Abstract
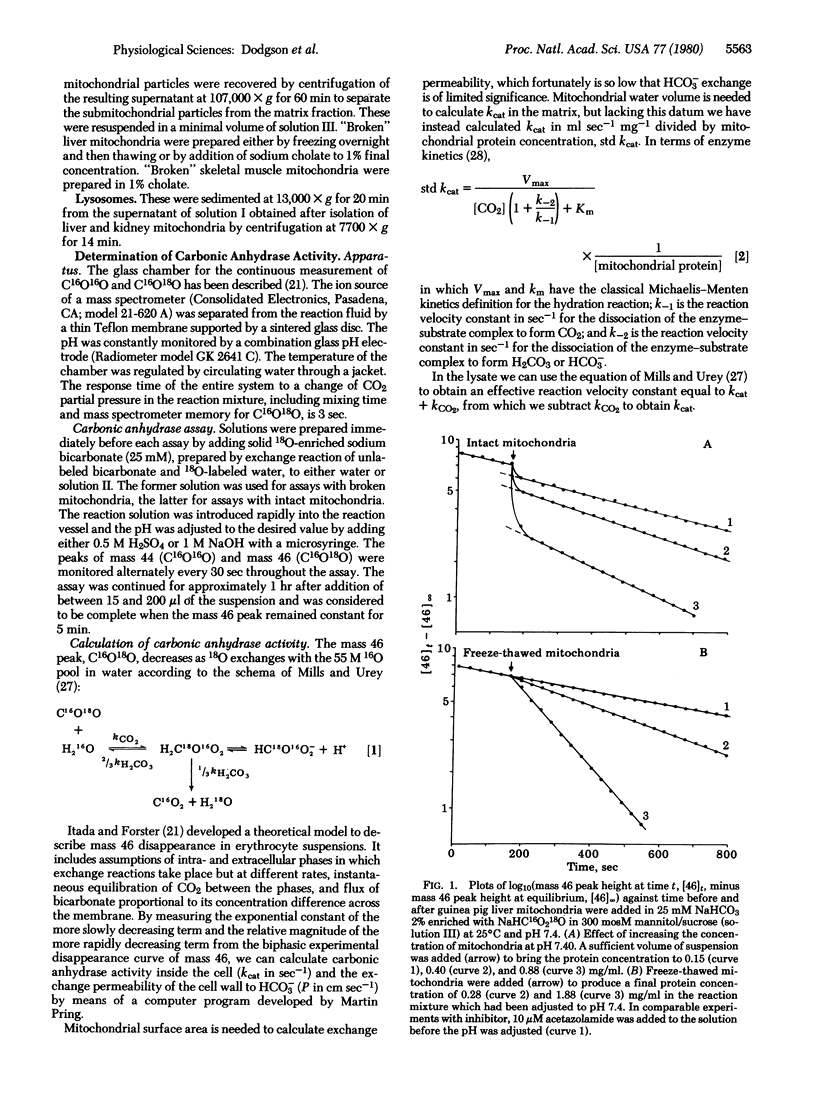
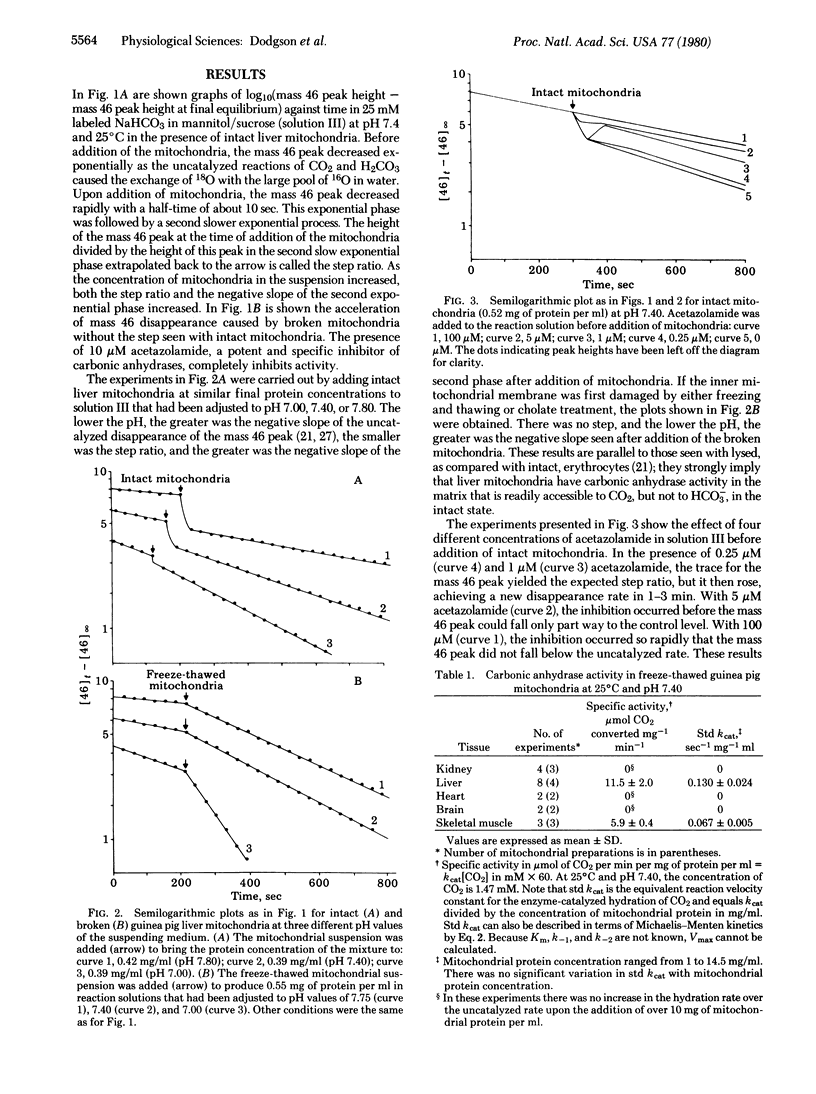
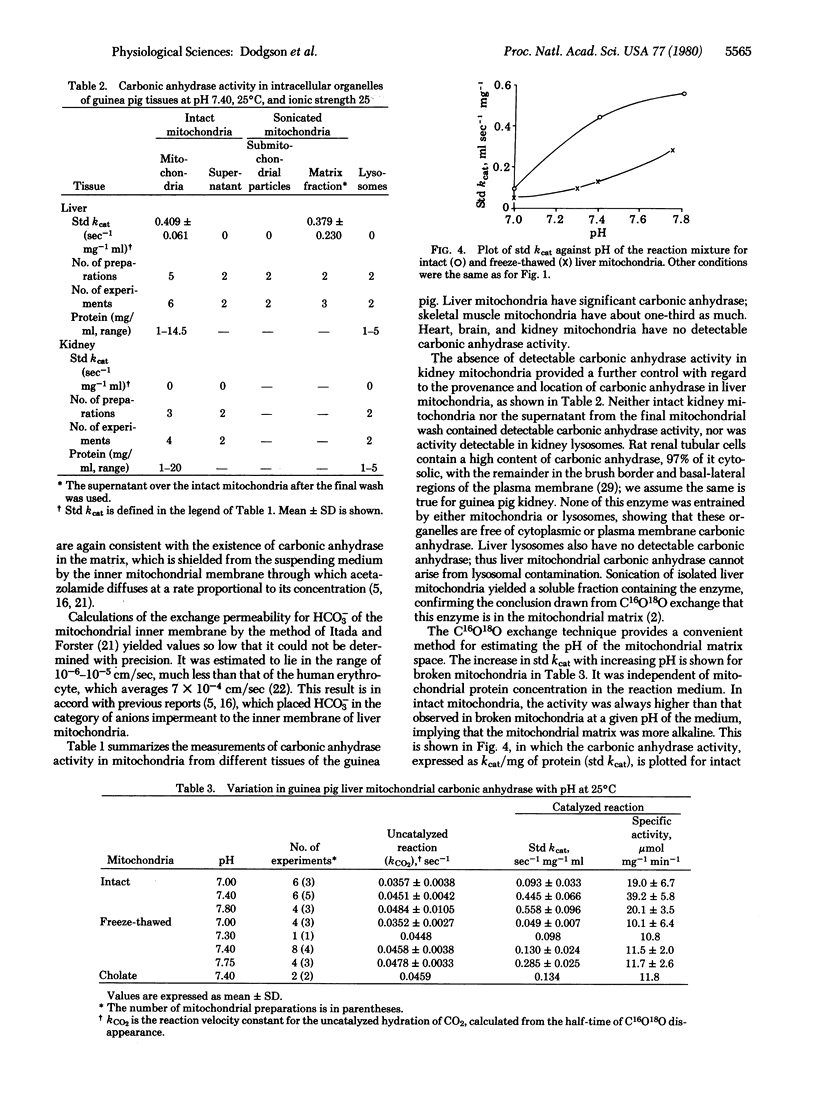
We have assayed carbonic anhydrase activity (carbonate dehydratase, carbonate hydro-lyase, EC 4.2.1.1) and bicarbonate permeability in suspensions of broken and intact guinea pig mitochondria by monitoring the disappearance of C16O18O. We found significant activity in preparations from liver and skeletal muscle, but not in preparations from heart muscle, brain, and kidney. Intact mitochondria containing carbonic anhydrase produce a two-phase acceleration of the disappearance of the labeled CO2, which indicates that the enzyme is located in a region more accessible to CO2 than to HCO3-. Acetazolamide inhibits the enzyme activity instantly in broken mitochondria but only after a delay in intact mitochondria, indicating that the enzyme is in a region not immediately accessible to the inhibitor. Sonication of mitochondria containing carbonic anhydrase activity releases the enzyme, which remains in the supernatant after sedimentation of the submitochondrial particles. This shows that mitochondrial carbonic anhydrase is in the matrix compartment and not in, or bound to, the inner membrane. The activity of the enzyme increases markedly with increasing pH. The enzyme activity of intact mitochondria is greater than that of the broken mitochondria at the same pH of the suspending fluid, corresponding to an intramitochondrial pH that is 0.2-0.5 unit more alkaline.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER P. D. Uses and limitations of measurements of rates of isotopic exchange and incorporation in catalyzed reactions. Arch Biochem Biophys. 1959 Jun;82(2):387–410. doi: 10.1016/0003-9861(59)90136-5. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P. K., SHEPARD T. H., 2nd Intracellular localization of carbonic anhydrase in rat liver and kidney tissues. Arch Biochem Biophys. 1959 Mar;81(1):124–129. doi: 10.1016/0003-9861(59)90182-1. [DOI] [PubMed] [Google Scholar]
- Deprez C., François C. Ion transport and oxidative metabolism. II. Absence of the enzyme carbonic anhydrase in mitochondria isolated from different tissues of the dog and the rat. Biochimie. 1972;54(1):103–107. doi: 10.1016/s0300-9084(72)80043-9. [DOI] [PubMed] [Google Scholar]
- Elder J. A., Lehninger A. L. Respiration-dependent transport of carbon dioxide into rat liver mitochondria. Biochemistry. 1973 Feb 27;12(5):976–982. doi: 10.1021/bi00729a029. [DOI] [PubMed] [Google Scholar]
- Harris E. J. Anion/calcium ion ratios and proton production in some mitochondrial calcium ion uptakes. Biochem J. 1978 Dec 15;176(3):983–991. doi: 10.1042/bj1760983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J. The importance of CO2 for Ca2+ uptake by some mitochondria. Nature. 1978 Aug 24;274(5673):820–821. doi: 10.1038/274820a0. [DOI] [PubMed] [Google Scholar]
- Holton F. A. Presence and spatial localization of carbonic anhydrase in rat liver mitochondria. Biochem J. 1970 Feb;116(4):29P–30P. doi: 10.1042/bj1160029pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itada N., Forster R. E. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. J Biol Chem. 1977 Jun 10;252(11):3881–3890. [PubMed] [Google Scholar]
- KARLER R., WOODBURY D. M. Intracellular distribution of carbonic anhydrase. Biochem J. 1960 Jun;75:538–543. doi: 10.1042/bj0750538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun E., Volfin P., Loh H. H. Studies with specific enzyme inhibitors. XI. Identification of tissue-specific metabolic organization of mitochondria by monofluorocitrate. Mol Pharmacol. 1967 Nov;3(6):497–502. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E., Rossi C. S. Energy-linked ion movements in mitochondrial systems. Adv Enzymol Relat Areas Mol Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Ellison A. C., Fellner S. K., Graham W. B. A study of hepatic carbonic anhydrase. Mol Pharmacol. 1966 Mar;2(2):144–157. [PubMed] [Google Scholar]
- Mela L., Seitz S. Isolation of mitochondria with emphasis on heart mitochondria from small amounts of tissue. Methods Enzymol. 1979;55:39–46. doi: 10.1016/0076-6879(79)55006-x. [DOI] [PubMed] [Google Scholar]
- Storey B. T., Scott D. M., Lee C. Energy-linked quinacrine fluorescence changes in submitochondrial particles from skeletal muscle mitochondria. Evidence for intramembrane H+ transfer as a primary reaction of energy coupling. J Biol Chem. 1980 Jun 10;255(11):5224–5229. [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Wistrand P. J., Kinne R. Carbonic anhydrase activity of isolated brush border and basal-lateral membranes of renal tubular cells. Pflugers Arch. 1977 Aug 29;370(2):121–126. doi: 10.1007/BF00581684. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Utter M. F. The role of CO2 fixation in metabolism. Essays Biochem. 1965;1:1–27. [PubMed] [Google Scholar]


