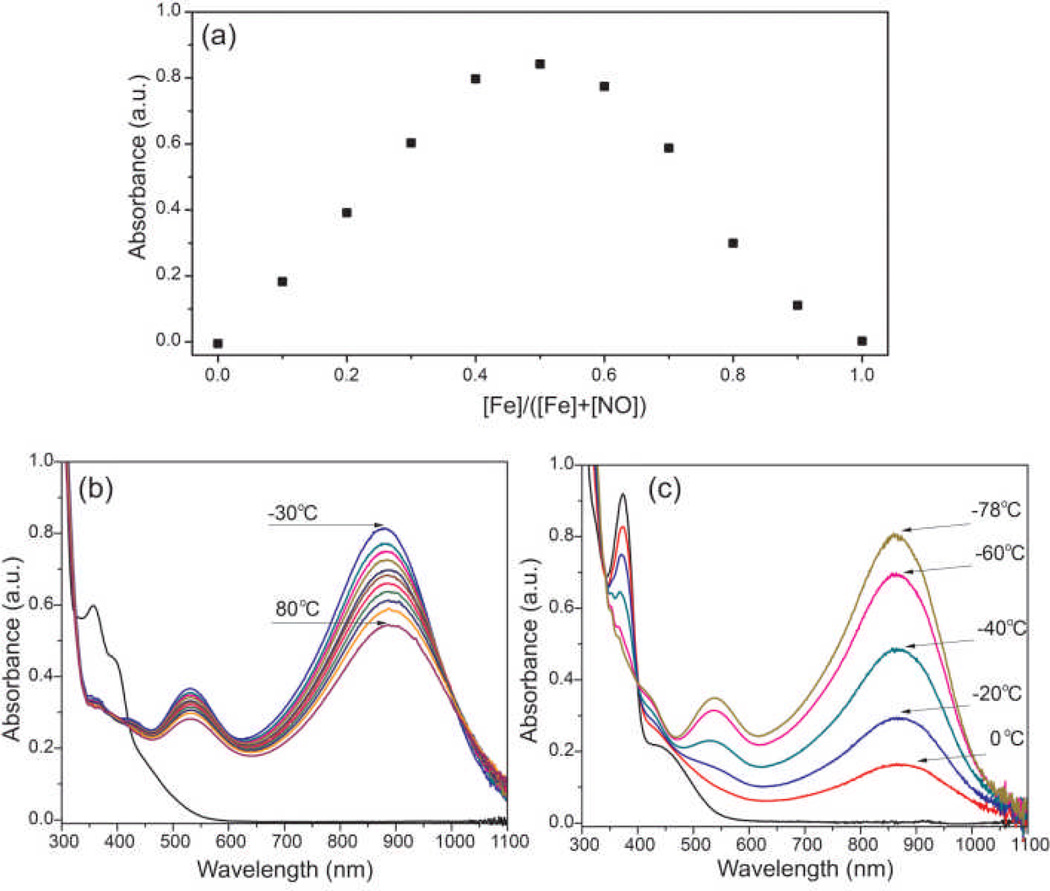
[Fe(TAML)]− has two available axial coordination sites, and reaction with NO could potentially afford both mono- and/or dinitrosyl adducts. To probe the stoichiometry of NO binding, a Job’s plot was prepared by varying the molar ratios of [Fe(TAML)]− to NO. Monitoring the absorbance at 888 nm as a function of the altered molar ratio of reactants revealed a clear break at 0.5, which is characteristic of 1:1 adduct formation (Figure 2a). This result is consistent with formation of a mononitrosyl, in which iron could either be a five-coordinate or octahedral six-coordinate with solvent coordinated at an axial site trans to the nitrosyl ligand.
Figure 2.
(a) Job’s plot of NO binding to [Fe(TAML)]− confirming a 1:1 binding stoichiometry. (b) Variable temperature UV-vis spectrum of 200 µM [Fe(TAML)]− and 20 mM NO in 100:1 CH3CN:MeOH. (c) Variable temperature UV-vis spectrum of 200 µM [Fe(TAML)]− and 20 mM NO in MeOH.
To further investigate NO binding, variable temperature UV-vis spectroscopic experiments were performed. The strong visible and near-IR absorptions of [Fe(TAML)NO]− at 533 and 888 m, respectively, are qualitatively similar to absorptions at 565 and 970 nm recently reported for the S=1 {Fe-NO}6 adduct of a tetra-anionic N2S2 ligand scaffold from which NO could be released upon heating.9 In the N2S2 system, the near-IR absorptions were assigned to electronic transitions in the S=1 manifold, but cooling the nitrosyl to 0 °C bleached these bands owing to spin crossover from the S=1 to the S=0 spin state. To perform similar studies with [Fe(TAML)NO]−, variable temperature UV-vis experiments from −30 to 80 °C in 100:1 CH3CN:MeOH or 0 to −78 °C in MeOH were performed (Figure 3b,c). In both cases, the intensities of the near near-IR absorptions changed but the wavelength of the absorbance maxima was not appreciably altered. These results suggest that the change in UV-vis intensity reflects the solvent dependence of the NO binding thermodynamics rather than [Fe(TAML)NO]− spin state interconversion. The solvent dependence of NO binding can further be observed by addition of H2O to a solution of [Fe(TAML)-NO]−, which leads to partial displacement of coordinated NO (Figure S1).
Figure 3.
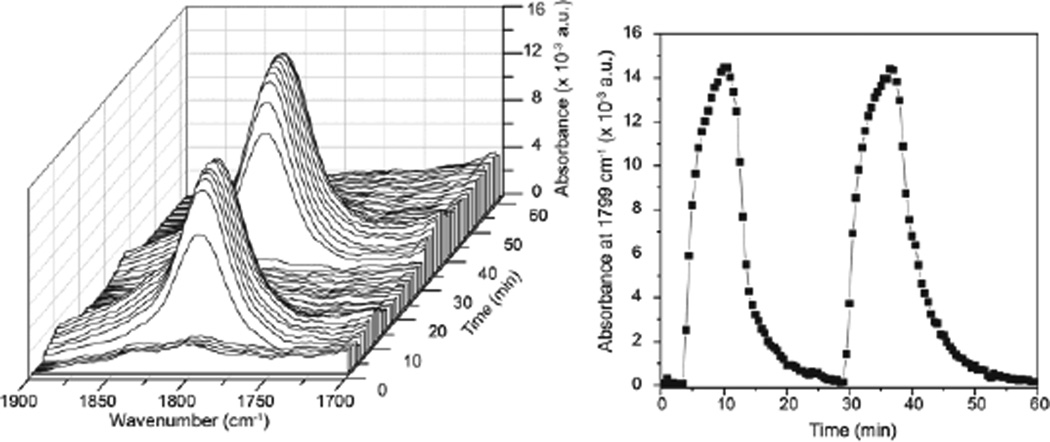
Left: React-IR trace of the reaction of 20 mM [Fe(TAML)]− with 5 equiv of NO. Two cycles of NO addition and subsequent purging with N2 are shown. Right: Intensity of the 1799 cm−1 (vNO) stretch as a function of time. Two cycles of NO/N2 cycling are depicted.
To provide direct evidence of iron nitrosyl bond formation, solution IR studies were performed, monitoring the growth and disappearance of a nitrosyl stretch as a function of NO addition and N2 purging. Addition of 5 equiv of NO to a 20 mM solution of [Fe(TAML)]− in 5:1 CH3CN:MeOD resulted in the appearance of a new band at 1799 cm−1, consistent with formation of an Fe(III) nitrosyl (Figure 3). Purging the headspace of the reaction vessel with N2 to remove the NO completely bleached the nitrosyl stretch. Readdition of NO regenerated the 1799 cm−1 stretch with an identical intensity as the initial cycle (Figure 3). Isotopic labeling with 15NO resulted in an observed NO stretch at 1761 cm−1, which agrees well with the predicted isotopic shift of 1767 cm−1 based on the simple harmonic oscillator model.
To further explore the electronic structure of the resultant iron nitrosyl, EPR and NMR spectroscopy were employed. Samples of 10 mM [Fe(TAML)]− in MeOH were treated with 10 equiv of NO and frozen at 20 K. Upon reaction with NO, the characteristic S=3/2 EPR signal of [Fe(TAML)]− bleached completely.10 These results are consistent with formation of an EPR-silent, integer spin iron product (Figure S2).
NMR spectroscopy was used to further probe the spin state of the nitrosyl product. Although [Fe(TAML)]− is paramagnetic, the 1H NMR spectrum shows characteristic resonances that can be monitored upon reaction with NO. If the metal nitrosyl product is diamagnetic, as is the case with many {Fe-NO}6 complexes, the 1H NMR spectrum should sharpen significantly and show resonances consistent with a typical diamagnetic compound. Alternatively, if the product is a less-common paramagnetic S=1 or S=2 {Fe-NO}6 species, the NMR spectrum may show new characteristic features of the {Fe-NO}6. To test these possibly outcomes, a 10 mM solution of [Fe(TAML)]− in either 9:1 CD3CN:CD3OD or neat CD3OD was titrated with different amounts of NO. Diamagnetic sharpening was not observed in either solvent system, but new paramagnetic resonances were observed. For example, in CD3OD, the resonance at −13 ppm in the NMR spectrum of [Fe(TAML)]− disappeared upon treatment with NO, and the shape of the spectrum from 0 to −5 ppm changed upon NO addition (Figure S3). These results are consistent with formation of a paramagnetic [Fe(TAML)NO]− species.11
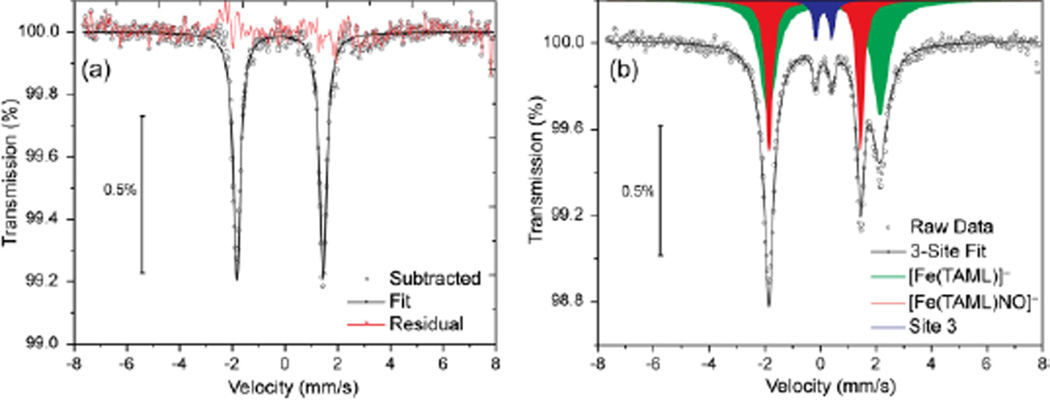
We further characterized the nitrosyl product by Mössbauer spectroscopy. Isolating the nitrosyl adduct in solid form proved problematic owing to the ease by which the coordinated NO was displaced. Solid material containing [Fe(TAML)NO]− was isolated by bubbling NO through a solution of [Fe(TAML)]− in methanol at a rate sufficient to evaporate the solvent. The IR spectrum of the resultant black solid revealed a nitrosyl stretch at 1797 cm−1 (KBr pellet) consistent with the solution IR measurements. Mössbauer spectroscopy at 80 K was performed, and the resultant data were fit to a three-site model corresponding to the [Fe(TAML)]− starting material (64%), the nitrosyl complex (36%), and a minor uncharacterized impurity (0.07%) (Figure 4).12 The parameters corresponding to the nitrosyl adduct were δ = −0.20 mm/s, Δeq = 3.29 mm/s, γL,R = 0.31. The observed negative isomer shift is similar to those of several Fe(IV) compounds coordinated by similar tetra-amido ligands,7e,7j suggesting the formation of an FeIV-NO− core.
Figure 4.
(a) Isolated spectrum of [Fe(TAML)NO]− after subtraction of the signal from [Fe(TAML)]− and a minor impurity. (b) Three site fit of the raw data.
In addition to the solution and solid state measurements of the structure and properties of [Fe(TAML)NO]−, we performed DFT calculations to probe the electronic configuration of the complex at the B3LYP/6-311+g(d,p) level of theory. Previous calculations of the coordinatively-unsaturated [Fe(TAML)]− complex showed high spin density in the dz2 orbital, poised to react with NO.7g Our calculations confirmed this prior result and suggest that the S=1 nitrosyl complex is 4.0 kcal/mol more stable than the analogous S=0 complex.13 This energetic difference is consistent with the solution data, which suggest an S=1 ground state electronic configuration for [Fe(TAML)NO]−. Time dependent DFT (TDDFT) calculations were performed using PB1PBE/6-311+g(d,p) to calculate the UV-vis spectra of the optimized S=0 and S=1 [Fe(TAML)NO]− structures. The calculated UV-spectrum of the S=1 complex (Figure S4, S5) qualitatively reproduces the experimentally observed near-IR absorbance, whereas the calculated S=0 spectrum was featureless above 680 nm. Taken together with the spectroscopic evidence, the energy and TDDTF calculations support an S=1 spin state for [Fe(TAML)NO]−.
Conclusions
A rare example of reversible binding of NO to a synthetic non-heme Fe(III) complex is presented. Upon addition of NO, the S=3/2 [Fe(TAML)]− complex binds NO with a 1:1 stoichiometry and spectroscopic parameters consistent with formation of an S=1 {Fe-NO}6 complex. Upon purging with N2, the coordinated NO is released to regenerate the parent Fe(III) complex.
Supplementary Material
Acknowledgement
The work was supported by the National Science Foundation (CHE-061194 to SJL) and National Institutes of Health (K99GM092970 to MDP). The authors thank Dr. Loi Do and Mr. Justin Wilson for helpful discussions in addition to assistance with Mössbauer spectroscopy and DFT calculations, respectively.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details, spectroscopic methods, and details of DFT calculations. See DOI: 10.1039/b000000x/
references
- 1.a.) Tennyson AG, Lippard SJ. Chem. Biol. 2011;18:1211–1220. doi: 10.1016/j.chembiol.2011.09.009. [DOI] [PubMed] [Google Scholar]; b.) Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. Free Radical Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; c.) Goodrich LE, Paulat F, Praneeth VKK, Lehnert N. Inorg. Chem. 2010;49:6293–6316. doi: 10.1021/ic902304a. [DOI] [PubMed] [Google Scholar]; d.) Roncaroli F, Videla M, Slep LD, Olabe JA. Coord. Chem. Rev. 2007;251:1903–1930. [Google Scholar]
- 2.a.) Schopfer MP, Wang J, Karlin KD. Inorg. Chem. 2010;49:6267–6282. doi: 10.1021/ic100033y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b.) McCleverty JA. Chem. Rev. 2004;104:403–418. doi: 10.1021/cr020623q. [DOI] [PubMed] [Google Scholar]; c.) Hayton TW, Legzdins P, Sharp WB. Chem. Rev. 2002;102:935–991. doi: 10.1021/cr000074t. [DOI] [PubMed] [Google Scholar]
- 3.Enemark JH, Feltham RD. Coord. Chem. Rev. 1974;13:339–406. [Google Scholar]
- 4.For selected examples, see: Schweitzer D, Ellison JJ, Shoner SC, Lovell S, Kovacs JA. J. Am. Chem. Soc. 1998;120:10996–10997. Patra AK, Afshar R, Olmstead MM, Mascharak PK. Angew. Chem. Int. Ed. 2002;41:2512–2515. doi: 10.1002/1521-3773(20020715)41:14<2512::AID-ANIE2512>3.0.CO;2-7. Sellmann D, Blum N, Heinemann FW, Hess BA. Chem- Eur. J. 2001;7:1874–1880. doi: 10.1002/1521-3765(20010504)7:9<1874::aid-chem1874>3.0.co;2-2. Serres RG, Grapperhaus CA, Bothe E, Bill E, Weyhermuller T, Neese F, Wieghardt K. J. Am. Chem. Soc. 2004;126:5138–5153. doi: 10.1021/ja030645+.
- 5.For selected examples, see: Merkle AC, McQuarters AB, Lehnert N. Dalton Trans. 2012;41:8047–8059. doi: 10.1039/c2dt30464c. Fry NL, Mascharak PK. Acc. Chem. Res. 2011;44:289–298. doi: 10.1021/ar100155t. Fry NL, Wei J, Mascharak PK. Inorg. Chem. 2011;50:9045–9052. doi: 10.1021/ic201242d. Ghosh K, Kumar S, Kumar R, Singh UP, Goel N. Inorg. Chem. 2010;49:7235–7237. doi: 10.1021/ic1009847. De P, Sarkar B, Maji S, Das AK, Bulak E, Mobin SM, Kaim W, Lahiri GK. Eur. J. Inorg. Chem. 2009:2702–2710. Rose MJ, Mascharak PK. Inorg. Chem. 2009;48:6904–6917. doi: 10.1021/ic900899j. Halpenny GM, Mascharak PK. Inorg. Chem. 2009;48:1490–1497. doi: 10.1021/ic801748t. Rose MJ, Mascharak PK. Coord. Chem. Rev. 2008;252:2093–2114. doi: 10.1016/j.ccr.2007.11.011.
- 6.a.) Collins TJ, Powell RD, Slebodnick C, Uffelman ES. J. Am. Chem. Soc. 1991;113:8419–8425. [Google Scholar]; b.) Bartos MJ, Gordon-Wylie SW, Fox BG, Wright LJ, Weintraub ST, Kauffmann KE, Münck E, Kostka KL, Uffelman ES, Rickard CEF, Noon KR, Collins TJ. Coord. Chem. Rev. 1998;174:361–390. [Google Scholar]
- 7.a.) Popescu DL, Vrabel M, Brausam A, Madsen P, Lente G, Fabian I, Ryabov AD, van Eldik R, Collins TJ. Inorg. Chem. 2010;49:11439–11448. doi: 10.1021/ic1015109. [DOI] [PubMed] [Google Scholar]; b.) Ellis WC, McDaniel ND, Bernhard S, Collins TJ. J. Am. Chem. Soc. 2010;132:10990–10991. doi: 10.1021/ja104766z. [DOI] [PubMed] [Google Scholar]; c.) Ellis WC, Tran CT, Denardo MA, Fischer A, Ryabov AD, Collins TJ. J. Am. Chem. Soc. 2009;131 doi: 10.1021/ja9086837. 18052-+; [DOI] [PubMed] [Google Scholar]; d.) Ghosh A, Mitchell DA, Chanda A, Ryabov AD, Popescu DL, Upham EC, Collins GJ, Collins TJ. J. Am. Chem. Soc. 2008;130:15116–15126. doi: 10.1021/ja8043689. [DOI] [PubMed] [Google Scholar]; e.) Chanda A, Shan XP, Chakrabarti M, Ellis WC, Popescu DL, de Oliveira FT, Wang D, Que L, Collins TJ, Münck E, Bominaar EL. Inorg. Chem. 2008;47:3669–3678. doi: 10.1021/ic7022902. [DOI] [PMC free article] [PubMed] [Google Scholar]; f.) de Oliveira FT, Chanda A, Banerjee D, Shan XP, Mondal S, Que L, Bominaar EL, Münck E, Collins TJ. Science. 2007;315:835–838. doi: 10.1126/science.1133417. [DOI] [PubMed] [Google Scholar]; g.) Chanda A, Popescu DL, de Oliveira FT, Bominaar EL, Ryabov AD, Münck E, Collins TJ. J. Inorg. Biochem. 2006;100:606–619. doi: 10.1016/j.jinorgbio.2005.12.016. [DOI] [PubMed] [Google Scholar]; h.) Collins TJ. Acc. Chem. Res. 2002;35:782–790. doi: 10.1021/ar010079s. [DOI] [PubMed] [Google Scholar]; i.) Kostka KL, Fox BG, Hendrich MP, Collins TJ, Rickard CEF, Wright LJ, Münck E. J. Am. Chem. Soc. 1993;115:6746–6757. [Google Scholar]; j.) Collins TJ, Fox BG, Hu ZG, Kostka KL, Münck E, Rickard CEF, Wright LJ. J. Am. Chem. Soc. 1992;114:8724–8725. [Google Scholar]
- 8.MeOH was added to help solubilize the Fe(III) complex
- 9.Rose MJ, Betterley NM, Oliver AG, Mascharak PK. Inorg. Chem. 2010;49:1854–1864. doi: 10.1021/ic902220a. [DOI] [PubMed] [Google Scholar]
- 10.A minor signal was observed at g = 2.01 which we attribute to either a minor impurity, or potential formation of a small amount an S=1/2 six-coordinate trans-dinitrosyl. The overall spin of this species accounts for less than 5% of the total sample by spin integration.
- 11.Attempts to measure the spin multiplicity directly using the Evans method proved unsuccessful, probably due the excess free NO required to drive the equilibrium to the nitrosyl product
- 12.Mossbauer parameters for the starting material were δ = 0.15 mm/s, Δeq = 4.02 mm/s, γL,R = 0.68, 0.74, and for the minor impurity were δ = 0.12 mm/s, Δeq = 0.57 mm/s, γL,R = 0.25.
- 13.Attempts to optimize the geometry of the S=2 nitrosyl from numerous initial geometries always resulted in dissociation of the NO ligand, suggesting that the S=2 spin state was energetically favorable.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.