Background: VEGF is central to cancer angiogenesis; however, we have a poor understanding of how VEGF is regulated in lung tumors.
Results: High levels of SP-1 transcription factor expression amplify basal and hypoxia-induced VEGF expression.
Conclusion: SP-1 plays a key role in both genetic and hypoxic microenvironment regulation of VEGF in cancer.
Significance: Targeting of both VEGF and SP-1 may provide a more effective cancer therapy.
Keywords: Angiogenesis, Lung Cancer, Sp1, Transcription Factors, Vascular Endothelial Growth Factor (VEGF)
Abstract
VEGF plays a central role in angiogenesis in cancer. Non-small cell lung cancer (NSCLC) tumors have increased microvascular density, localized hypoxia, and high VEGF expression levels; however, there is a lack of understanding of how oncogenic and tumor microenvironment changes such as hypoxia lead to greater VEGF expression in lung and other cancers. We show that NSCLC cells secreted higher levels of VEGF than normal airway epithelial cells. Actinomycin D inhibited all NSCLC VEGF secretion, and VEGF minimal promoter-luciferase reporter constructs were constitutively active until the last 85 base pairs before the transcription start site containing three SP-1 transcription factor-binding sites; mutation of these VEGF promoter SP-1-binding sites eliminated VEGF promoter activity. Furthermore, dominant negative SP-1, mithramycin A, and SP-1 shRNA decreased VEGF promoter activity, whereas overexpression of SP-1 increased VEGF promoter activity. Chromatin immunoprecipitation assays demonstrated SP-1, p300, and PCA/F histone acetyltransferase binding and histone H4 hyperacetylation at the VEGF promoter in NSCLC cells. Cultured NSCLC cells expressed higher levels of SP-1 protein than normal airway epithelial cells, and double-fluorescence immunohistochemistry showed a strong correlation between SP-1 and VEGF in human NSCLC tumors. In addition, hypoxia-driven VEGF expression in NSCLC cells was SP-1-dependent, with hypoxia increasing SP-1 activity and binding to the VEGF promoter. These studies are the first to demonstrate that overexpression of SP-1 plays a central role in hypoxia-induced VEGF secretion.
Introduction
Unlimited tumor growth is dependent upon a combination of conditions, such as self-sufficiency in growth, limitless replication, lack of anti-growth signals, lack of apoptosis, metastasis, suppression of immune surveillance, and deregulated angiogenesis (1). Rapid tumor growth causes localized hypoxia leading to angiogenesis, the formation of new blood vessels. Histological examination of lung tumors has established that non-small cell lung cancers (NSCLCs)2 have increased blood vessel formation (greater microvessel density), elevated levels of VEGF expression (2), and increased expression of cellular markers of hypoxia; all of these measurements are associated with decreased survival rates (2–6).
VEGF plays a central role in the angiogenic response to a diverse range of stimuli such as growth factors, prostanoids, hypoxia, and hypoglycemia (7–10). The VEGF family of four genes (A, B, C, and D) and their numerous splice variants exert their physiological role through the endothelial cell based receptor tyrosine kinases, VEGFR1 (Flt-1), VEGFR2 (KDR/FLk-1), and VEGFR3. The loss of the VEGF-A gene is lethal to the developing embryo (11, 12), and antibody blockade of the VEGF/VEGFR interactions both in vitro and in vivo or small molecular weight chemical inhibition of the VEGFRs prevents angiogenesis (13, 14).
Tumor cell control of VEGF expression is a combination of tumor microenvironment (hypoxia, hypoglycemia) and genetic/epigenetic factors (oncogenes), where VEGF-A gene expression can be controlled at transcription, post-transcription, and post-translation prior to secretion or integration into the extracellular matrix (15–17). Under normoxic conditions the proline residues of the transcription factor HIF-1α are hydroxylated by the prolyl-hydroxylase complex PHD1/2/3 creating a target for the ubiquitination of HIF-1α and consequent HIF-1α degradation. Rapid tumor growth past 2 mm in diameter leads to localized hypoxia (18). Hypoxia decreases PHD2 activity, leading to reduced HIF-1α hydroxylation/ubiquitination and increasing HIF-1α stability (19–21). Stable HIF-1α and Aryl Hydrocarbon Receptor Nuclear Translocator complexes bind to the hypoxia recognition element of the VEGF promoter leading to increased VEGF transcription. Hypoxia-induced VEGF expression can also be HIF-1α-independent with hypoxia-induced VEGF expression in colon cancer (22) and hypoxia activation of PGC-1 driving VEGF expression independent of HIF-1α in muscle cells (23). Tumor cells can increase VEGF expression by the oncogenic transformation associated with loss of cell cycle control, elevated p53 tumor suppressor, loss of the von Hippel-Lindau gene (24) and gain of function mutation of the GTPase Ras (25). In addition oncogene-driven growth factor overexpression, such as EGF (8) or endothelin-1 (26), led to elevated VEGF transcription, expression, and secretion.
SP-1 has been shown to drive VEGF secretion in some cancers, but its potential role in driving hypoxia-induced VEGF secretion has not been studied previously. Furthermore mechanisms responsible for elevated VEGF expression in NSCLC are unknown. Here we demonstrate in several NSCLC cell lines that under normoxic conditions constitutive VEGF expression was the result of increased SP-1 transcription factor expression, activity, and binding to the VEGF proximal promoter. In addition VEGF expression correlated with the levels of SP-1 overexpression in human NSCLC tumor tissue ex vivo. Furthermore, hypoxic induction of VEGF expression was also SP-1-dependent with increased SP-1 transcription factor activity and increased SP-1 binding to the VEGF promoter under hypoxic conditions with no hypoxic induction of the VEGF promoter when SP-1-binding sites were mutated, suggesting that these sites were critical. VEGF therefore joins a group of genes including β-enolase, cyclooxygenase 2, and carbonic anhydrase 9, whose transcription control in response to hypoxia requires the SP-1 transcription factor (27–29). This is novel and has not been shown in any type of cancer cell previously. In conclusion, our studies suggest that SP-1 expression plays a central role in hypoxia-induced VEGF expression, angiogenesis, and the consequent progression of tumor growth.
EXPERIMENTAL PROCEDURES
Lung Cancer Tissue Samples
Human lung tumor samples were obtained from patients undergoing surgery at Queen's Medical Centre in Nottingham between June 2010 and April 2011 with informed patient consent and under ethical approval for this use from Nottingham Research Ethics Committee One (REC 08/H0403). The samples were obtained from the School of Clinical Science tissue bank (REC 10/H0405/6) approved by the Trent Research Ethics Committee. Samples obtained from the surgical resection of 30 patients were composed of 14 adenocarcinoma, 15 squamous cell carcinoma, and 1 adeno/squamous carcinoma.
Fluorescence Triple Stain Immunohistochemistry and Data Analysis
Sections were taken from embedded lung tissue samples for control (preimmune IgG) and test (SP-1 and VEGF IgG) immunohistochemistry. Antigen retrieval was performed by boiling sections in 10 mm citric acid (pH 6) for 20 min. Test sections were blocked then incubated with rabbit anti-SP-1 (SC-14027; Santa Cruz Biotechnologies, Santa Cruz, CA) (1 μg/ml) and mouse anti-VEGF (SC-729; Santa Cruz) (1 μg/ml) with 10 ηg/ml DAPI. Control sections were incubated with rabbit IgG and mouse IgG (R & D Systems, Abingdon, UK) at 1 μg/ml each then goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594 (Invitrogen UK). The images were taken on a Nikon 90i microscope with FITC (465–495-nm excitation) and Texas Red (540–580-nm excitation) filters. Seven fields of view were recorded for each test slide at 20× magnification. Control slide fluorescence was subtracted from test slide fluorescence for each image to give total fluorescence units for VEGF (Texas Red) and SP-1 (FITC).
Cell Culture
Human NSCLC cell lines, A549, NCI-H460, and NCI-H1299 were obtained from the ATCC collection via LGC and MOR/P were obtained from Prof. Penella Woll (University of Sheffield). All cell lines were grown in RPMI 1640 medium with 10% fetal bovine serum (Harlan, UK), 100 units/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin-B, 4 mm l-glutamine (Sigma) at 37 °C, 5% CO2, and 100% humidity. Cultures were grown to 100% confluence and plated to 24-well tissue culture dishes at a density of 5 × 104 cells/ml for 18 h. Culture medium was removed and replaced with RPMI 1640 with 4 mm l-glutamine (serum-free) for 8 h prior to transfection or 24 h prior to further assay. Normal human bronchial epithelial cells were obtained from Lonza (Wokingham, UK) at passage 3 and were grown to passage 5 in BEGM medium (Lonza, UK). Hypoxic conditions were created in a nitrogen-fed incubator at 37 °C, 100% humidity, 5% CO2, and 1% O2 for either 4 or 20 h.
Luciferase Reporter Gene Transfection and Assays
Luciferase reporter gene transfection and assays were performed as published previously (30).
RNA Isolation and RT-QPCR
RNA extraction and first strand cDNA synthesis and quantitative real time PCR were performed as described previously (JBC endothelin-1). Quantitative real time PCR was performed with the following primers sets: for VEGF, VEGF165abF GAGCAAGACAAGAAAATCCC and VEGF165/189AR CCTCGGCTTGTCACATCTG; for SP-1, SP-1F GCATGCACCTGCCCCTACTGTAAAGAC and SP-1R CGTTTGTGCCTCTGTAGCTCATCC; for SP-2, SP-2F GCCTGAATGCAGCCCAGTTGGCGG and SP-2R GCAGTTGGGACACGTGCAGGCCATGC; for SP-3, SP-3F GCACAGACAGTGACCCCTTCTGG and SP-3R GCAGAATCTATACAGTTCACTGTAACT; for GAPDH, GAPDHF CGGAGTCAACGGATTTGGTTCGTATTGG and GAPDHR GCTCCTGGAAGATGGTGATGGGATTTCC; and for 18 S ribosomal RNA, 18 S RNAf CGGCTACCACCACATCCAAGGAA and 18SrRNAR GCTGGAATTACCGCGGGCT. All gene specific quantification was calculated as Δct (target ct− housekeeping Cctt) relative to control or untreated cell experiment control to give a final Δct (test)/Δct (basal). All ct calculations were performed by Stratagene, MxPro 3.2.
Protein Isolation and Western Blot Analysis
Protein extraction and Western blot analysis of SP-1 were performed as described previously (31).
Enzyme-linked Immunosorbant Assay
Cell lines were plated to 24-well plates and grown to 100% confluence, and the medium was replaced with RPMI 1640 with l-glutamine only for 24 h. ELISA for VEGF-A (R & D Systems, Abingdon, UK) was performed according to the manufacturer's protocol. All assay points were performed in triplicate on 24-well plates in a final medium volume of 500 μl. All of the measurements were normalized to cell counts after supernatants were taken for assay.
mRNA Stability
The cell lines were grown to 100% confluence, with (test) or without (control) 5 μg/ml of actinomycin D (Sigma-Aldrich) for up to 24 h. Total RNA was extracted, and RT-QPCR was performed with either VEGF or SP-1 cDNA primers as detailed above. All gene-specific quantification were calculated as Δct (target ct − housekeeping ct) with the 18 S RNA “housekeeping” primer set. mRNA was quantified as percentage of the zero hour sample (100%) for both control and actinomycin D-treated cells. mRNA decay rates were calculated as the time taken to reach 50% of 0 h control (t½).
Chromatin Immunoprecipitation Assay
Cell lines were grown to 100% confluence in 150-cm2 flasks, and the medium was removed and replaced with serum-free RPMI 1640 for 24 h. Chromatin was prepared using the ChIP IT Express kit (Active Motif, Rixensart, Belgium) following the manufacturer's instructions. Briefly, the cells were “fixed” with 1% formaldehyde, chromatin was sheared in 1 ml of shearing buffer at 35% amplitude for 10 min (30 s on, 60 s off) at 3 °C in an Active Motif Epishear Sonicator. Immunoprecipitations consisted of 2 μg of antibody, 20 μg of chromatin, protein G magnetic beads in 200 μl incubated at 4 °C for 18 h on a bottle roller. IPs were washed and treated as per the manufacturer's instructions (“output” samples). 10 μl of sheared chromatin provided the input DNA. Quantitative real time PCR was performed with the following conditions; 1× KAPA SYBR FAST (Anachem, Luton, UK), 1 m betaine, 250 nm forward and reverse primers. Primers for −85 to +50 relative to the VEGF gene transcription start site were as in Ref. 32. Quantitative real time PCR was performed in a Stratagene Mx3000P® real time PCR thermo-cycler with 1 cycle of 95 °C for 3 min and 55 cycles of 95 °C for 5 s and 62 °C for 1 min, with fluorescence integration for product quantitation during the 62 °C segment. Dissociation curves were performed for each reaction set to confirm that ct values were derived from a single PCR product. IP PCR products were quantified as Δct (output/input) for each experiment. Control QPCRs were performed with the “input” and “output” samples with identical PCR conditions except primers designed +8000 bp from the VEGF transcription start site with the following sequences, VEGF 8000+ F, GCAGCCATGTCTTGGCCTCAAG and VEGF 8000+R, GGAAGGAAGCAGATCACAGAGG. These “OFF-ChIP” reactions act as controls for nonpromoter region-specific immunoprecipitation. Antibodies used were as follows; SP-1 (17-601; Millipore), SP-3 (SC-644; Santa Cruz Biotechnologies), polyacetyl-histone H4 (06-598; Millipore), polyacetyl-histone H3 (06-599; Millipore), histone H3 (SC-10809; Santa Cruz Biotechnologies), histone H4 (SC-8657; Santa Cruz Biotechnologies), PCAF (SC-8999; Santa Cruz Biotechnologies), CBP (SC-583; Santa Cruz Biotechnologies), p300 (SC-584; Santa Cruz Biotechnologies), and normal rabbit IgG (12-370; Millipore).
Plasmid Constructs
VEGF promoter reporter constructs (33), including VEGF-135 with EGR and SP-1-binding site mutations (34) were a kind gift from Prof. Dieter Marmé (Institute of Molecular Oncology, Tumor Biology Centre, Freiburg, Germany). The Sp-1 reporter constructs containing Sp-1-binding sites (plasmid 194) and the control vector (plasmid 191) were a kind gift from Prof. Jeffrey E. Kudlow (School of Medicine, University of Alabama at Birmingham). The SP-1 DNA-binding domain construct, pEBGN-SP1, and empty vector pEBGN were kind gifts from Dr. Gerard Thiel (University of Saarland, Saarbrücken, Germany). The SP1 overexpression vector, pCMV-SP1 was a kind gift from Dr. Gilles Pages (Institut de biologie du developpement et cancer, University of Nice, Nice, France). The shRNA construct (pURSP-1) targeting SP-1 was a kind gift from Prof. J McCormick (Carcinogenesis Laboratory, Michigan State University).
Data Analysis
All of the assays were performed in triplicate with data presented as the standard errors of the mean. Statistical analysis of correlation was tested with a Pearson's correlation coefficient test using GraphPad Prism (GraphPad, San Diego, CA). The p values were scored as significant for 0.01–0.05 (*), 0.001–0.01 (**), and <0.001 (***).
RESULTS
NSCLC Cell Lines Secreted Greater Amounts of VEGF-A than Normal Human Bronchial Epithelial (NHBE) cells, VEGF Protein Secretion Required Active VEGF mRNA Transcription, and the Last 33 Base Pairs of the VEGF Promoter Were Required for VEGF Promoter Reporter Activity
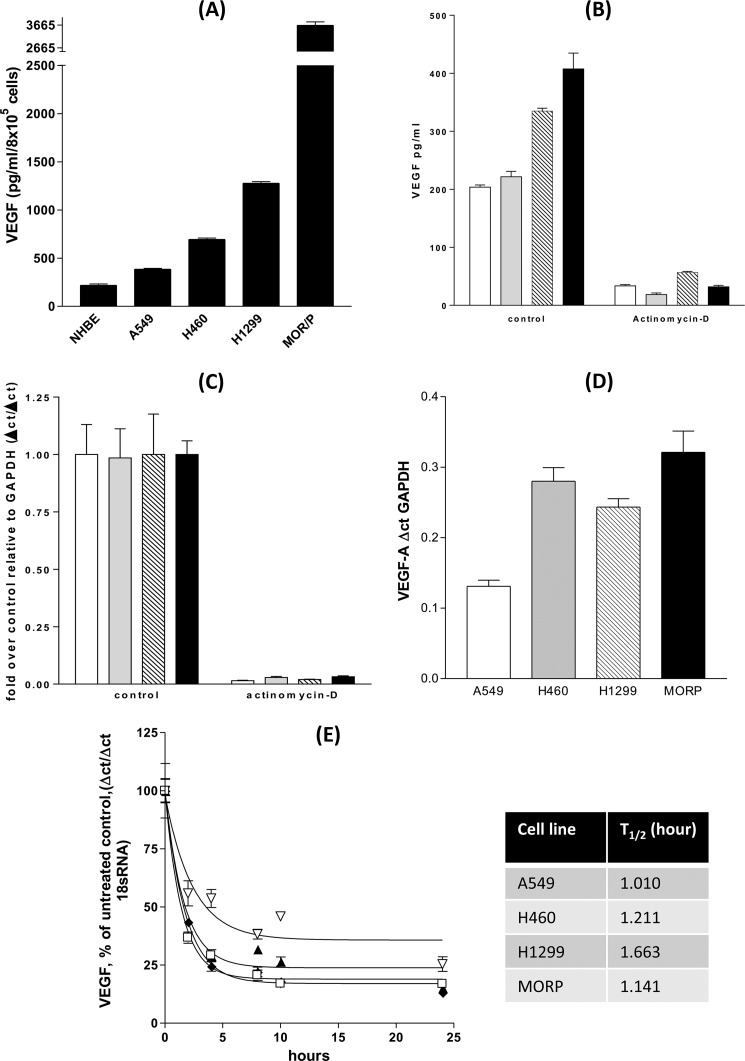
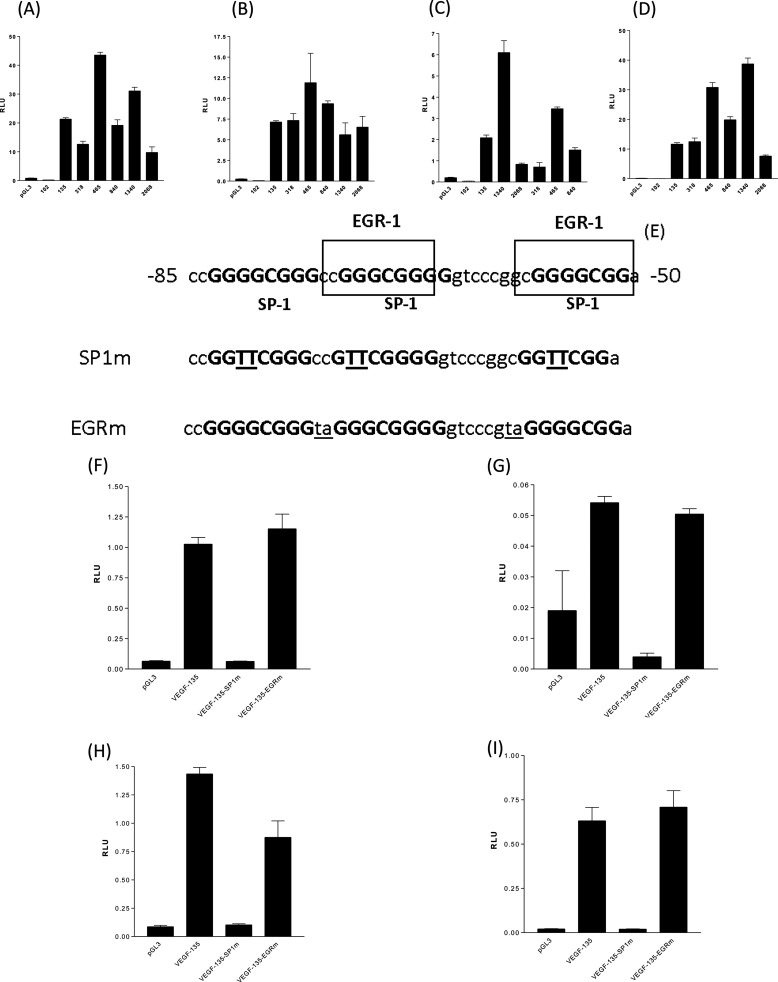
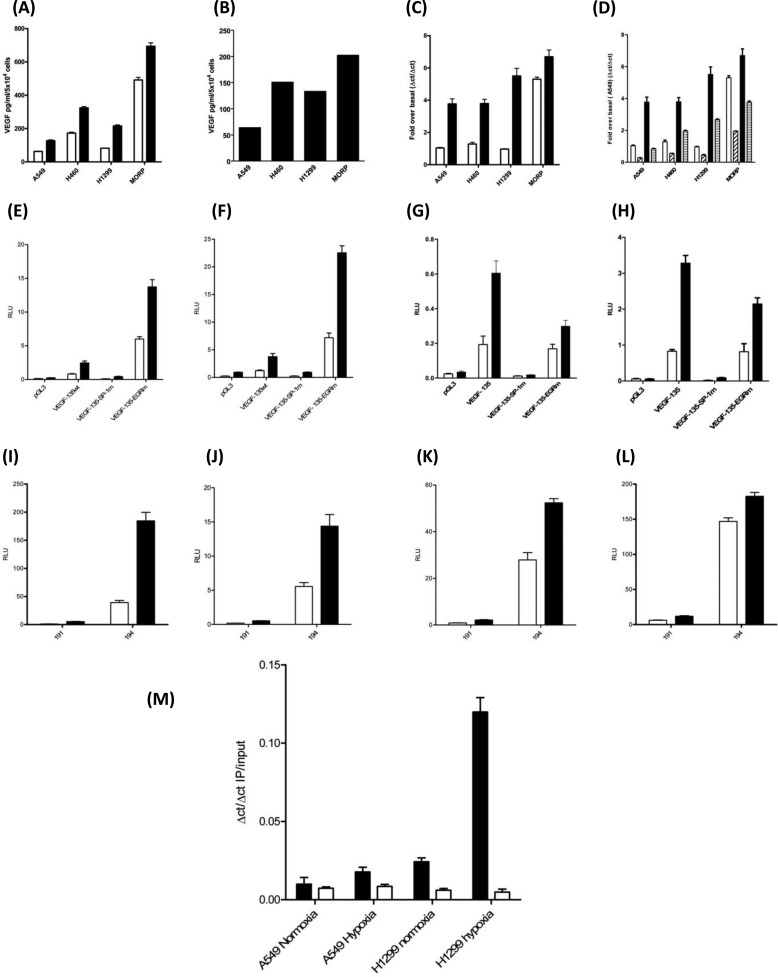
Serum-free NSCLC cell lines secreted more VEGF-A than equivalent numbers of primary normal human bronchial epithelial cells over 24 h. Among the NSCLC cell lines, VEGF output was ranked highest to lowest as A549<H460<H199<MOR/P (Fig. 1A). The addition of the RNA polymerase II inhibitor, actinomycin D, for 24 h prior to VEGF ELISA analysis, caused 80% depletion of VEGF-A secretion from all four NSCLC lines under basal conditions (Fig. 1B). Parallel analysis of VEGF-A mRNA demonstrates that there was 90% depletion of VEGF mRNA within 24 h of actinomycin D treatment (Fig. 1C). Total mRNA in each NSCLC cell line was proportional to VEGF protein secretion (Fig. 1D) with A549 having the lowest VEGF mRNA level and MOR/P the greatest VEGF mRNA quantity, with the exception of H460 possessing a relatively higher mRNA than VEGF protein when compared with H1299. All VEGF mRNA had a short half-life of <2 h, with little difference between the cell lines (Fig. 1E), suggesting that altered VEGF mRNA stability did not explain the difference in mRNA accumulation between cell lines. All four NSCLC cell lines were transfected with VEGF promoter reporter constructs from positions +50 to −2018 (2068), +50 to −1286 (1340), +50 to −789 (840), +50 to −414 (465), +50 to −265 (318), +50 to −85 (135), and +50 to −52 (102) (all numbers are positions relative to the VEGF-A gene transcription start site) and assayed for luciferase activity after 24 h. 35 bp (from −85 to −50 bp prior to the transcription start site) of the VEGF promoter were required for promoter reporter activity in all four cell lines (Fig. 2, A–D).
FIGURE 1.
Increased VEGF transcription drives elevated VEGF secretion from non-small cell lung cancer cells. NHBE cells and four non-small cell lung cancer cell lines were cultured for 24 h with serum-free medium. A, culture supernatants were analyzed by VEGF ELISA. B and C, A549 (open bars), H460 (gray bars), H1299 (lined bars), and MORP (black bar) were treated with actinomycin D (5 μg/ml) for 24 h, the culture supernatants analyzed by VEGF ELISA (B) and cell RNA extracted for RT-QPCR analysis of VEGF mRNA accumulation (C). D, RT-QPCR of untreated controls demonstrated that VEGF secretion was paralleled by VEGF mRNA levels in NSCLC cells. mRNA stability is roughly equivalent in each of the NSCLC lines with mRNA half-lives between 1.0 and 1.6 h. E, NSCLC lines were treated with 5 μg/ml actinomycin D for 24 h with samples taken at 0, 2, 4, 8, 12, and 24 h after addition with RT-QPCR for VEGF mRNA levels with A549 (▴), H460 (□), H1299 (♦), and MORP (▿). All of the measurements represent the means ± S.E. of three independent experiments. All of the ELISAs are normalized to cell counts or subsequent MTT assay for cellular cytotoxicity.
FIGURE 2.
Two SP-1 transcription factor-binding sites, in the VEGF proximal gene promoter, are essential for VEGF transcription in NSCLC cells. A–D, four NSCLC cell lines, A549 (A), H460 (B), H1299 (C), and MORP (D) were transfected with 0.1 μg of VEGF promoter reporter-luciferase construct and pGL3 “promoter-less” for 24 h and assayed for luciferase activity (VEGF promoter constructs are +50 bp upstream of the VEGF transcription start site to −52 (102), −85 (135), −268 (318), −415 (465), −790 (840), 1290 (1340), and 2018 (2068)), the 33-bp section of VEGF promoter (between −52 and −85 bp) were required for VEGF promoter activity in NSCLC cells. E, mutation of all three SP-1-binding sites in the VEGF-135 promoter reporter construct (mutated residues underlined for SP1m), but not mutation of EGR binding sites (mutated residues underlined for EGRm), eliminated VEGF-135 promoter reporter activity in all four NSCLC lines. F–I, four NSCLC cell lines, A549 (F), H460 (G), H1299 (H), and MORP (I) were transfected with 0.1 μg of VEGF promoter reporter-luciferase construct (VEGF-135, nonmutated, VEGF-135-SP1m, all SP-1 sites mutated, VEGF-135-EGRm, all EGR sites mutated) and pGL3 for 24 h and assayed for luciferase activity. All of the measurements represent the means ± S.E. of three independent experiments. RLU, relative light units.
SP-1 Transcription Factor-binding Sites Were Required for Constitutive VEGF Promoter Activity in NSCLC, SP-1 Transcription Factor Reporter Activity Correlated with Increased VEGF mRNA Expression and SP-1 Overexpression Can Drive VEGF Promoter Activity
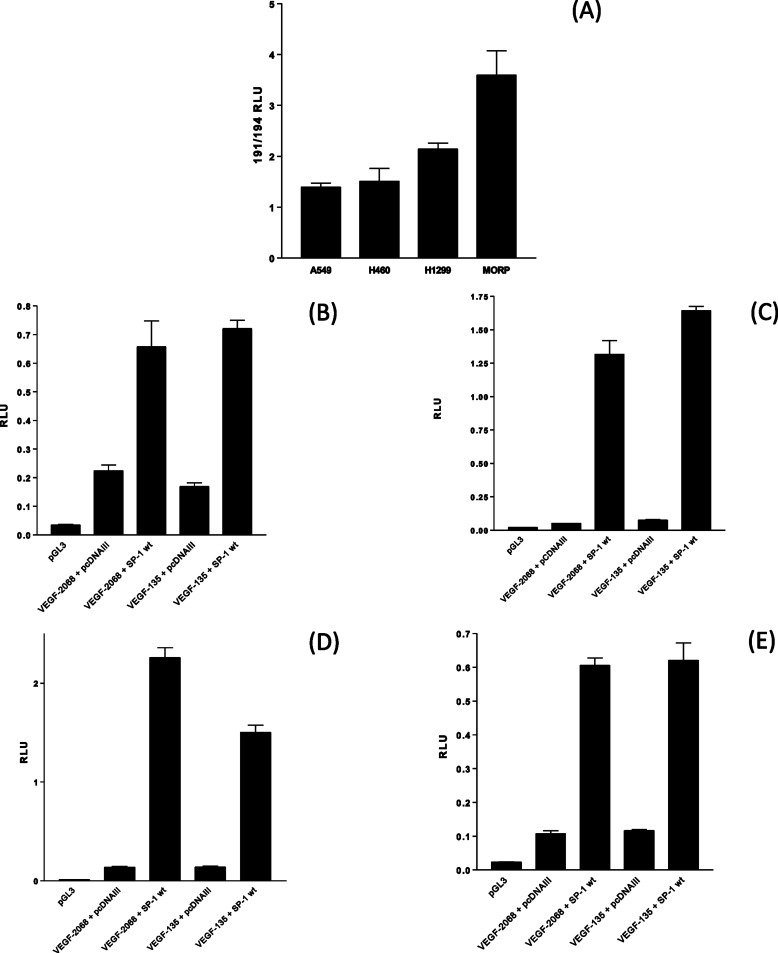
The last 855 bp of the VEGF promoter (from −85 to −0 bp prior to the transcription start site) possesses a single AP-1 transcription factor-binding site, two EGR transcription factor-binding sites, and three SP1 transcription factor-binding sites (Fig. 2E). Site-directed mutagenesis of the two EGR binding sites (VEGF-135-EGRm) did not affect VEGF-135 activity in all four cell lines (Fig. 2, F–I), site-directed mutagenesis of all three SP-1-binding sites (VEGF-135-SP1m) completely eliminated VEGF promoter reporter activity in all four NSCLC cell lines (Fig. 2, F–I). We have established that the NSCLC cell lines produce differing quantities of VEGF-A protein and that the order of increased VEGF secretion (A549 < H460 < H199 < MOR/P) was paralleled by increasing VEGF transcript (Fig. 1D). Analysis of VEGF promoter constructs has established that the SP-1 binding within the last 85 bp prior to the transcription start site was essential for VEGF promoter reporter activity. Transfection of the “194” SP-1 activity reporter gene construct (in parallel with the same vector with all SP-1 sites inactivated by mutation, “191”) demonstrated that there is greater SP-1 transcription factor activity in the order A549 < H460 < H199 < MOR/P (Fig. 3A), an order of activity reflective of both VEGF mRNA accumulation and protein secretion for a 24-h period for each cell line. To establish that increased SP-1 activity could drive VEGF promoter activity, the 2068-VEGF and 135-VEGF promoter reporters were co-transfected with a vector overexpressing the SP-1 protein (Fig. 3, B–E). In all four cell lines, SP-1 overexpression increases VEGF promoter reporter activity.
FIGURE 3.
NSCLC cells have increasing SP-1 transcription factor activity in parallel with elevated VEGF secretion, and SP-1 overexpression can increase NSCLC VEGF promoter activity. NSCLC cell lines were transfected with 0.1 μg of the SP-1 transcription factor reporter, plasmid 194, and the control SP-1 reporter, with all SP-1 sites functionally mutated (plasmid 191), for 24 h before assaying for luciferase activity. A, SP-1 transcription factor activity is expressed as a ratio of reporter to negative control (194/191). B–E, overexpression of SP-1 increased VEGF promoter-reporter construct activity in NSCLC cell lines A549 (B), H460 (C), H1299 (D), and MORP (E). NSCLC were co-transfected with 0.1 μg of VEGF-2068-luciferase or VEGF-135-luciferase plasmids with 1.0 μg of pCDNAIII empty vector or pCDNA-SP-1 for 24 h followed by a luciferase assay. All of the measurements represent the means ± S.E. of three independent experiments. RLU, relative light units.
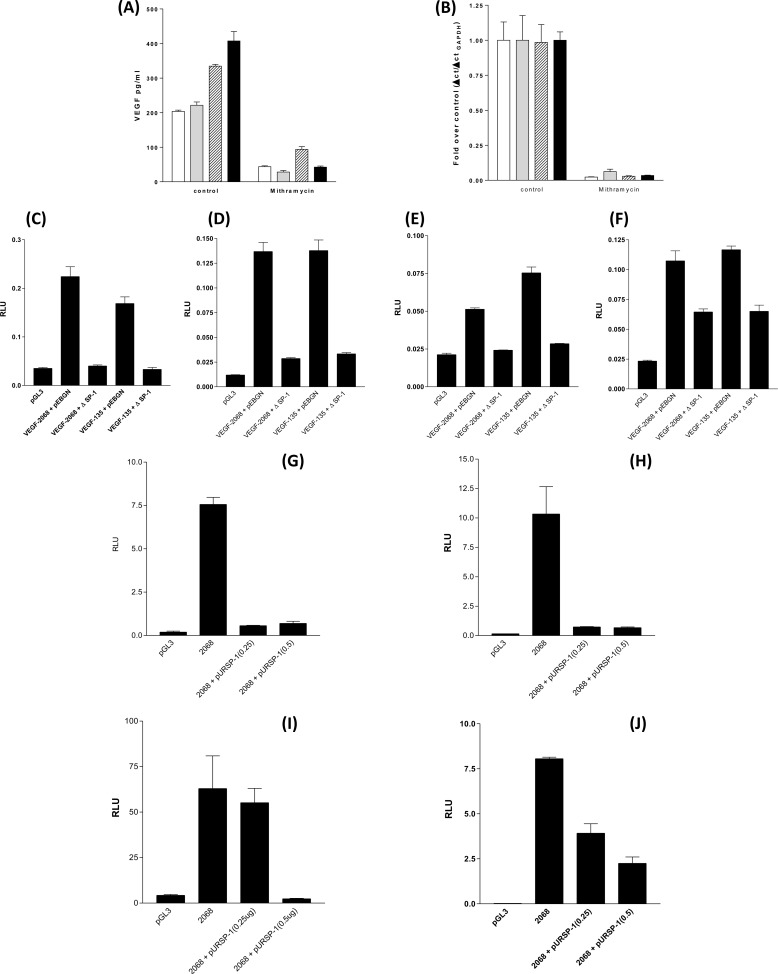
Mithramycin A Treatment and Either Dominant Negative SP-1 or SP-1 Ribozyme Construct Co-transfection Decreased Constitutive VEGF Promoter Activity
The pentagycosidic antibiotic mithramycin A blocks SP-1 binding to DNA by virtue of its ability to disrupt protein binding to GC-rich DNA (such as the SP-1 interaction with GGcGGGGcc binding site) (35). Treatment of all four NSCLC cell lines with mithramycin A for 24 h after medium replacement decreased VEGF-A secretion (Fig. 4A) and inhibited VEGF mRNA accumulation (Fig. 4B). We extended this observation by co-transfecting the VEGF-2068 and VEGF-135 promoter luciferase reporter constructs with the pEBGN-ΔSP-1 plasmid expressing the DNA-binding domain (amino acids 592–758) of SP-1. The SP-1 C-terminal protein can bind to the SP-1 recognition motifs of promoter DNA but by virtue of its inactivity will displace native SP-1 transcription factor activity. In each NSCLC cell line, pEBGN-ΔSP-1 decreased VEGF promoter activity for both VEGF-2068 and VEGF-135 (Fig. 4, C–F). The GC-rich binding site recognized and bound by SP-1 can bind a large family of SP-1-related proteins, the SP-1/Kruppel-like factors (36). The suppression of VEGF expression and secretion by mithramycin A and the reduction of VEGF promoter activity by a transactivation-inactive SP-1 construct does not eliminate the possibility that both have displaced an SP-1/Kruppel-like factor family member and not SP-1 itself from the VEGF promoter. The SP-1 shRNA/ribozyme construct pUR-SP1 provides a direct method of SP-1 inhibition by decreasing SP-1 expression (37). Co-expression of the pURSP-1 construct with the VEGF-2068 luciferase reporter for 48 h decreased VEGF promoter activity in A549, H460, H1299, and MORP cell lines (Fig. 4, G–J).
FIGURE 4.
Inhibition of SP-1 DNA binding, introduction of a nonfunctional SP-1 protein (ΔSP-1) or an SP-1 shRNA construct will prevent VEGF secretion and transcription. A and B, NSCLC, VEGF secretion (A) and mRNA accumulation (B) are inhibited in by mithramycin A. NSCLC were treated with 1 × 10−6 m mithramycin A for 24 h, and the supernatants were assayed by ELISA for VEGF. Total RNA was extracted, and the levels of VEGF mRNA were assessed by RT-QPCR with reference to GAPDH as a fold over untreated control. Open bars, A549; gray bars, H460; lined bars, H1299; black bars, MORP. NSCLC were co-transfected with 0.1 μg of VEGF-2068-luciferase or VEGF-135-luciferase plasmids with 1.0 μg of SP-1 DNA-binding domain construct (ΔSP-1) for 24 h followed by a luciferase assay. C–F, A549 (C), H460 (D), H1299 (E), and MORP (F). NSCLC were co-transfected with 0.1 μg of VEGF-2068-luciferase (2068) with 0.25 or 0.5 μg of SP-1 shRNA construct (pURSP-1) for 48 h followed by a luciferase assay. G–J, A549 (G), H460 (H), H1299 (I), and MORP (J). All of the measurements represent the means ± S.E. of three independent experiments. RLU, relative light units.
NSCLC Have Elevated SP-1 Protein Levels, and SP-1 Protein Association with the Native VEGF Promoter Is Greater in Cell Lines Expressing More SP-1 Protein, Both Correlating with Increased Chromatin Remodelling and Histone Acetyltransferase Binding
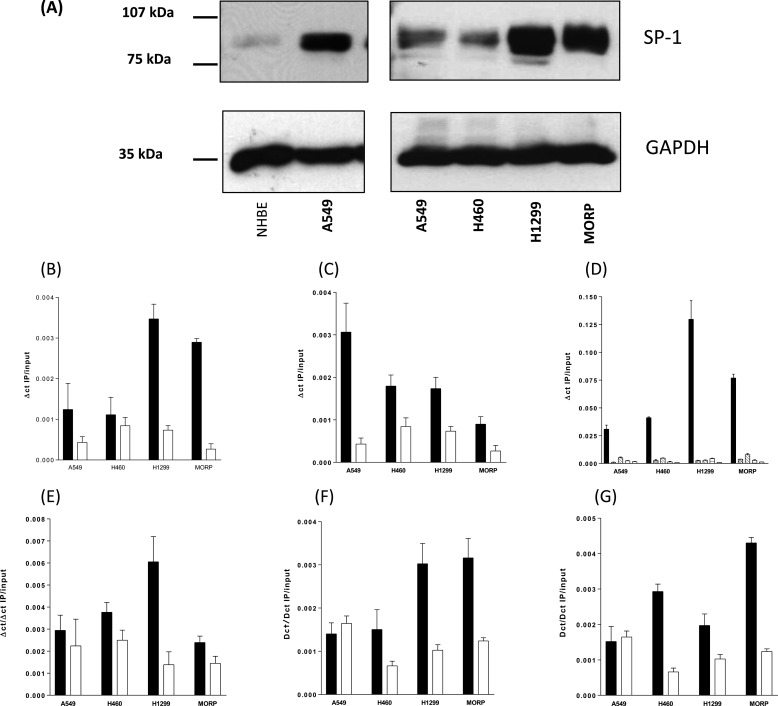
To assess SP-1 protein levels in NSCLC cell lines, total protein extracts were analyzed by Western blot with a comparison with an equivalent protein loading from NHBE cells (Fig. 5A). The SP-1 protein was visible as a doublet of 96 and 102kDa. The NSCLC cell lines had a far higher level of SP-1 protein than that of NHBE, and there were greater amounts of SP-1 in H1299 and MORP cell lines than that of the A549 and H460 cell lines. The level of SP-1 protein was in the order NHBE [tlt] A549 = H460 < H1299 < MORP. With elevated SP-1 protein expression correlating with increased SP-1 transcription factor activity and the VEGF promoter demonstrating dependence on SP-1-binding sites within VEGF-reporter gene constructs, we sought to confirm that the native VEGF promoter has a greater association with SP-1. ChIP assays were performed in all four cell lines with SP-1 antisera. QPCR was performed from −85 to +50 bp of the VEGF promoter (relative to the transcription start site) against total chromatin and the output IPs. The VEGF promoter from all four cell lines associated with the SP-1 protein with increased binding in cell lines expressing greater quantities of SP-1 protein (H1299 and MOR/P) (Fig. 5B). “Off ChIP” control QPCR at +8000 bp (data not shown) showed no nonspecific chromatin SP-1 interaction. Of the family of Kruppel-like factor transcription factors, SP-3 has the same site specificity and affinity as SP-1 (38), and the SP3 protein is constitutively expressed at the same level in all four cell lines (data not shown). To provide a further control for the correlation between SP-1 expression and SP-1 VEGF promoter binding, we performed ChIP for SP-3 and found a reduction in SP-3/VEGF promoter binding as SP-1 binding increases (Fig. 5C). With the high sequence homology between the SP-1 and SP-3 transcription factors and similar affinities for GC rich binding site on the VEGF promoter, ChIP for SP-3 provides a control for the specificity of SP-1 ChIP, and in this case higher SP-1 binding correlated with greater SP-1 expression. By virtue of its ability to compete for the same binding site as SP-1, SP-3 may act as an inhibitor of SP-1 transcription factor activity (39). Although we have not further investigated the role of SP-3 in VEGF expression, high SP-3 binding correlates with low SP-1 binding (Fig. 5C), and the least VEGF transcription of all four cell lines was studied. Transcription initiation is dependent upon an open, remodelled, chromatin structure around the transcription start site. Acetylation of core histones such as histone H4 and histone H3 by the histone acetyltransferase family (such as CBP, p300, and PCA/F) is a component of the remodelling process. We compared the ChIP products for acetyl-H4, acetyl-H3, total H4, and total H3 at the VEGF promoter (Fig. 5D). Active VEGF gene transcription and SP-1 binding was associated with an increase in histone H4 acetylation and a small increase in histone H3 acetylation. Increased histone acetylation was paralleled by increased histone acetyltransferase binding at the active promoter. We have found that there was increasing PCA/F (Fig. 5F) and CBP (Fig. 5G) binding across all four cell-lines correlated with greater VEGF transcription and VEGF secretion. “Off-ChIP” controls confirmed that these binding events were localized to the proximal VEGF promoter (data not shown).
FIGURE 5.
NSCLC cells express higher levels of SP-1 total cell protein than normal human bronchial epithelial cells. Native SP-1 transcription factor binding to the VEGF promoter increases with VEGF expression as SP-3 transcription factor binding decreases. Increased SP-1 binding is paralleled by increased histone H4 acetylation and histone acetyltransferase binding to the VEGF promoter of NSCLC cells. A, 30 μg of total cellular protein from NHBE and A549 were Western blotted and probed with an anti-SP-1 antibody (left panel). 10 μg of total cellular protein from A549, H460, H1299, and MORP were Western blotted and probed with an anti-SP-1 antibody (right panel), and the blots were stripped and reprobed with an anti-GAPDH antibody. B and C, the images are representative blots from experiments repeated three times. ChIP was performed for transcription factors SP-1 (B) and SP-3 (C) from chromatin samples from NSCLC cells maintained in serum-free medium for 24 h. IP and input DNAs were subjected to QPCR for the VEGF promoter between −85 and + 50 bp relative to the transcription start site of the VEGF promoter. Control ChIP was performed with equivalent rabbit IgG for all chromatin samples. D, ChIP for identical samples and their relevant control IgGs (open bars) were performed for acetylated (black bars) and nonacetylated histone H4 (cross-hatched bars), acetylated (diagonal line bars), and nonacetylated histone H3 (horizontal line bars). E, p300 and histone acetyltransferase. F, P/CAF and histone acetyltransferase. G, CBP and histone acetyltransferase. All of the measurements represent the means ± S.E. of three independent experiments.
VEGF and SP-1 Protein Co-localize and Expression Levels Show a Strong Correlation in Human NSCLC Tumor Tissues
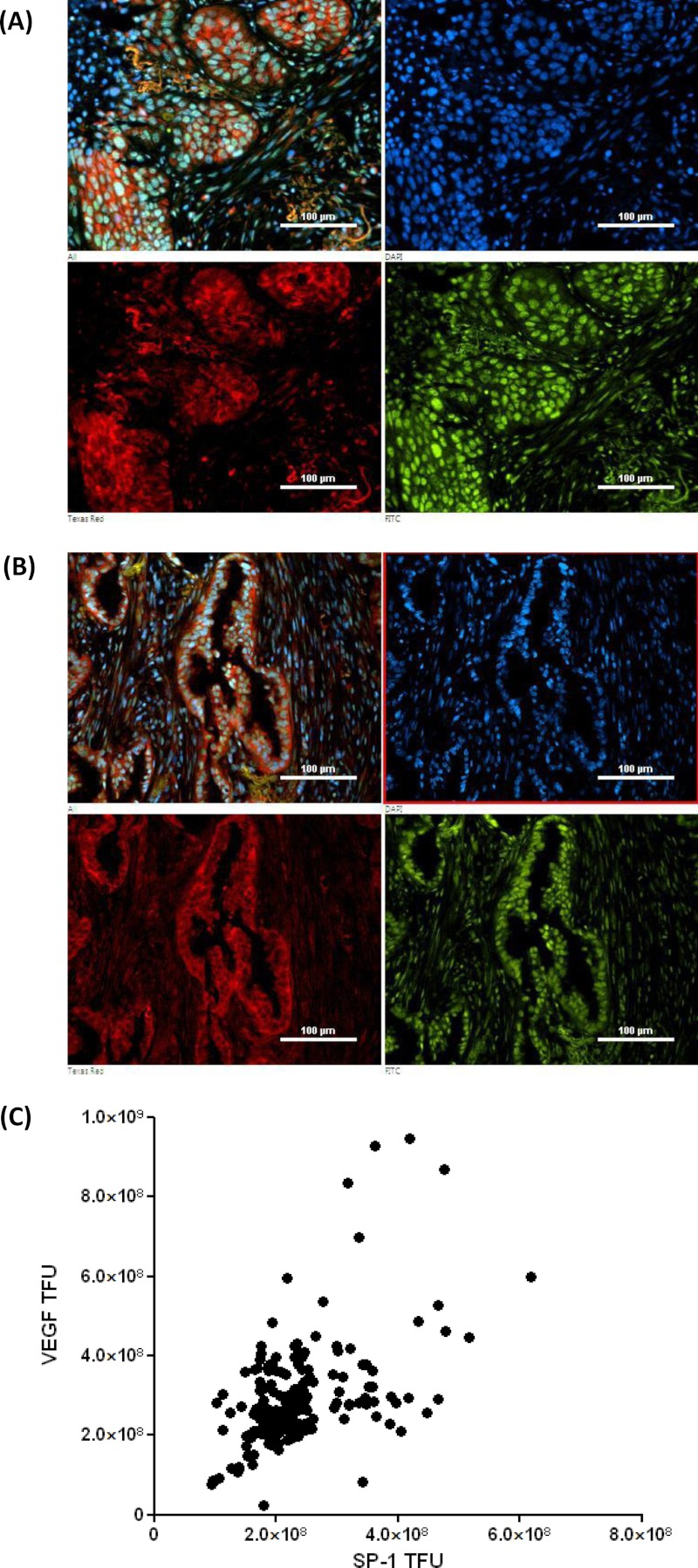
NSCLC tumor samples were analyzed for VEGF and SP-1 protein expression by dual fluorescence immunohistochemistry (Fig. 6, A and B). VEGF co-localizes with SP-1 in squamous cell carcinoma (Fig. 6A) and in adenocarcinoma (Fig. 6B). VEGF was cytoplasmic in distribution, and SP-1 is nuclear in distribution. Total fluorescence counts for Texas Red (VEGF) and FITC (SP-1) were taken, and the background counts for each slide were subtracted from parallel slice controls incubated with preimmune IgG (seven fields of view for each patient sample). VEGF and SP-1 total fluorescence units were analyzed by Pearson's correlation coefficient test, giving a Pearson R value of 0.503, p ≤ 0.0001, suggesting a highly significant correlation between VEGF and SP-1 protein expression levels in NSCLC tissue of both squamous and adenocarcinoma morphology (Fig. 6C).
FIGURE 6.
VEGF and SP-1 protein expression correlate and co-localize in human non-small cell lung cancer tumors. Immunohistochemistry, 30 patient tumor samples were triple stained with anti-VEGF antibodies (Texas Red), anti-SP-1 antibodies (FITC), and DAPI. A, an example squamous cell carcinoma shows a combined triple image (upper left), DAPI (upper right), cytoplasmic VEGF (lower left, Texas Red filter), and nuclear SP-1 (lower right, FITC filter). B, an example adenocarcinoma shows a combined triple image (upper left), DAPI (upper right), cytoplasmic VEGF (lower left, Texas Red filter), and nuclear SP-1 (lower right, FITC filter). C, total VEGF and total SP-1 fluorescence measurements from 30 tumors (seven random fields of view, with preimmune antibody control fluorescence subtracted) were analyzed by a two-tailed Pearson correlation plot giving a Pearson R score of 0.503 and P score of <0.0001. TFU, total fluorescence units.
The SP-1 Transcription Factor-binding Site of the Minimal VEGF Promoter Is Required for Hypoxia-induced VEGF Expression in NSCLC with SP-1 Transcription Factor Activity and SP-1 Binding, to the VEGF Promoter, Increased during NSCLC Hypoxia
NSCLC cell lines were incubated for 20 h under normoxic and hypoxic conditions with a VEGF ELISA performed on the resulting culture supernatants (Fig. 7A). The total hypoxia-stimulated VEGF output (hypoxia-normoxia) increased from A549 to MORP (Fig. 7B), and hypoxia increased VEGF mRNA accumulation in all four cell lines (Fig. 7C). With SP-1 central to basal VEGF transcription, we investigated the role of SP-1 in hypoxia-induced VEGF transcription. Pretreatment with mithramycin A decreased the hypoxic accumulation of VEGF mRNA in all four cell lines, implying a role for SP-1 in the hypoxic induction of VEGF mRNA accumulation (Fig. 7D). The minimal VEGF promoter contains three essential SP-1-binding sites (Fig. 2). NSCLC were transfected with the all of the VEGF promoter reporter constructs followed by 20 h of normoxia or hypoxia with subsequent luciferase assay for each cell line (Supplemental data S1), the minimal hypoxia-induced VEGF promoter was within the VEGF-135 construct. To establish what role SP-1 has in hypoxia-induced VEGF transcription the wild-type, SP-1 mutant, and EGR mutant VEGF-135-luciferase constructs were transfected into NSCLC cell lines, and the luciferase activity was measured after exposure to normoxic or hypoxic conditions for 20 h. The VEGF-135 construct had no hypoxic response without SP-1-binding sites (Fig. 7, E–H). To confirm that a cellular hypoxic response had taken place, the hypoxia response element luciferase plasmid (40) was transfected into each NSCLC cell line, and a luciferase assay was performed after 20 h of hypoxia (Supplemental data S2). If SP-1 plays a role in hypoxia-induced VEGF transcription, then the activity of SP-1 and binding of SP-1 to the VEGF promoter should increase under hypoxic conditions. NSCLC cells were transfected with the SP-1 reporter-luciferase construct (194) and its control vector (191) with 20 h of normoxia or hypoxia. In all four cell lines, hypoxia increased SP-1 reporter gene activity (Fig. 7, I–L). Chromatin immunoprecipitation assays were conducted for SP-1 (with control preimmune IgG) against the VEGF promoter with chromatin from normoxia or 4-h hypoxia-treated A549 or H1299 cell lines. The A549 cell line had the lowest SP-1 activity and SP-1 basal binding to the VEGF promoter (Figs. 3A and 5A) with the H1299 cell line the highest in both cases. Hypoxia induced a small but significant binding of SP-1 to the native VEGF promoter in A549 cells. In contrast there was a large increase in SP-1 binding to the native VEGF promoter in H1299 cells (Fig. 7M).
FIGURE 7.
SP-1 transcription factor binding is required for hypoxia-induced VEGF expression in NSCLC. SP-1 reporter activity and Sp-1 transcription factor binding to the native VEGF promoter increased with hypoxia. Hypoxia increased VEGF secretion and mRNA accumulation. A and B, four NSCLC cell lines were cultured for 24 h under normoxic (open bars) or hypoxic conditions (closed bars) (1% O2), and the culture supernatants were analyzed by VEGF ELISA (A) with total VEGF secretion induced by hypoxia in each cell line (B). C, after 4 h of hypoxia (closed bars) or control (open bars) cell RNA were extracted for RT-QPCR analysis of VEGF mRNA accumulation. Mithramycin blocks VEGF mRNA accumulation in response to hypoxia. D, NSCLC cell lines were treated with mithramycin A (1 × 10−6 m) for 30 min prior to 4 h of control conditions (open bars, untreated; hatched bars, treated) or 4 h of hypoxia (filled bars, untreated; cross-hatched bars, treated). Mutation of SP-1-binding sites in the VEGF minimal (135) promoter luciferase-reporter eliminates the hypoxic response in all four NSCLC cell lines. E–H, NSCLC cell lines A549 (E), H460 (F), H1299 (G), and MORP (H) transfected with wild-type (VEGF-135wt), SP-1 site mutant (VEGF-SP-1m), and EGR site mutant (VEGF-EGRm) VEGF-135-luciferase reporter constructs (Fig. 2E) with normoxia (open bars) and hypoxia (closed bars) for 20 h. Hypoxia increases SP-1 reporter-luciferase activity in NSCLC cell lines. I–L, all four NSCLC cell lines, A549 (I), H460 (J), H1299 (K), and MORP (L), were transfected with the control plasmid with mutant SP-1-binding sites (191) and the wild-type SP-1 reporter construct (194) followed by normoxia (open bars) or hypoxia (closed bars) for 20 h. Hypoxia-induced SP-1 binding to the native VEGF promoter is greater in NSCLC expressing higher SP-1 levels. A549 (low Sp-1 expression) and H1299 (high SP-1 expression) were exposed to normoxia or hypoxia for 4 h. M, ChIP was performed for transcription factor SP-1 (filled bars) from chromatin samples from NSCLC. IP and input DNAs were subjected to QPCR for the VEGF promoter between −85 and +50 bp relative to the transcription start site of the VEGF promoter. Control ChIP were performed with equivalent rabbit IgG for all chromatin samples (open bars). All of the measurements represent the means ± S.E. of three independent experiments. RLU, relative light units.
DISCUSSION
Immunohistochemical studies of non-small cell lung cancer tumors have established that there is a strong correlation between tumor-associated blood vessel formation, an increase in microvascular density, and the expression of VEGF-A protein (2–5). The aim of this study was to provide a molecular mechanism for the observed increased expression of VEGF in non-small cell lung cancer tumor and assess the role of this in the general mechanism of hypoxic regulation of VEGF expression. There are a number of novel features, some of which give greater insight into the mechanisms involved in VEGF secretion in lung cancer and some of which have more general novelty in respect to the role of SP-1 in hypoxic induction of VEGF.
With regard to fresh insight into the mechanisms involved in VEGF secretion in lung cancer, our study is the first to demonstrate that under basal conditions in NSCLC cells, the VEGF promoter is SP-1-dependent and that NSCLC cells express higher levels of active SP-1 protein than normal cells. We are also the first to establish in any cancer that increased SP-1 binding to the native VEGF promoter correlates with increased histone acetyltransferase binding and histone H4 polyacetylation. Furthermore, we are the first to demonstrate that there is a strong correlation between VEGF and SP-1 protein levels in human NSCLC tumor tissue.
With regard to more generalizable biological novelty, we have shown for the first time a central role for SP-1 in hypoxia-induced VEGF expression. SP-1-binding sites were required for hypoxic induction of VEGF promoter reporter constructs, hypoxia-increased SP-1 activity, and hypoxic induction of SP-1 binding to the VEGF promoter. This is the first such observation of a role for SP-1 in hypoxia regulation of VEGF in any type of cancer.
Our observation of lower VEGF secretion from NHBE than NSCLC is from a single NHBE cell line. Although it could be argued that this reflects genetic variation, we feel this is unlikely, because Lee et al. (41) and Takayama et al. (42) demonstrated that noncancerous airway epithelial cells secrete less VEGF than NSCLC cells.
In our study deletion analysis of the VEGF-A promoter defined constitutive activity in NSCLC cells in the last 85 bp prior to the transcription start site. Mutation of the three SP-1-binding sites, within this region, eliminates all promoter activity, and mutation of interdigitating EGR-1 binding sites did not affect VEGF promoter reporter activity. In a recent study Shimoyamada et al. (43) defined a similar region of the VEGF promoter when studying constitutive VEGF promoter activity in NSCLC cell lines (one of which, A549, forms part of this study). In their study all cells were cultured in 10% fetal calf serum, and the authors point out that growth factors such as EGF and PDGF (found in fetal calf serum) can induce EGR-1 activity (44–46). In contrast our study was conducted in serum-free conditions to remove environmental factors and better define any constitutive genetic factors responsible for VEGF expression. Under serum-deprived conditions, the SP-1-binding sites were essential for VEGF expression, and mutation of the EGR-1 binding site had no effect. Therefore it is likely that EGR-1 plays a role in VEGF expression in NSCLC, in response to growth factors present in the tumor microenvironment, whereas SP-1 overexpression and increased activity drives a high base-line VEGF expression in NSCLC tumor tissue. There has been only one previous study showing an increase in SP-1 expression in human lung tumors that showed that high SP-1 protein levels were correlated with CD147 expression (a cancer biomarker glycoprotein with a role in tumor metastasis) (47). The CD147 promoter possesses four SP-1-binding sites, and these studies showed that treatment with mithramycin or an SP-1 siRNA reduces CD147 RNA accumulation. Furthermore plasmid-driven SP-1 overexpression increased CD147 mRNA accumulation in a similar manner to our study of the VEGF promoter. Increased expression of the SP-1 correlates with a poor prognosis in multiple cancer types (48–52). In pancreatic and gastric carcinomas, there was a positive correlation between SP-1 and VEGF expression levels. High SP-1 expression was also found to correlate with components of cellular invasiveness in breast cancer and glioma. Thyroid cancer SP-1 expression levels are linked to expression of the sodium/iodide transporter (reducing the efficacy of radio-iodide therapy). The SP-1 transcription factor can interact with the promoters of many genes involved in self-sufficiency in growth, limitless replication, lack of anti-growth signals, lack of apoptosis, metastasis, deregulated angiogenesis, and suppression of immune surveillance (39); for this reason the consequences of SP-1 overexpression are far reaching in terms of malignant cell transformation. Lou and colleagues (37) overexpressed SP-1 in human fibroblasts leading to the creation of fibrosarcoma. Subsequent removal of SP-1 with an SP-1 ribozyme caused fibrosarcoma reversion to a nonmalignant state.
To date there is very little molecular detail of the epigenetic changes that take place at the VEGF promoter in NSCLC or other cancers. In this study we have shown that the VEGF promoter binds the histone acetyltransferases CBP and PCA/F. Localized histone polyacetylation is a required modification for active transcription; in this study we show that polyacetylation occurs at the VEGF promoter in all four NSCLC cell lines, specifically at histone H4 with polyacetylation between 5 and 25 times that of histone H3 at the VEGF promoter. This observation is promoter-specific because QPCR analysis of the same ChIP immunoprecipitations, at the SP-1 gene promoter (data not shown), have greater polyacetylation at histone H3 than at histone H4.
There is strong evidence, both histochemical and biochemical, for the occurrence of hypoxia in lung tumors (6). In this study, we have identified SP-1 overexpression as a cause of deregulated VEGF expression and have demonstrated that SP-1 plays a key role in hypoxia-induced VEGF expression from the same cell lines; however, we have not fully defined the mechanism of SP-1-dependent hypoxia-induced VEGF transcription. The study of colon cancer by Mizukami et al. (22) defined a region of the VEGF promoter capable of HIF-1α-independent hypoxic stimulation that contained all of the three SP-1-binding sites we have characterized, although further direct characterization of the role of SP-1 in hypoxia-induced VEGF expression in this study did not take place. HIF-1α-independent hypoxic regulation of genes other than VEGF has been described with HIF-1α-independent activation of β-enolase, pyruvate kinase-M (52), carbonic anhydrase IX (27), and cyclooxygenase 2 (28) promoters are being SP-1-dependent. In contrast hypoxic suppression of the MSH2 and MSH6 (complexes of which form the MutSα DNA mismatch repair marker protein) by HIF-1α is dependent upon the interaction of SP-1 and HIF-1α (29). Future work should focus on potential interactions between HIF-1α and SP-1 at the VEGF promoter in lung cancer angiogenesis.
In summary, constitutive VEGF secretion in NSCLC cells is dependent upon the SP-1 transcription factor activity under normoxic and serum-free conditions in a panel of NSCLC cell lines. VEGF mRNA expression correlated with SP-1 transcription factor activity and SP-1 binding to the native VEGF promoter in NSCLC cells. NSCLC cells express high levels of the SP-1 protein when compared with the levels present in normal cells, and VEGF/SP-1 protein levels have a significant correlation in human non-small cell lung cancer tumors. Of far greater significance to general mechanisms of hypoxia-induced gene expression is our finding that hypoxia increased SP-1 activity, and binding to the VEGF promoter is required for hypoxia-induced VEGF expression. These studies suggest that SP-1 will be an important target in future cancer angiogenesis research.
Supplementary Material

This article contains supplemental Figs. S1 and S2.
- NSCLC
- non-small cell lung cancer
- HIF
- hypoxia-inducible factor
- QPCR
- quantitative PCR
- IP
- immunoprecipitation
- NHBE
- normal human bronchial epithelial.
REFERENCES
- 1. Hanahan D., Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 2. Yuan A., Yu C. J., Chen W. J., Lin F. Y., Kuo S. H., Luh K. T., Yang P. C. (2000) Correlation of total VEGF mRNA and protein expression with histologic type, tumor angiogenesis, patient survival and timing of relapse in non-small-cell lung cancer. Int. J. Cancer 89, 475–483 [DOI] [PubMed] [Google Scholar]
- 3. Yuan A., Yu C. J., Kuo S. H., Chen W. J., Lin F. Y., Luh K. T., Yang P. C., Lee Y. C. (2001) Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J. Clin. Oncol. 19, 432–441 [DOI] [PubMed] [Google Scholar]
- 4. Han H., Silverman J. F., Santucci T. S., Macherey R. S., d'Amato T. A., Tung M. Y., Weyant R. J., Landreneau R. J. (2001) Vascular endothelial growth factor expression in stage I non-small cell lung cancer correlates with neoangiogenesis and a poor prognosis. Ann. Surg. Oncol. 8, 72–79 [DOI] [PubMed] [Google Scholar]
- 5. Decaussin M., Sartelet H., Robert C., Moro D., Claraz C., Brambilla C., Brambilla E. (1999) Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs). Correlation with angiogenesis and survival. J. Pathol. 188, 369–377 [DOI] [PubMed] [Google Scholar]
- 6. Graves E. E., Vilalta M., Cecic I. K., Erler J. T., Tran P. T., Felsher D., Sayles L., Sweet-Cordero A., Le Q. T., Giaccia A. J. (2010) Hypoxia in models of lung cancer. Implications for targeted therapeutics. Clin. Cancer Res. 16, 4843–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsai J. C., Goldman C. K., Gillespie G. Y. (1995) Vascular endothelial growth factor in human glioma cell lines. Induced secretion by EGF, PDGF-BB, and bFGF. J. Neurosurg. 82, 864–873 [DOI] [PubMed] [Google Scholar]
- 8. De Luca A., Carotenuto A., Rachiglio A., Gallo M., Maiello M. R., Aldinucci D., Pinto A., Normanno N. (2008) The role of the EGFR signaling in tumor microenvironment. J. Cell Physiol. 214, 559–567 [DOI] [PubMed] [Google Scholar]
- 9. Gately S. (2000) The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 19, 19–27 [DOI] [PubMed] [Google Scholar]
- 10. Textor B., Sator-Schmitt M., Richter K. H., Angel P., Schorpp-Kistner M. (2006) c-Jun and JunB are essential for hypoglycemia-mediated VEGF induction. Ann. N.Y. Acad. Sci. 1091, 310–318 [DOI] [PubMed] [Google Scholar]
- 11. Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J., Moore M. W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 [DOI] [PubMed] [Google Scholar]
- 12. Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M., Fahrig M., Vandenhoeck A., Harpal K., Eberhardt C., Declercq C., Pawling J., Moons L., Collen D., Risau W., Nagy A. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 [DOI] [PubMed] [Google Scholar]
- 13. Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362, 841–844 [DOI] [PubMed] [Google Scholar]
- 14. Gerber H. P., Ferrara N. (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 65, 671–680 [PubMed] [Google Scholar]
- 15. Yoo P. S., Mulkeen A. L., Cha C. H. (2006) Post-transcriptional regulation of vascular endothelial growth factor. Implications for tumor angiogenesis. World J. Gastroenterol. 12, 4937–4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrara N. (2010) Binding to the extracellular matrix and proteolytic processing. Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 21, 687–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ladomery M. R., Harper S. J., Bates D. O. (2007) Alternative splicing in angiogenesis. The vascular endothelial growth factor paradigm. Cancer Lett. 249, 133–142 [DOI] [PubMed] [Google Scholar]
- 18. Folkman J., Long D. M., Jr., Becker F. F. (1963) Growth and metastasis of tumor in organ culture. Cancer 16, 453–467 [DOI] [PubMed] [Google Scholar]
- 19. Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation. Implications for O2 sensing. Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 20. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 21. Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 22. Mizukami Y., Li J., Zhang X., Zimmer M. A., Iliopoulos O., Chung D. C. (2004) Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res. 64, 1765–1772 [DOI] [PubMed] [Google Scholar]
- 23. Arany Z., Wagner B. K., Ma Y., Chinsomboon J., Laznik D., Spiegelman B. M. (2008) Gene expression-based screening identifies microtubule inhibitors as inducers of PGC-1α and oxidative phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 4721–4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark P. E. (2009) The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 76, 939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rak J., Yu J. L., Klement G., Kerbel R. S. (2000) Oncogenes and angiogenesis. Signaling three-dimensional tumor growth. J. Investig. Dermatol. Symp. Proc. 5, 24–33 [DOI] [PubMed] [Google Scholar]
- 26. Spinella F., Rosanò L., Di Castro V., Natali P. G., Bagnato A. (2004) Endothelin-1-induced prostaglandin E2-EP2, EP4 signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J. Biol. Chem. 279, 46700–46705 [DOI] [PubMed] [Google Scholar]
- 27. Kaluz S., Kaluzová M., Stanbridge E. J. (2003) Expression of the hypoxia marker carbonic anhydrase IX is critically dependent on SP1 activity. Identification of a novel type of hypoxia-responsive enhancer. Cancer Res. 63, 917–922 [PubMed] [Google Scholar]
- 28. Xu Q., Ji Y. S., Schmedtje J. F., Jr. (2000) Sp1 increases expression of cyclooxygenase-2 in hypoxic vascular endothelium. Implications for the mechanisms of aortic aneurysm and heart failure. J. Biol. Chem. 275, 24583–24589 [DOI] [PubMed] [Google Scholar]
- 29. Koshiji M., To K. K., Hammer S., Kumamoto K., Harris A. L., Modrich P., Huang L. E. (2005) HIF-1α induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol. Cell 17, 793–803 [DOI] [PubMed] [Google Scholar]
- 30. Deacon K., Knox A. J. (2010) Endothelin-1 (ET-1) increases the expression of remodeling genes in vascular smooth muscle through linked calcium and cAMP pathways. Role of a phospholipase A2 (cPLA2)/cyclooxygenase-2 (COX-2)/prostacyclin receptor-dependent autocrine loop. J. Biol. Chem. 285, 25913–25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deacon K., Blank J. L. (1997) Characterization of the mitogen-activated protein kinase kinase 4 (MKK4)/c-Jun NH2-terminal kinase 1 and MKK3/p38 pathways regulated by MEK kinases 2 and 3. MEK kinase 3 activates MKK3 but does not cause activation of p38 kinase in vivo. J. Biol. Chem. 272, 14489–14496 [DOI] [PubMed] [Google Scholar]
- 32. Bradbury D., Clarke D., Seedhouse C., Corbett L., Stocks J., Knox A. (2005) Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J. Biol. Chem. 280, 29993–30000 [DOI] [PubMed] [Google Scholar]
- 33. Finkenzeller G., Technau A., Marmé D. (1995) Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem. Biophys. Res. Commun. 208, 432–439 [DOI] [PubMed] [Google Scholar]
- 34. Finkenzeller G., Sparacio A., Technau A., Marmé D., Siemeister G. (1997) Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene 15, 669–676 [DOI] [PubMed] [Google Scholar]
- 35. Blume S. W., Snyder R. C., Ray R., Thomas S., Koller C. A., Miller D. M. (1991) Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J. Clin. Invest. 88, 1613–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Black A. R., Black J. D., Azizkhan-Clifford J. (2001) Sp1 and Kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell Physiol. 188, 143–160 [DOI] [PubMed] [Google Scholar]
- 37. Lou Z., O'Reilly S., Liang H., Maher V. M., Sleight S. D., McCormick J. J. (2005) Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 65, 1007–1017 [PubMed] [Google Scholar]
- 38. Li L., Davie J. R. (2010) The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. 192, 275–283 [DOI] [PubMed] [Google Scholar]
- 39. Wierstra I. (2008) Sp1. Emerging roles. Beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun 372, 1–13 [DOI] [PubMed] [Google Scholar]
- 40. Brown L. M., Cowen R. L., Debray C., Eustace A., Erler J. T., Sheppard F. C., Parker C. A., Stratford I. J., Williams K. J. (2006) Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol. Pharmacol. 69, 411–418 [DOI] [PubMed] [Google Scholar]
- 41. Lee C. G., Yoon H. J., Zhu Z., Link H., Wang Z., Gwaltney J. M., Landry M., Elias J. A. (2000) Respiratory syncytial virus stimulation of vascular endothelial cell growth factor/vascular permeability factor. Am. J. Respir. Cell Mol. Biol. 23, 662–669 [DOI] [PubMed] [Google Scholar]
- 42. Takayama K., Reynolds P. N., Adachi Y., Kaliberova L., Uchino J., Nakanishi Y., Curiel D. T. (2007) Vascular endothelial growth factor promoter-based conditionally replicative adenoviruses for pan-carcinoma application. Cancer Gene. Ther. 14, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimoyamada H., Yazawa T., Sato H., Okudela K., Ishii J., Sakaeda M., Kashiwagi K., Suzuki T., Mitsui H., Woo T., Tajiri M., Ohmori T., Ogura T., Masuda M., Oshiro H., Kitamura H. (2010) Early growth response-1 induces and enhances vascular endothelial growth factor-A expression in lung cancer cells. Am. J. Pathol. 177, 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bouchard F., Bélanger S. D., Biron-Pain K., St-Pierre Y. (2010) EGR-1 activation by EGF inhibits MMP-9 expression and lymphoma growth. Blood 116, 759–766 [DOI] [PubMed] [Google Scholar]
- 45. Lim J. H., Jung C. R., Lee C. H., Im D. S. (2008) Egr-1 and serum response factor are involved in growth factors- and serum-mediated induction of E2-EPF UCP expression that regulates the VHL-HIF pathway. J. Cell Biochem. 105, 1117–1127 [DOI] [PubMed] [Google Scholar]
- 46. Kamimura M., Bea F., Akizawa T., Katus H. A., Kreuzer J., Viedt C. (2004) Platelet-derived growth factor induces tissue factor expression in vascular smooth muscle cells via activation of Egr-1. Hypertension 44, 944–951 [DOI] [PubMed] [Google Scholar]
- 47. Kong L. M., Liao C. G., Fei F., Guo X., Xing J. L., Chen Z. N. (2010) Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 101, 1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yao J. C., Wang L., Wei D., Gong W., Hassan M., Wu T. T., Mansfield P., Ajani J., Xie K. (2004) Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin. Cancer Res. 10, 4109–4117 [DOI] [PubMed] [Google Scholar]
- 49. Shi Q., Le X., Abbruzzese J. L., Peng Z., Qian C. N., Tang H., Xiong Q., Wang B., Li X. C., Xie K. (2001) Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 61, 4143–4154 [PubMed] [Google Scholar]
- 50. Zannetti A., Del Vecchio S., Carriero M. V., Fonti R., Franco P., Botti G., D'Aiuto G., Stoppelli M. P., Salvatore M. (2000) Coordinate up-regulation of Sp1 DNA-binding activity and urokinase receptor expression in breast carcinoma. Cancer Res. 60, 1546–1551 [PubMed] [Google Scholar]
- 51. Guan H., Cai J., Zhang N., Wu J., Yuan J., Li J., Li M. (2012) Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int. J. Cancer 130, 593–601 [DOI] [PubMed] [Google Scholar]
- 52. Discher D. J., Bishopric N. H., Wu X., Peterson C. A., Webster K. A. (1998) Hypoxia regulates β-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J. Biol. Chem. 273, 26087–26093 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.