Abstract
The use of fluorescent staining and flow cytometry to assess sperm quality in aquatic species has increased over the past decade, but comparisons among studies are difficult or impossible due to variation in application, analysis, and reporting of protocols and data.The goal of the present study was to determine the effect of exposure to two cryoprotectants commonly used for cryopreservation of sperm from aquatic species on the accuracy of flow cytometric assessment of sperm quality.Membrane integrity of zebrafish (Danio rerio) sperm exposed to 10% and 20%methanol and dimethyl sulfoxide (DMSO)in 300 mOsm kg−1 Hanks’ balanced salt solution (HBSS) or calcium-free HBSSwas determined using SYBR 14/propidium iodide staining. Both cryoprotectants significantly affected forward-scatter and side-scatter characteristics of sperm samples, resulting in significant changes in the number of total and gated events, and in the number and percentage of intact cells. These results indicate that it cannot be assumed that the approach to flow cytometric analysis of fresh sperm will be applicable to cryoprotectant-treated or cryopreserved sperm. In total, we document examples of five potentially interacting factors that produce errors of 5 to 50% each, resulting in underestimates and overestimates of total and intact sperm (actual numbers and percentages) in the presence of the two most commonly used cryoprotectants at the concentrations used most often for cryopreservation of sperm from aquatic species. This study provides methods to reduce or eliminate these errors and recommendations necessary for standardization and reporting.
Keywords: flow cytometry, SYBR 14/PI, zebrafish, membrane integrity, sperm viability
1. Introduction
Despite the common use of fluorescent staining and flow cytometry for the assessment of gamete quality in mammalian species (i.e. hundreds of published studies), the use of this technique in aquatic species is relatively new but becoming increasingly important (e.g., Ogier de Baulny et al., 1997; Liu et al., 2007; Guthrie et al., 2008). Unfortunately, comparison of flow cytometry methodology and results among studies in aquatic species is difficult or impossible due to the large amount of variability in the application of this technique. Standardization to minimize variation is important to ensure that flow cytometry can be utilized and interpreted correctly for gamete quality in aquatic species in the way it is currently used in mammalian species. Flow cytometry enables rapid screening of large numbers (e.g., thousands) of cells within a sample to detect structural or functional changes, providing an objective method of quality assessment. This generally involves the addition of fluorescent dyes that target specific cell structures or processes in conjunction with analysis of light-scattering characteristics to discriminate among cell populations. One of the most commonly tested parameters in flow cytometric assessment of sperm quality is plasma membrane integrity, using the two-color SYBR 14/propidium iodide (PI) “sperm viability” assay(Garner et al., 1994). SYBR 14 is a nucleic acid stain that readily crosses the plasma membrane, while PI is membrane impermeant and can only stain the nuclei of cells with compromised plasma membranes. Excitation of both fluorophores occurs at 488 nm (a laser wavelength commonly used in flow cytometers), but the emission spectra (SYBR 14 (green): 525 nm; PI (red): 610 nm) are sufficiently divergent to allow detection with separate photomultiplier tubes (PMT) with most flow cytometry systems.
The resulting scatter plots often include signals from debris and other cells along with sperm cells, all of which are interpreted as ‘events’ by the flow cytometer. Identification of the sperm population within this total accumulation of events is usually based on analysis of forward scatter (FSC; a rough measure of cell size) and side scatter (SSC; a rough measure of cell granularity) characteristics, and segregation of sperm and non-sperm events is achieved by creating a “gate” around the events corresponding to the sperm population to exclude non-sperm events from data analysis. This approach relies on the assumption that sperm and non-sperm events remain completely separate on FSC vs. SSC scatter plots, and that subsequent analysis of fluorescence characteristics of gated events occurs without interference from non-sperm events. A recent study examining the use of flow cytometry to assess sperm quality in mammalian species demonstrated that this is not necessarily the case, and that failure to properly distinguish between sperm and non-sperm events can lead to misestimations in determination of cell concentrations and in identifying the proportion of viable cells (Petrunkina and Harrison, 2010).
In aquatic species, population gating in flow cytometric analysis of sperm quality can be affected by several factors. One of the most important factors to consider is contamination of sperm samples with non-sperm material, which includes particulate contamination during buffer preparation and cellular contamination during sperm collection. This is particularly problematic in species that require dissection and crushing of the testes to collect a sperm sample. This is the case for several commercially important aquatic species, including ictalurid catfishes (Christensen and Tiersch, 2005) and oysters (Dong et al., 2005), and small-bodied biomedical model species such as zebrafish (Danio rerio) and swordtails (Xiphophorus species)(Yang and Tiersch, 2009). Dissection and crushing of the testes results in contamination of the sperm sample with blood and other somatic cells, and presents difficulties in gating and analysis of flow cytometry data. Cryopreservation and related processes can also affect flow cytometric analyses by damaging or destroying sperm, which affects sperm concentration but not necessarily the observed proportion of intact cells. These processes could also alter the FSC and SSC profiles of sperm populations (Martínez-Pastor et al., 2008) and affect subsequent gating and analysis profiles. This could cause misinterpretation of data by affecting the numbers or percentages observed for intact cells.
Methods for reliable and accurate assessment of sperm quality will be important for future breeding programs and the development of genetic repositories for aquatic species, and thus it is essential to address current variability in the application and interpretation of flow cytometric assays to fully utilize these technologies. We chose the zebrafish as a model for this work. It is a small fish (< 4 cm length) native to South Asia (Engeszer et al., 2007), an area of rapid disappearance of such fishes, and is an extremely popular model used for biomedical research around the world (Yang and Tiersch, 2009). The goal of the present study was to determine the effect of exposure to two cryoprotectants commonly used for cryopreservation of sperm from aquatic species on the accuracy of flow cytometric assessment of sperm quality. The specific objectives were to: 1)characterize the effects of 10% and 20% methanol (MeOH) and dimethyl sulfoxide (DMSO) on scatter plots of FSC and SSC and S14 vs. PI in Hanks’ balanced salt solution (HBSS) and calcium-free HBSS (C-F HBSS) with and without zebrafish sperm, and determine whether these effects were reversible following removal of cryoprotectants (washing) or filtration of cryoprotectant solutions; 2)determine the effects of 10% and 20% MeOH and DMSO on the concentration and percentage of intact zebrafish sperm in HBSS and C-F HBSS, and determine whether these effects were reversible following removal of cryoprotectants (washing); and 3) identify factors related to cryoprotectant exposure affecting the accuracy of flow cytometric assessment of sperm quality and gauge the level of error introduced by these factors, and make recommendations to minimize their effect. In this report, using two of the most commonly used cryoprotectants at the concentrations used most often for cryopreservation of sperm from aquatic species, we document examples of five potentially interacting factors that produce errors of 5 to 50% each, resulting in underestimates and overestimates of total and intact sperm number and percentage. This study provides methods to reduce or eliminate these errors and provides necessary recommendations for standardization and reporting.
2. Materials and methods
2.1 Zebrafish
Male zebrafish of the AB wild-type line (25 – 30 mm standard body length) were obtained from the Zebrafish International Resource Center (ZIRC, Eugene, OR, USA) and maintained in 10-l aquaria (Aquatic Habitats, Apopka, FL, USA) (two fish per l) at the Aquaculture Research Station of the Louisiana State University Agricultural Center. Water flow in the aquaria was recirculated through a fluidized plastic media filter (Aquatic Habitats, Apopka, FL, USA) at 60 l h−1, which was back-flushed weekly. Water temperature was held at 26°C and photoperiod was set at 14 h light: 10 h dark. Fish were fed twice daily with commercial pellets (Aquatic Eco-Systems, Apopka, FL, USA). Animal care and use conformed to the guidelines provided by the Institutional Animal Care and Use Committees of the Louisiana State University Agricultural Center.
2.2 Sperm collection
Zebrafish (three per replicate) were stunned by immersion in crushed ice for 1 min, blotted dry, and killed by decapitation. Testes were viewed with the aid of a dissecting microscope (X 10 magnification), separated from the surrounding tissues, and placed into1.5-ml microcentrifuge tubes. The testes were suspended in300 mOsm kg−1 HBSS (137 mM NaCl, 5.4 mM KCl, 1.3 mM CaCl2·2H2O, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.55 mM glucose, pH 7.2) or 300 mOsm kg−1C-F HBSS(137 mM NaCl, 5.4 mM KCl, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.55 mM glucose, pH 7.2)at a ratio of 1:20 (testes weight [mg]:HBSS orC-F HBSS HBSS300 volume [μl]). Osmolalities of HBSS and C-F HBSS were measured using a vapor-pressure osmometer (Vapro 5520, Wescor, Logan, UT, USA) and adjusted with ultrapure water (Barnstead Nanopure™ Analytical, Thermo Scientific, Dubuque, IA, USA). Sperm were released by squeezing the testes with a pair of forceps, and residual tissues were removed from the sperm suspension and discarded. Because of the small volumes collected, sperm suspensions from three males were pooled to provide sufficient volume for experiments, which were performed in triplicate. A total of 27 male zebrafish were used in these experiments. Sperm samples were kept on ice throughout the experiments.
Sperm concentration of the pooled samples was determined using a micro-spectrophotometer (NanoDrop, Thermo Fisher Scientific, Wilmington, DE, USA). Briefly, a 2 μl aliquot of the sperm suspension was placed on the lower sample pedestal of the instrument and the absorbance was measured at a wavelength of 400 nm. This was repeated three times and the sperm concentration was determined from the mean absorbance value using an equation previously determined in our laboratory (Tan et al., 2010). Sperm concentration was adjusted to 2×107cells ml−1 by dilution with HBSS or C-F HBSS and filtered through a 40-μm cell strainer (BD Biosciences, San Jose, CA, USA) to remove any remaining tissues.
2.3 Fluorescent staining and flow cytometry
Sperm membrane integrity was assessed using the SYBR 14/PI assay (Molecular Probes, Eugene, OR, USA). SYBR 14 was prepared by diluting 1 μl of stock solution with 49 μl of HBSS or C-F HBSS, and the PI was used undiluted. Aliquots of 250 μl of each sperm treatment were placed into 1.5-ml microcentrifuge tubes and 1.25 μl of each stain were added. The final concentration of SYBR 14 was 100 nM and PI was 12 μM. Samples were incubated in the dark for 10 min at room temperature. Staining of aliquots from each sperm treatment was performed in duplicate and repeated for each time point. The staining protocol was validated by assessing membrane integrity of three ratios (100:0, 50:50, and 0:100) of fresh and heat-treated (70°C for 5 min) zebrafish sperm.
Flow cytometry was performed using a flow cytometer (Accuri C6, Accuri Cytometers Inc., Ann Arbor, MI, USA) equipped with a 488 nm, 50 mW solid-state laser. This instrument is designed to measure the volume of sample collected, enabling direct calculation of concentration without the addition of counting beads (http://www.accuricytometers.com). Prior to experimentation the fluidics system of the Accuri C6 was calibrated for the sample container and volume used, and volumetric validation was performed using AccuCheck counting beads (Molecular Probes, Eugene, OR, USA) to ensure that actual counts were within ±10% of the expected count. Flow cytometer performance was assessed each day using fluorescent validation beads (Spherotech beads, Accuri Cytometers Inc., Ann Arbor, MI, USA) to ensure that coefficient of variation (CV) values were < 3.0% (calculated based on full peak height by the flow cytometry software (specified below) and used to indicate the precision of event measurements) for the fluorescence detectors (designated as FL1, FL2, FL3, and FL4, and sensitive to increasing ranges of wavelengths based on use of bandpass and longpass filters). Immediately before analysis, the microcentrifuge tube containing each sample was flicked gently three times with a finger to ensure suspension of the cells, and 10 μl of sample were analysed at a flow rate of 35 μl min−1using CFlow Plus analysis software (version1.0.202.1, Accuri Cytometers Inc., Ann Arbour, MI, USA). Fluorescence of SYBR 14 was detected with a 530 ± 15 nm bandpass filter (FL1) and PI was detected with a >670 nm longpass filter (FL3). During analysis, total events were collected as FSC vs. SSC scatter plots with the FSC threshold set atthe default value of 80,000 of a total 16.7 million channels. Although there is considerable numerical difference in the FSC (and SSC) range between this and other flow cytometry systems, the threshold settings used are roughly comparable to those of other instruments. This numerical difference in range is due to the 24-bit signal processing and seven decades of visible data used by the Accuri C6, which allows for a greater dynamic range than most other flow cytometry systems and removes the need for PMT voltage adjustment (http://www.accuricytometers.com).Gating settings for the sperm population (gated events) used to exclude non-sperm events were based on the FSC and SSC profile of fresh zebrafish sperm diluted to 1×106ml−1 with HBSS and stained with SYBR 14 and PI as described above. Gated events were viewed on a scatter plot showing FL1 (SYBR 14) vs. FL3 (PI) with fluorescence compensation based on the computed median fluorescence of single dye control samples to reduce spectral overlap. Fluorescent events were segregated into those stained with SYBR 14 alone (intact cells), and those stained with both SYBR 14 and PI or PI alone (damaged cells) using CFlow Plus analysis software.
2.4 Cryoprotectant exposure
Sperm suspensions (2 × 107 ml−1) were aliquoted into 1.5-ml microcentrifuge tubes and diluted 1:1 with HBSS or C-F HBSS containing 0, 20, or 40% (v/v) MeOH or DMSO in triplicate. The final cryoprotectant concentrations were 0, 10, and 20%, and the final sperm concentration was 1 × 107 ml−1 in all treatments. After 15 min of exposure to treatments, a 50-μl aliquot was removed from each treatment and diluted 1:9 with HBSS or C-F HBSS containing cryoprotectant (0%, 10%, or 20% v/v sufficient to maintain the treatment concentration) to achieve a final assay sperm concentration of 1 × 106 ml−1. Samples were divided into two aliquots of 250μl each, stained with SYBR 14 and PI, and analysed by flow cytometry at 30 min after initial treatment exposure.
2.5 Cryoprotectant removal
A second set of aliquots (50μl each) were removed from each cryoprotectant treatment at 30 min after initial exposure, centrifuged at 1000 ×g for 1 min, and the supernatant removed. The sperm pellet was resuspended in 500 μl of HBSS orC-F HBSS to achieve sperm concentrations of ~1 × 106 ml−1. Samples were divided into two aliquots of 250 μl each, stained with SYBR 14 and PI, and analyzed by flow cytometry as described above.
2.6 Assessment of cryoprotectant solution debris
Cryoprotectant solutions containing DMSO or MeOH at final concentrations of 0%, 10%, and 20% (v/v) without sperm were prepared in triplicate in HBSS, ultrapure water, and C-F HBSSat a final volume of 5 ml each. Two ml were removed from each cryoprotectant solution and placed in 5-ml pop-top tubes, and the remaining 3 ml of each were filtered through 0.45-μm pore nylon membrane syringe filters (Whatman Inc., Florham Park, NJ, USA). Three aliquots of 250 μl were removed from each of the filtered and non-filtered cryoprotectant solutions, SYBR 14 (100 nM) and PI (12 μM) were added, and samples were analyzed by flow cytometry as described above.
2.7 Data analysis
Sperm concentration data were normalized by square-root transformation(Handelsman, 2002), and percentage data were normalized by arcsine-square-root transformation prior to statistical analyses. Data were analyzed using ANOVA with Tukey’s post-tests, and paired-sample t-tests using the statistical program SYSTAT 12 (Ver. 12.02.00, Systat Software Inc., Chicago, IL, USA). Results were considered significantly different at P< 0.05. Data are presented as mean ± SEM.
3. Results
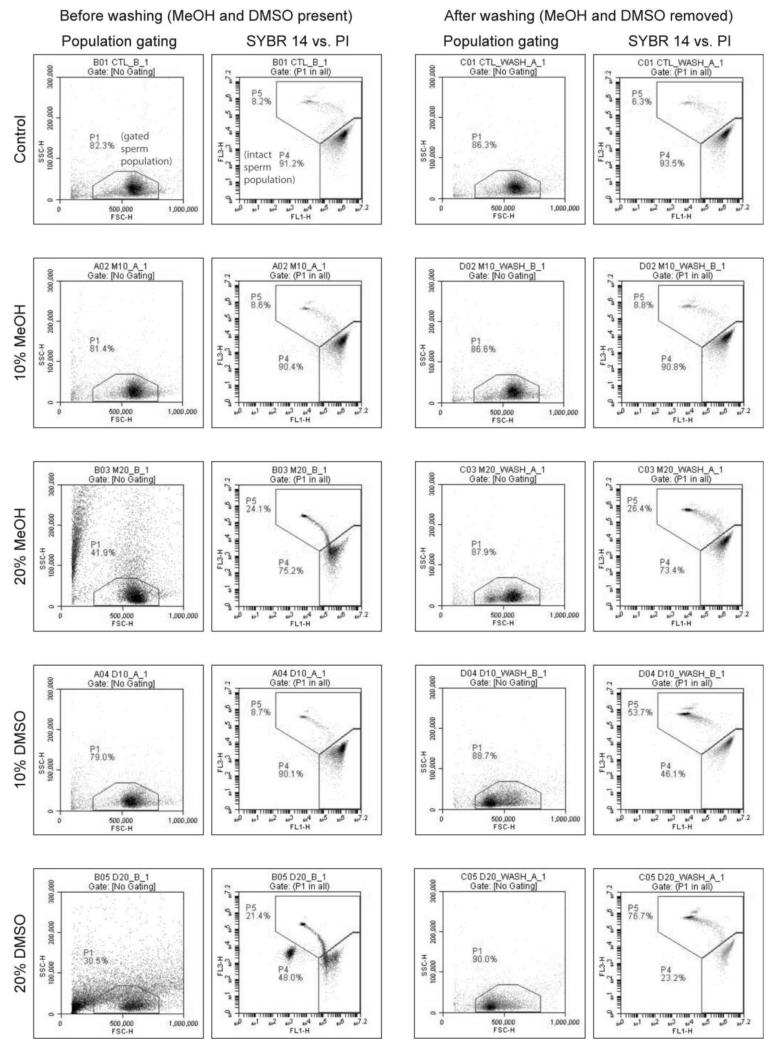
3.1 Effect of MeOH and DMSO on FSC vs. SSC and FL1 vs. FL3 scatter plots
The addition of cryoprotectants to HBSS altered the FSC and SSC characteristics of zebrafish sperm, and had a noticeable effect on the analysis of events based on fluorescence (FL1 vs. FL3) characteristics. In the absence of cryoprotectant (control), the majority of FSC vs. SSC events (>80%) fell within the gated region (P1; based on fresh zebrafish sperm) used to define the sperm population (Fig. 1, top row, before washing). The addition of 20% MeOH resulted in the formation of a second population of events close to the FSC threshold (Fig. 1, third row from top, before washing), together with an increase in variation in SSC (“granularity”) and to a lesser extent FSC (“size”). The addition of 20% DMSO resulted in the formation of a second population of events close to the FSC threshold and extending across the gated region used to define the sperm population. In the control treatment FL1 vs. FL3 scatter plots, the majority of events (> 98%) appeared as two separate populations within the gated regions used to define intact (P4) and damaged (P5) cells (Fig. 1, top row, before washing). The addition of cryoprotectants resulted in changes to the proportions of events falling within the P4 and P5 regions, and reduced the distinction between these populations compared to the control (Fig. 1, before washing). The addition of 20% DMSO resulted in the appearance of a third population of events outside the P4 and P5 regions in the FL1 vs. FL3 scatter plot, affecting the overall proportion of events falling within these gated regions.
Figure 1.
Forward scatter vs. side scatter (FSC vs. SSC) and FL1 vs. FL3 (SYBR 14 vs. PI) scatter plots of zebrafish sperm exposed to treatments containing HBSS alone, and HBSS containing10% and 20% methanol (MeOH) ordimethyl sulfoxide (DMSO). The region designated as“P1” in the FSC vs. SSC plot is the gated sperm population, and the region designated as “P4” in the SYBR 14 vs. PI plot is the intact sperm population.
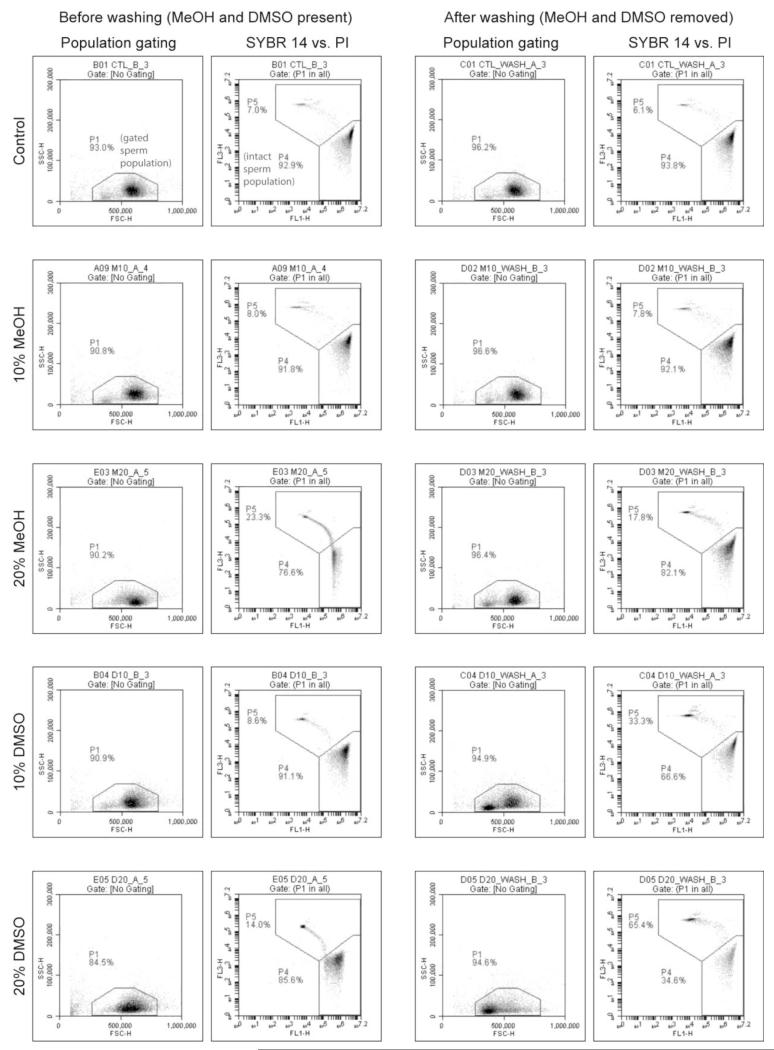
The addition of cryoprotectants to C-F HBSS had a noticeable effect on the FSC vs. SSC and FL1 vs. FL3 scatter plots of zebrafish sperm compared to the control, but unlike the HBSS treatments, the addition of 20% MeOH or DMSO did not result in the formation of a second event population near the FSC threshold (Fig. 2). In all treatments, the majority of FSC vs. SSC events (> 80%) fell within the gated region used to define the sperm population (Fig. 2, before washing). In the FL1 vs. FL3 scatter plots, the addition of cryoprotectants affected the proportions of events falling within the P4 and P5 regions and the distinction between these populations compared to the control, but unlike the HBSS treatments there were only two event populations in all treatments (Fig. 2, before washing).
Figure 2.
Forward scatter vs. side scatter (FSC vs. SSC) and FL1 vs. FL3 (SYBR 14vs. PI) scatter plots of zebrafish sperm exposed to treatments containing Calcium-free HBSS (C-F HBSS) alone, and C-F HBSS containing 10% and 20% methanol (MeOH) ordimethyl sulfoxide (DMSO). The region designated as“P1” in the FSC vs. SSC plot is the gated sperm population, and the region designated as “P4” in the SYBR 14 vs. PI plot is the intact sperm population.
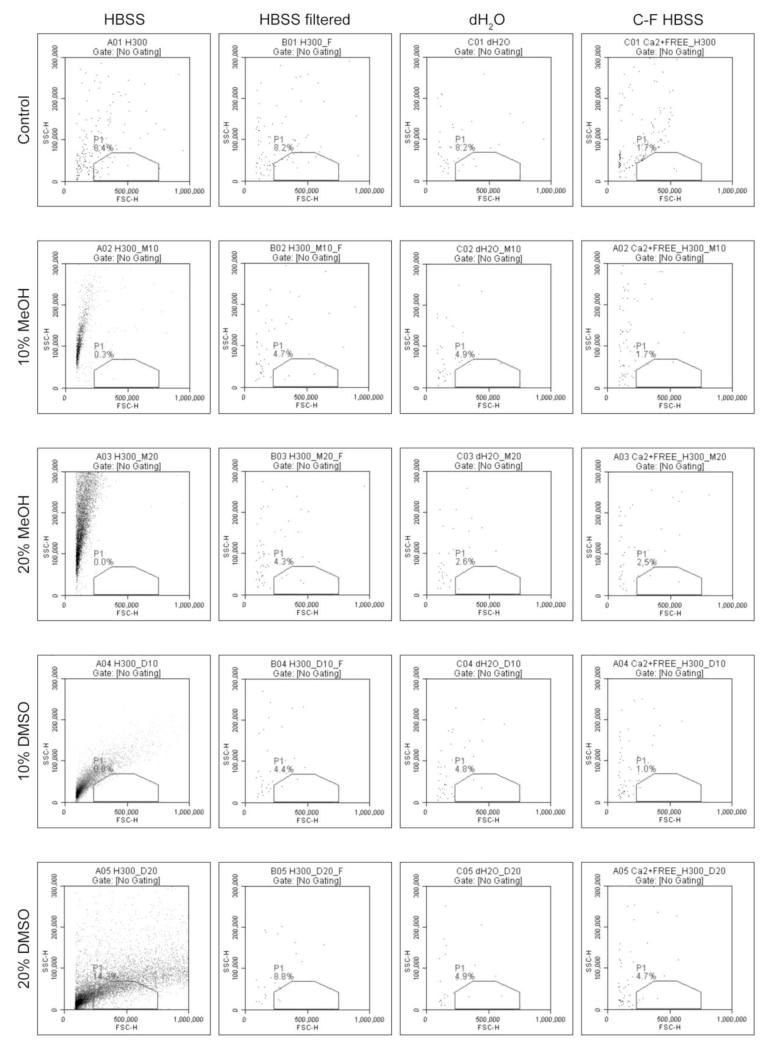
In media that did not contain zebrafish sperm, the addition of 10% or 20% MeOH or DMSO affected FSC vs. SSC characteristics in HBSS, but had no effect on ultrapure water or C-F HBSS scatter plots (Fig. 3). In the absence of cryoprotectant, there was no significant difference between HBSS, ultrapure water, or C-F HBSS in the number of FSC vs. SSC events (P> 0.05) (Table 1). The addition of 10 and 20% MeOH to HBSS resulted in the formation of a population of events near the FSC threshold and extending across a wide SSC range (Fig. 3, second and third rows from the top, HBSS). The addition of 10 and 20% DMSO to HBSS resulted in the formation of a population of events near the FSC threshold, extending across a wide FSC range and across the gated region used to define the sperm population (Fig. 3, fourth and fifth rows from the top, HBSS). There was a significant increase in the FSC vs. SSC event count in HBSS containing 10% MeOH (P = 0.0033), 20% MeOH (P = 0.0014), 10% DMSO (P = 0.0018), and 20% DMSO (P = 0.0003) compared to the control (Table 1). In ultrapure water and C-F HBSS, the addition of 10 or 20% MeOH or DMSO did not result in the formation of a population of events.
Figure 3.
Forward scatter vs. side scatter plots of solution debris in HBSS alone and containing 10% and 20% methanol (MeOH) ordimethyl sulfoxide (DMSO), before (first column) and after (second column) filtration through 0.45 μm syringe filters. Ultrapure water (dH2O, third column) and C-F HBSS (fourth column) alone and containing 10% and 20% methanol (MeOH) ordimethyl sulfoxide (DMSO) are shown for comparison.
Table 1.
Total events (×103 ml−1) recorded in FSC vs. SSC scatter plots of HBSS, ultrapure water, and C-F HBSS containing 0% (control), 10%, and 20% MeOH and DMSO, before and after filtration with 0.45-μm syringe filters.
| Unfiltered |
Filtered |
|||||
|---|---|---|---|---|---|---|
| HBSS | Ultrapure H2O | C-F HBSS | HBSS | Ultrapure H2O | C-F HBSS | |
| (0%) Control | 9.1± 0.2 | 8.5 ± 1.2 | 16.3 ± 2.6 | 7.0 ± 2.7 | 5.3 ± 0.4 | 6.7 ± 1.0 |
| 10% MeOH | 125.7 ± 13.0# | 5.1 ± 0.5* | 10.8 ± 0.7* | 6.9 ± 1.3 | 3.6 ± 0.3 | 5.1 ± 1.5 |
| 10% DMSO | 721.4 ± 51.4# | 6.5 ± 0.6* | 9.6 ± 0.5#* | 4.6 ± 0.6 | 5.7 ± 1.0 | 7.2 ± 0.2* |
| 20% MeOH | 575.3 ± 40.4# | 3.5 ± 0.5#* | 7.2 ± 0.5#* | 7.7 ± 0.9 | 3.6 ± 0.7 | 8.8 ± 0.2 |
| 20% DMSO | 1321.2 ± 30.3# | 5.5 ± 1.4* | 7.7 ± 0.7#* | 5.1 ± 1.4 | 3.7 ± 0.3 | 4.7 ± 0.6 |
Indicates significant difference between washed and corresponding treatment values (P < 0.05).
Indicates significant difference between control and treatments within in the same column (P < 0.05).
After centrifugation and re-suspension in HBSS or C-F HBSS (washing), there was a single population of events in all sperm treatments, and the majority of FSC vs. SSC events (> 80%) fell within the gated region used to define the sperm population (Fig. 1 and Fig. 2, after washing). After filtration of media through 0.45-μm syringe filters there were no distinct event populations observed in HBSS containing MeOH or DMSO (Fig. 3, HBSS filtered), suggesting that the events observed in the presence of cryoprotectants were removed by filtration. There was no significant difference in FSC vs. SSC event count between cryoprotectant treatments and controls in HBSS, ultrapure water, or C-F HBSS, and all treatments had less than 1 × 104 ml−1total events(Table 1).
3.2. Effect of MeOH and DMSO on event concentrations and intact cells
3.2.1. In the presence of cryoprotectants
The concentration of intact cells was calculated as the number of cells per ml that stained positively for SYBR 14 but did not stain with PI. The percentage intact was the intact concentration calculated as a proportion of the gated event concentration. In HBSS, the addition of 10% MeOH had no significant effect on the total (P = 0.995) or gated (P = 0.813) event counts, or on the number (P = 0.699) or percentage (P = 0.967) of intact cells compared to control (Table 2, HBSS). The addition of 10% DMSO caused a significant reduction in the number of gated events (P = 0.030) and in the number of intact cells (P = 0.020), but had no significant effect on the percentage of intact cells (P = 1.000) (Table 2, HBSS). The addition of 20% MeOH caused a slight but not significant increase in total event count, and a significant reduction in the gated event count (P = 0.000), intact cell count (P = 0.000), and percentage of intact cells (P = 0.000) compared to the control (Table 2, HBSS). The addition of 20% DMSO caused a significant increase in the total number of events (P = 0.000), but a significant reduction in the gated event count (P = 0.000), and in the number (P = 0.000) and percentage of intact cells (P = 0.000) (Table 2, HBSS).
Table 2.
Concentration of total events and gated events, and intact cell concentration and percentage(expressed as a proportion of gated event concentration) in zebrafish sperm samples in HBSS or C-F HBSS containing 0% (control), 10%, and 20% MeOH or DMSO (treatment),and after centrifugation and re-suspension in HBSS or C-F HBSSto remove cryoprotectants (washed).
| Total events (×105 ml−1) |
Gated events (×105 ml−1) |
Intact cells (×105 ml−1) |
Intact cells (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| HBSS | Treatment | Washed | Treatment | Washed | Treatment | Washed | Treatment | Washed |
| 0% (Control) | 10.2 ± 0.3 | 9.6 ± 0.2 | 8.8 ± 0.3 | 8.6 ± 0.2 | 8.0 ± 0.3 | 8.0 ± 0.2 | 91.0 ± 0.3 | 93.1 ± 0.4* |
| 10% MeOH | 10.0 ± 0.4 | 9.1 ± 0.3 | 8.3 ± 02 | 8.2 ± 0.3 | 7.4 ± 0.2 | 7.4 ± 0.3 | 90.2 ± 0.5 | 90.4 ± 0.7 |
| 10% DMSO | 9.1 ± 0.3 | 8.6 ± 0.4 | 7.3 ± 0.3# | 7.8 ± 0.4 | 6.6 ± 0.3# | 3.9 ± 0.3*# | 90.9 ± 0.3 | 50.3 ± 1.5*# |
| 20% MeOH | 11.4 ± 0.3 | 8.4 ± 0.3* | 4.0 ± 0.3# | 7.6 ± 0.4* | 2.9 ± 0.2# | 5.4 ± 0.4*# | 72.0 ± 1.7# | 70.6 ± 2.9# |
| 20% DMSO | 16.6 ± 0.3# | 8.2 ± 0.4* | 4.6 ± 0.3# | 7.5 ± 0.4* | 2.0 ± 0.2# | 1.8 ± 0.1# | 43.3 ± 2.4# | 23.9 ± 0.6*# |
|
| ||||||||
| Calcium–Free HBSS | ||||||||
| 0% (Control) | 10.7 ± 0.3 | 9.9 ± 0.5 | 9.7 ± 0.3 | 9.4 ± 0.4 | 9.0 ± 0.3 | 8.8 ± 0.4 | 92.3 ± 0.2 | 93.7 ± 0.5 |
| 10% MeOH | 10.1 ± 0.3 | 9.7 ± 0.3 | 9.2 ± 0.3 | 8.5 ± 0.3 | 8.5 ± 0.3 | 8.4 ± 0.3 | 91.8 ± 0.2 | 92.1 ± 0.6 |
| 10% DMSO | 9.9 ± 0.2 | 8.4 ± 0.6 | 8.8 ± 0.2 | 7.9 ± 0.5 | 8.0 ± 0.2* | 5.1 ± 0.5*# | 90.7 ± 0.5* | 63.6 ± 2.1*# |
| 20% MeOH | 9.0 ± 0.2# | 9.3 ± 0.5 | 8.1 ± 0.2# | 8.8 ± 0.5 | 6.0 ± 0.2*# | 7.3 ± 0.5* | 73.9 ± 1.9*# | 82.6 ± 1.1*# |
| 20% DMSO | 8.3 ± 0.3# | 8.3 ± 0.3 | 6.9 ± 0.3# | 7.8 ± 0.3 | 5.8 ± 0.3*# | 2.6 ± 0.3*# | 83.5 ± 1.0*# | 33.5 ± 0.7*# |
Indicates significant difference between washed and corresponding treatment values (P < 0.05).
Indicates significant difference between control and treatments within in the same column and medium (P < 0.05).
In C-F HBSS the addition of 10% MeOH had no significant effect on the total (P = 0.548) or gated (P = 0.656) event counts, or on the number (P = 0.645) or percentage (P = 0.977) of intact cells compared to control (Table 2, C-F HBSS). The addition of 10% DMSO had no significant effect on total (P = 0.264) or gated (P = 0.135) event counts, but there was a significant reduction in the number of intact cells (P = 0.000) and no significant effect on the percentage of intact cells (P = 0.501) (Table 2, C-F HBSS). The addition of 20% MeOH or 20% DMSO resulted in a significant reduction in total events (MeOH, P = 0.001; DMSO, P = 0.000), gated events (MeOH, P = 0.002; DMSO, P = 0.000), intact cell counts (MeOH, P = 0.000; DMSO, P = 0.000), and percentage of intact cells (MeOH, P = 0.000; DMSO, P = 0.000) compared to the control (Table 2, C-F HBSS).
3.2.2. After washing to remove cryoprotectants
In the HBSS treatments after washing to remove cryoprotectants, there was no significant difference in the number of total or gated events compared to the control in any of the cryoprotectant treatments (P> 0.05)(Table 2, HBSS). There was no significant difference in the concentration (P = 0.819) or percentage (P = 0.610) of intact cells in the 10% MeOH treatment after washing compared to in the presence of cryoprotectant, but there was a significant decline in the concentration (P = 0.000) and percentage (P = 0.000) of intact cells in the 10% DMSO treatment after washing(Table 2, HBSS). After washing there was a significant reduction in the total number of events in the 20% MeOH (P = 0.0001) and 20% DMSO (P = 0.0001) treatments, and there was a significant increase in the number of gated events (MeOH, P = 0.001; DMSO, P = 0.009) in these treatments (Table 2, HBSS). Washing resulted in a significant increase in the intact cell concentration in the 20% MeOH treatment (P = 0.002) but no significant difference in the percentage of intact cells (P = 0.770), and intact cell concentration remained significantly lower than the control (P = 0.000) (Table 2, HBSS). There was no significant difference in the intact cell concentration in the 20% DMSO treatment after washing (P = 0.417), but there was a significant decline in the percentage of intact cells (P = 0.001) (Table 2, HBSS).
In the C-F HBSS treatmentsafter washing to remove cryoprotectants, there was no significant difference in the number of total or gated events compared to the controlin any of the cryoprotectant treatments (P< 0.05) (Table 2, C-F HBSS). There was a significant decline in both the concentration and percentage of intact cells after washing in the 10% DMSO (concentration P = 0.003; percentage P = 0.000) and 20% DMSO (concentration P = 0.000; percentage P = 0.000) treatments (Table 2, C-F HBSS). In the 10% MeOH treatment, washing did not significantly affect the intact cell concentration (P = 0.951) or percentage (P = 0.564 (Table 2, C-F HBSS). In the 20% MeOH treatment there was a significant increase in the concentration of intact cells after washing (P = 0.015) and the concentration of intact cells did not differ significantly from the control (P = 0.150) (Table 2, C-F HBSS). After washing the percentage of intact cells in the 20% MeOH treatment increased significantly (P = 0.005), but remained significantly lower than the control (P = 0.000) (Table 2, C-F HBSS).
4. Discussion
The use of fluorescent staining and flow cytometry for the assessment of sperm quality in aquatic species has been steadily increasing over the past decade. Despite this, comparisons between studies are extremely difficult due to the large variation in the application, analysis, and reporting of protocols and data. The present study identified several sources of variation related to cryoprotectant exposure that can significantly affect flow cytometry scatter plots and the accuracy of subsequent sperm quality assessments. These sources of variation are presented in Table 3 along with recommendations for reducing the influence of these effects on data analyses. The cryoprotectants studied are two of the most commonly used for aquatic species and the concentrations used were within the range commonly used in most sperm cryopreservation protocols for aquatic species. DMSO is the most commonly used cryoprotectant (Tiersch, 2000) and has been tested in hundreds of studies in fish (e.g., Liu, et al., 2007; Horvarth et al., 2008; Taitson et al., 2008) and shellfish (e.g., Gwo et al., 2002; Salinas-Flores et al., 2005), while methanol has become increasingly popular in aquatic species (e.g., Lezcano et al., 2004; Horvath et al., 2005; Yang et al., 2010) notably zebrafish (Yang et al., 2007) because it has cryoprotective properties and low toxicity but, unlike DMSO, it is non-osmotic (i.e. does not increase osmolality of solutions). These cryoprotectants are typically used at concentrations of 5 to 20% (v/v) in traditional cryopreservation protocols (Suquet et al., 2000; Yang and Tiersch, 2009) (the concentration range examined in the present study) and at considerably higher concentrations for vitrification (typically >40% v/v) (Fahy et al., 1984; Denniston et al., 2000).
Table 3.
Summary of sources of variation in flow cytometry scatter plots and subsequent population gating of zebrafish sperm exposed to cryoprotectants reported in this study, and possible solutions to reduce their effects on data interpretation.
| Source of variation | Effect on sample | Effect on population gating | Magnitude of potential error* | Potential solution |
|---|---|---|---|---|
|
Cellular or particulate contamination |
Increased non-sperm events on FSC vs. SSC |
Decreased sperm count as a proportion of collected events |
+++ | Calculate intact percentages based on fluorescent count |
| Dependent on amount of contamination |
||||
| Precipitation of solutes | Increased non-sperm events on FSC vs. SSC |
Decreased sperm count as a proportion of collected events |
+++ | Omit calcium from cryopreservation buffers |
| Dependent on cryoprotectant concentration |
Filter cryoprotectant solution prior to sperm addition |
|||
| Calculate intact percentages based on fluorescent count |
||||
| Bulk volume effect | Reduce total solution volume | Increase relative sperm concentration |
+ | Unavoidable physical property of aqueous solutions containing cryoprotectants |
| Dependent on cryoprotectant concentration |
||||
| Change in FSC characteristics | Events fall outside sperm gating region |
Reduction in gated and fluorescent event counts |
++ | Wash to remove cryoprotectant prior to analysis |
| Events fall below threshold | Reduction in total event count | Dependent on cryoprotectant type and concentration |
Calculate intact concentration based on initial sperm concentration |
|
| Change in SSC characteristics | Events fall outside sperm gating region |
Reduction in gated and fluorescent event counts |
++ | Wash to remove cryoprotectant prior to analysis |
| Dependent on cryoprotectant type and concentration |
Calculate intact concentration based on initial sperm concentration |
The magnitude of potential error is expressed as≤5% (+),≤25%, (++), or ≤50% (+++).
In the present study, the addition of 20% MeOH or DMSO to HBSS led to the formation of a second population of events, distinct from but interacting with the sperm population. The fact that this second population was only observed in HBSS containing calcium (1.3 mM CaCl2) led to the hypothesis that interactions between the cryoprotectants and the buffer solution resulted in formation of a calcium-salt precipitate. One possible candidate is calcium carbonate (CaCO3) formed by a reaction between CaCl2 and NaHCO3 (a component of HBSS added to buffer pH) in the solution. This reaction has been used previously to model the formation of oyster shells (Wheeler et al., 1981) and pancreatic stones (Multigner et al., 1983), albeit at higher concentrations (20 mM each) than those present in HBSS (CaCl2 1.3 mM, NaHCO3 4.2 mM). Although there are typically no issues with the solubility of components in HBSS, it is possible that changes to solution properties following the addition of methanol or DMSO altered the interaction between the ionic components of CaCl2 and NaHCO3. It has been reported that a reduction in total volume is observed when methanol (Patel and Sandler, 1985) or DMSO (Egorov and Makarov, 2009) are dissolved in water, due to the bulk volume properties of the resulting solutions. In addition, ionic solubility in aqueous solutions is reduced in the presence of methanol (Pinho and Macedo, 2005), and is difficult to predict in the presence of DMSO (Balakin et al., 2004). It is possible that this reduction in total volume, together with a reduction in the proportion of the solution capable of solubilizing ionic components could result in the precipitation of compounds with low solubility such as CaCO3.The differences in FSC and SSC characteristics of the second population observed between HBSS containing MeOH and DMSO were unexplained, but may indicate differences in the composition of the calcium precipitates formed depending on which cryoprotectant was present. Further analysis of these interactions was beyond the scope of the present study, and should be examined further in future research.
The formation of precipitates in the presence of cryoprotectants, or the introduction of non-sperm particles by other means, could lead to inaccuracies in the collection and analysis of sperm quality data collected using flow cytometry. For example, in HBSS containing 20% MeOH or DMSO total event count was as much as 50% higher than control. Most flow cytometers are designed to collect a specific number of events for analysis, typically set at 10,000 events for sperm quality assessment in aquatic species (e.g., Segovia et al., 2000; Cabrita et al., 2005). If not accounted for, a significant increase in non-sperm events would result in a reduction in the actual number of sperm cells analyzed because a significant proportion of the events collected would be debris, effectively reducing the sample size of cells analyzed. This problem could potentially be addressed by adjusting the flow cytometer collection settings (e.g. PMT voltage or signal threshold) to reduce the incidence of non-sperm events, although these adjustments must be made during flow cytometer setup prior to sample analysis, and typically require a relatively high level of flow cytometry knowledge and expertise to ensure the accuracy of subsequent data collection. In addition, while this approach would reduce the number of non-sperm events collected, it also has the potential to eliminate cryoprotectant-affected sperm cells that fall below the voltage threshold, and would not account for debris that fall within the gated region used to define the sperm population.
In addition to increasing the total event count, a proportion of the debris in HBSS containing 20% DMSO fell within the gated region used to define the sperm population, and appeared as an additional population on the FL1 vs. FL3 scatter plots (Fig. 1, bottom row, before washing). Despite this, there was actually a decrease in the gated event count, and a significant reduction in both the concentration and percentage of intact cells (calculated as a proportion of the gated event concentration) (Table 2). In C-F HBSS, the concentration of intact cells was significantly lower in the 20% MeOH (6.0 × 105/ml) and 20% DMSO treatments (5.8 × 105/ml) compared to the control (8.0 × 105/ml), but both were still double the concentration of intact cells in the corresponding treatments in HBSS (20% MeOH: 2.9 × 105/ml; 20% DMSO: 2.0 × 105/ml). This suggests that the proportion of intact cells in the HBSS was affected both directly through cryoprotectant toxicity, and indirectly by solution effects resulting from the presence of cryoprotectant. It may be possible to reduce the effect of non-sperm events on analyses by subtracting them from calculations as described by Petrunkina et al. (2010), or by expressing intact cells as a percentage of the fluorescent event count (i.e. events stained with SYBR 14 and/or PI) instead of gated event count. Either way, it is extremely difficult to determine whether a decline in the intact cell count or percentage is caused by the effects of cryoprotectants on event counts due to solution effects, or the effect of cryoprotectant toxicity (or freezing injury in the case of cryopreserved sperm) on sperm membrane integrity.
After washing to remove cryoprotectants, there was a decrease in the total event counts in all HBSS treatments and in the C-F HBSS control and 10% cryoprotectant treatments. In the control and 10% MeOH and DMSO treatments in each buffer, this reduction was slight (< 10%), and was most likely associated with the incidental removal of sperm cells when the supernatant was removed. In HBSS containing 20% MeOH or DMSO, the large reduction in total events (25 – 50%) was mostly due to a reduction in the amount of debris, possibly due to removal with the supernatant or dissolution when the sperm pellet was re-suspended in fresh buffer. Despite the decline in total event count after washing, cryoprotectant removal resulted in an increase in the gated event count in the 20% MeOH and 20% DMSO treatments in both HBSS and C-F HBSS (Table 2). This suggests that a considerable proportion of the events that fell outside of the gated region in the presence of cryoprotectant were actually sperm cells that were consequently excluded from analyses, and that this effect was reversible following removal of the cryoprotectant. Interestingly, there was a significant increase in the number of intact cells in the 20% MeOH treatment after washing in HBSS and C-F HBSS, indicating that a significant proportion of the events excluded from analysis in the presence of MeOH were intact sperm. These observations on the effect of washing further highlight the potential for significant inaccuracies in the analysis of sperm quality in the presence of cryoprotectant, and indicate that cryoprotectant-treated and cryopreserved sperm cannot be considered or treated the same as fresh sperm, which is usually used to establish baseline instrument settings for analyses.
The most accurate way to report sperm membrane integrity data would be to provide the actual concentration of intact cells in a particular sample, rather than percentage data that can be affected by the range of factors described above. This approach requires calculation and control of sperm concentration in the original and treatment samples, which is an essential component of sperm cryopreservation protocols (Dong et al., 2007), but is almost always overlooked or ignored. The flow cytometry system used in this study allowed for the collection and analysis of specific volumes of sample, enabling direct calculation of total, gated, and intact event concentrations for every treatment. Although the ability to measure the volume of sample collected is not common to all flow cytometers, it is likely that this feature will be increasingly incorporated into flow cytometry systems in the future. An alternative for calculating concentrations using traditional flow cytometry systems would be to include a known concentration of fluorescent counting beads in sperm samples (Christensen et al., 2004). This would allow calculation of sperm concentrations relative to the concentration of fluorescent beads, although variations in the accuracy of this method have been reported (Lu et al., 2007), and it may become expensive or impractical for large numbers of samples. If reporting percentage data for membrane integrity in fresh sperm samples, the percentage of intact cells should be based on the fluorescent event count (i.e. SYBR 14 and PI positive events). This allows exclusion of non-sperm events from analysis, even if they fall within the gated region used to define the sperm population. When dealing with cryoprotectant-treated or cryopreserved sperm, adjustments need to be made to account for cryoprotectant (and other cryopreservation) effects on FSC and SSC characteristics and event counts. This could be achieved by basing percentage intact calculations on the initial (fresh) sperm concentration, or if possible on the concentration of fluorescent events in the fresh sperm sample. This would reduce the influence of cryoprotectant effects on FSC and SSC characteristics and event counts, and allow for accurate and realistic comparisons of fresh and treatment samples.
Given the prevalent use of these cryoprotectants in aquatic species sperm cryopreservation, the introduction of non-sperm events to flow cytometric analyses due to interactions between cryoprotectants and buffers could be a relatively common occurrence in the flow cytometry data reported in previous studies. In view of the apparent interaction between cryoprotectants and calcium-salt in the dilution buffer, it is advisable to filter cryoprotectant solutions through 0.45-μm syringe filters prior to the addition of sperm to remove any precipitates that may have formed. Future studies could also consider omitting calcium from cryopreservation buffers altogether when possible. If it is indeed CaCO3 that is precipitating in the presence of cryoprotectants, the calcium is likely not to be biologically available to the sperm cells anyway, and in the case of species that require extracellular calcium for motility (for example cyprinid species (Alavi and Cosson, 2006) calcium can be added in thawing or activation solutions. In addition, the removal carbonate ions would reduce the overall buffering ability of the solution, leading to reductions in pH (Wheeler et al., 1981) and a reduced ability to respond to the changes in pH that occur during cryopreservation (Van Den Berg and Soliman, 1969).
The results from the present study have highlighted a range of confounding factors related to cryoprotectant exposure that can significantly affect flow cytometry scatter plots and data analysis. Of particular importance are the observations stated above that cryoprotectants can significantly affect FSC, SSC, gating of sperm events, and subsequent data calculations by 1) increasing the total event count through the creation of debris, and 2) decreasing the gated event counts through reversible FSC and SSC changes to the sperm cells. Together, these two factors can significantly influence both the number and percentage of intact cells, through a combination of direct and indirect effects on the proportion of intact cells in the sperm sample. It is also important to note that these results indicate that it cannot be assumed that the approach used for analysis of flow cytometric data from fresh sperm will be applicable to cryoprotectant-treated sperm samples and, by extension, cryopreserved sperm. Future research should focus on identifying and addressing additional sources of variation in the collection and processing of sperm from aquatic species for cryopreservation. As sperm cryopreservation in aquatic species moves towards high-throughput applications and repository storage of genetic material (Hu and Tiersch, 2011), it is essential that minimal standards for reporting of flow cytometry data be applied to all studies on sperm quality to ensure accuracy and precision, and to enable comparison among studies.
Highlights.
Membrane integrity of zebrafish (Danio rerio) sperm exposed to 10% and 20% methanol and dimethyl sulfoxide (DMSO) in 300 mOsm kg−1 Hanks’ balanced salt solution (HBSS) or calcium-free HBSS was determined using SYBR 14/propidium iodide staining.
Both cryoprotectants significantly affected forward-scatter and side-scatter characteristics of sperm samples, resulting in significant changes in the number of total and gated events, and in the number and percentage of intact cells.
We document examples of five potentially interacting factors that produce errors of 5 to 50% each.
Acknowledgements
This research was funded in part by the National Institutes of Health, National Center for Research Resources (R24RR023998), and the Louisiana Sea Grant College Program.We thank H. Olivier for cross-instrument comparison of debris scatter plots, and J. Jenkins for manuscript review.This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 2010-244-7374.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavi SMH, Cosson J. Sperm motility in fishes. (II) Effects of ions and osmolality: a review. Cell. Biol. Int. 2006;30:1–14. doi: 10.1016/j.cellbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Balakin KV, Ivanenkov YA, Skorenko AV, Nikolsky YV, Savchuk NP, Ivashchenko AA. In silico estimation of DMSO solubility of organic compounds for bioscreening. J. Biomol. Screen. 2004;9:22–31. doi: 10.1177/1087057103260006. [DOI] [PubMed] [Google Scholar]
- Cabrita E, Robles V, Cuñado S, Wallace JC, Sarasquete C, Herráez MP. Evaluation of gilthead sea bream, Sparus aurata, sperm quality after cryopreservation in 5 ml macrotubes. Cryobiology. 2005;50:273–284. doi: 10.1016/j.cryobiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Christensen J, Tiersch T. Cryopreservation of channel catfish sperm: effects of cryoprotectant exposure time, cooling rate, thawing conditions, and male-to-male variation. Theriogenology. 2005;63:2103–2112. doi: 10.1016/j.theriogenology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Christensen P, Stenvang JP, Godfrey WL. A flow cytometric method for rapid determination of sperm concentration and viability in mammalian and avian semen. J. Androl. 2004;25:255–264. doi: 10.1002/j.1939-4640.2004.tb02786.x. [DOI] [PubMed] [Google Scholar]
- Denniston RS, Michelet S, Godke RA. Principles of cryopreservation. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, Louisiana, USA: 2000. [Google Scholar]
- Dong Q, Huang C, Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Dong Q, Eudeline B, Huang C, Allen SK, Tiersch TR. Commercial-scale sperm cryopreservation of diploid and tetraploid Pacific oysters, Crassostrea gigas. Cryobiology. 2005;50:1–16. doi: 10.1016/j.cryobiol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Egorov GI, Makarov DM. The bulk properties of the water-dimethylsulfoxide system at 278-323.15 K and atmospheric pressure. Russ. J. Phys. Chem. A. 2009;83:693–698. [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21:407–426. doi: 10.1016/0011-2240(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Garner DL, Johnson LA, Yue S, Roth B, Haugland R. Dual DNA Staining Assessment of Bovine Sperm Viability Using SYBR-14 and Propidium Iodide. J. Androl. 1994;15:620–629. [PubMed] [Google Scholar]
- Guthrie HD, Woods LC, Long JA, Welch GR. Effects of osmolality on inner mitochondrial transmembrane potential and ATP content in spermatozoa recovered from the testes of striped bass (Morone saxatilis) Theriogenology. 2008;69:1007–1012. doi: 10.1016/j.theriogenology.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Gwo J-C, Chen C-W, Cheng H-Y. Semen cryopreservation of small abalone (Haliotis diversicolor supertexa) Theriogenology. 2002;58:1563–1578. doi: 10.1016/s0093-691x(02)01055-5. [DOI] [PubMed] [Google Scholar]
- Handelsman DJ. Optimal power transformations for analysis of sperm concentration and other semen variables. J. Androl. 2002;23:629–634. [PubMed] [Google Scholar]
- Horvarth A, Wayman WR, Dean JC, Urbanyi B, Tiersch TR, Mims SD, Johnson D, Jenkins JA. Viability and fertilizing capacity of cryopreserved sperm from three North American acipenseriform species: a retrospective study. J. Appl. Ichthyol. 2008;24:443–449. [Google Scholar]
- Horvath A, Wayman WR, Urbanyi B, Ware KM, Dean JC, Tiersch TR. The relationship of the cryoprotectants methanol and dimethyl sulfoxide and hyperosmotic extenders on sperm cryopreservation of two North-American sturgeon species. Aquaculture. 2005;247:243–251. [Google Scholar]
- Hu E, Tiersch T. Development of High-throughput Cryopreservation for Aquatic Species. In: Tiersch T, Green C, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, Louisiana: 2011. pp. 995–1003. [Google Scholar]
- Lezcano M, Granja C, Salazar M. The use of flow cytometry in the evaluation of cell viability of cryopreserved sperm of the marine shrimp (Litopenaeus vannamei) Cryobiology. 2004;48:349–356. doi: 10.1016/j.cryobiol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Liu QH, Li J, Zhang SC, Xiao ZZ, Ding FH, Yu DD, Xu XZ. Flow cytometry and ultrastructure of cryopreserved red seabream (Pagrus major) sperm. Theriogenology. 2007;67:1168–1174. doi: 10.1016/j.theriogenology.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Lu J-C, Chen F, Xu H-R, Wu Y-M, Xia X-Y, Huang Y-F, Lu N-Q. Is flow cytometry really adapted to the determination of sperm concentration? Scand. J. Clin. Lab. Invest. 2007;67:394–401. doi: 10.1080/00365510601124032. [DOI] [PubMed] [Google Scholar]
- Martínez-Pastor F, Fernández-Santos MR, del Olmo E, Domínguez-Rebolledo AE, Esteso MC, Montoro V, Garde JJ. Mitochondrial activity and forward scatter vary in necrotic, apoptotic and membrane-intact spermatozoan subpopulations. Reprod. Fertil. Dev. 2008;20:547–556. doi: 10.1071/rd08002. [DOI] [PubMed] [Google Scholar]
- Multigner L, De Caro A, Lombardo D, Campese D, Sarles H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem. Biophys. Res. Commun. 1983;110:69–74. doi: 10.1016/0006-291x(83)91261-5. [DOI] [PubMed] [Google Scholar]
- Ogier de Baulny B, Le Vern Y, Kerboeuf D, Maisse G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout (Oncorhynchus mykiss) spermatozoa. Cryobiology. 1997;34:141–149. [Google Scholar]
- Patel NC, Sandler SI. Excess volumes of the water/methanol, n-heptane/ ethyl-acetate, n-heptane/ n-butyraldehyde, and n-heptane/ isobutyraldehyde systems. J. Chem. Eng. Data. 1985;30:218–222. [Google Scholar]
- Petrunkina AM, Harrison RAP. Systematic misestimation of cell subpopulations by flow cytometry: A mathematical analysis. Theriogenology. 2010;73:839–847. doi: 10.1016/j.theriogenology.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Petrunkina AM, Waberski D, Bollwein H, Sieme H. Identifying non-sperm particles during flow cytometric physiological assessment: a simple approach. Theriogenology. 2010;73:995–1000. doi: 10.1016/j.theriogenology.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Pinho SP, Macedo EA. Solubility of NaCl, NaBr, and KCl in water, methanol, ethanol, and their mixed solvents. J. Chem. Eng. Data. 2005;50:29–32. [Google Scholar]
- Salinas-Flores L, Paniagua-Chavez CG, Jenkins JA, Tiersch T. Cryopreservation of sperm of red abalone (Haliotis rufescens) J Shellfish Res. 2005;24:415–420. [Google Scholar]
- Segovia M, Jenkins JA, Paniagua-Chavez C, Tiersch TR. Flow cytometric evaluation of antibiotic effects on viability and mitochondrial function of refrigerated spermatozoa of Nile tilapia. Theriogenology. 2000;53:1489–1499. doi: 10.1016/S0093-691X(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Suquet M, Dreanno C, Fauvel C, Cosson J, Billard R. Cryopreservation of sperm in marine fish. Aquac. Res. 2000;31:231–243. [Google Scholar]
- Taitson PF, Chami E, Godinho HP. Gene banking of the neotropical fish Leporinus obtusidens (Valenciennes, 1836): a protocol to freeze its sperm in the field. Animal Reproduction Science. 2008;105:283–291. doi: 10.1016/j.anireprosci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tan E, Yang H, Tiersch TR. Determination of sperm concentration for small-bodied biomedical model fishes by use of microspectrophotometry. Zebrafish. 2010;7:233–240. doi: 10.1089/zeb.2010.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiersch TR. Introduction. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, Louisiana: 2000. pp. xix–xxvi. [Google Scholar]
- Van Den Berg L, Soliman FS. Effect of glycerol and dimethyl sulfoxide on changes in composition and pH of buffer salt solutions during freezing. Cryobiology. 1969;6:93–97. doi: 10.1016/s0011-2240(69)80469-4. [DOI] [PubMed] [Google Scholar]
- Wheeler A, George JW, Evans C. Control of calcium-carbonate nucleation and crystal-growth by soluble matrix of oyster shell. Science. 1981;212:1397–1398. doi: 10.1126/science.212.4501.1397. [DOI] [PubMed] [Google Scholar]
- Yang H, Tiersch TR. Current status of sperm cryopreservation in biomedical research fish models: Zebrafish, medaka, and Xiphophorus. Comp. Biochem. Physiol. C. 2009;149:224–232. doi: 10.1016/j.cbpc.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Carmichael C, Varga Z, Tiersch T. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Norris M, Winn R, Tiersch T. Evaluation of cryoprotectant and cooling rate for sperm cryopreservation in the euryhaline fish medaka Oryzias latipes. Cryobiology. 2010 doi: 10.1016/j.cryobiol.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]