Abstract
Objective
Sasang constitution (SC) medicine, a branch of Korean traditional medicine, classifies the individual into one of four constitutional types (Taeum, TE; Soeum, SE; Soyang, SY; and Taeyang, TY) based on physiologic characteristics. The authors of the current article recently reported individual genetic elements associated with SC types via genome-wide association (GWA) analysis. However, to understand the biologic mechanisms underlying constitution, a comprehensive approach that combines individual genetic effects was applied.
Design
Genotypes of 1222 subjects of defined constitution types were measured for 341,998 genetic loci across the entire genome. The biologic pathways associated with SC types were identified via GWA analysis using three different algorithms—namely, the Z-static method, a restandardized gene set assay, and a gene set enrichment assay.
Results
Distinct pathways were associated (p<0.05) with each constitution type. The TE type was significantly associated with cytoskeleton-related pathways. The SE type was significantly associated with cardio- and amino-acid metabolism–related pathways. The SY type was associated with enriched melanoma-related pathways. TY subjects were excluded because of the small size of that sample. Among these functionally related pathways, core-node genes regulating multiple pathways were identified. TJP1, PTK2, and SRC were selected as core-nodes for TE; RHOA, and MAOA/MAOB for SE; and GNAO1 for SY (p<0.05), respectively.
Conclusions
The current authors systematically identified the biologic pathways and core-node genes associated with SC types from the GWA study; this information should provide insights regarding the molecular mechanisms inherent in constitutional pathophysiology.
Introduction
Sasang constitution (SC) medicine is a branch of Korean traditional medicine in which an individual is classified into one of four constitutional types (Taeum, TE; Soeum, SE; Soyang, SY; and Taeyang, TY) based on the nature of his/her physiologic and physical characteristics.1,2 Specifically, the balance among the physiologic functions of four representative internal organs—the Lung, Spleen, Liver, and Kidney (which represent the respiratory, digestive, preservative, and excretory functions, respectively)—is the most important factor for determining SC type. The balance among these organs determines the physiologic characteristics of SC types.3 For example, the TY type is associated with the developed nape of the neck and slender waist, the TE type is associated with large body and waist size, the SY type is associated with developed chest and small hip, and the SE type is associated with the hip and a weak chest.2,3 Recent genetic studies also found that polymorphisms of some genes were associated with SC types. For example, FTO and MC4R polymorphisms are associated with control of body mass, according to SC type.4 Interleukin-1α and -β polymorphisms were also associated with the SC type in obese women.5,6 These results support the notion of genetic involvement in determining constitution type.3–6
Considering that constitution is a concept that encompasses a diverse set of physiologic characteristics, such a single-gene approach is not sufficient to delineate the complex nature of the physiology underlying constitution. Therefore, Yin and colleagues reported previously that diverse genetic loci are associated with SC types in a genome-wide association (GWA) study of 60 subjects.7 Recently, the current authors greatly expanded the number of subjects to 1222 to identify genetic elements associated with SC.8 In that report, single-nucleotide polymorphisms (SNPs) associated with each constitution type were identified, and the relevant biologic functions were measured on the basis of these selected SNPs, but not on the whole genome level. Therefore, in this study, the current authors identified the biologic pathways involved in SC types, using a GWA study on the whole genome level. This pathway-based approach could provide novel insights into the molecular delineation of SC.
Materials and Methods
Subjects and diagnosis of constitution type
Peripheral blood samples were collected from a total of 1348 subjects who visited Korean Oriental hospitals between 2006 and 2009. All subjects provided informed consent to the use of their blood samples and clinicopathologic data for research purposes. All experiments were approved by the ethics committee of the Korea Institute of Oriental Medicine (KIOM) and conducted in accordance with the Institutional Guidelines for Human Experimentation. All samples and clinical information were deposited in the Sasang Constitutional Medicine databank (DB-SCM) in the KIOM.
The SC type of an individual was determined via three procedures. First, prediagnosis of the SC type of each individual was conducted based on the physical body shape, appearance, temperament, and pathologic symptoms of the individual by a licensed medical specialist who had been in clinical practice in SC medicine for at least 5 years. Second, subjects who were constitutionally prediagnosed were treated with constitution-specific herbal formulae, including Panax ginseng, Ephedra herba, Schisandra chinensis, etc., according to the individual's constitution.4,9,10 After taking the medicine for 30 days or more, any reductions in preexisting symptoms or occurrence of adverse effects (AEs) were recorded. It has been reported that prescriptions that were not appropriate for the SC type induced adverse reactions, such as indigestion, stomach pain, and evacuation troubles.11 Finally, the SC type of individual was determined by a review of at least 3 specialists in SC medicine. Therefore, subjects included only if they had good medication responses, showing clear reductions in their chief complaints and ordinary symptoms without any adverse effects. Among 1348 subjects, 16 subjects diagnosed with an obscure constitution type were excluded from the analysis.
Genotyping and quality control
The genotypes of genomic DNA isolated from the subjects' peripheral blood were determined using an Affymetrix Genome Wide Human SNP array 5.0, as previously described.8,9 From a total of 440,092 genotypes of SNPs (>95% call rate), the current authors discarded 12,039 markers following a Hardy-Weinberg equilibrium test (p<0.001) and 86,324 markers following minor allele frequency (MAF; p<0.01), which left 341,998 SNPs for subsequent analysis. Eighty-three (83) subjects with a high degree of similarity among genotypes (>0.8 of identity by state [IBS] index), and 27 subjects with heterogeneous genotypes (>0.05 on a multidimensional scaling [MDS] index) were excluded, leaving a final total of 1222 samples.
Association analysis
Differences in allele frequencies of each SNP among each constitution type (case) and other constitution types (control) were measured via a χ2 test using PLINK,12 and the list of SNPs significantly associated with each constitution type was provided in the authors' previous report.8
Pathway analysis
Three different algorithms were used—the Z-statistic; a restandardized gene set assay (GSA); and a gene set enrichment assay (GSEA). These were incorporated into GSA-SNP software to evaluate the pathways from GWA data.13 Initially, each SNP was assigned to a gene whose extent encompassed the SNP within a range of±20 kb in the neighborhood of each gene. In the Z-statistic algorithm, each gene set (GS) was assigned to the Z-statistic, which was defined as:
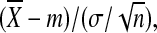 |
in which 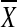 is the average of the gene scores, −log(k-th best p-value) in a gene set, m and σ are the mean and the standard deviation (SD) of all the gene scores, and n is the number of genes in the gene set. The k-th best p-value means the k-th smallest value of the association p-values from GWA analysis. The present study used a k number of 2. The current authors also considered two sample permutation-based methods, which also incorporated into GSA-SNP. One of these is the recently developed restandardizated GSA and the other is a modified version of the GSEA. Both algorithms use the maxmean statistic (nonnegative mean) rather than the Kolmogorov-Smirnov statistic originally used in GSEA to summarize gene sets. Integrative analysis of pathways was carried out using KEGGgraph—specifically, the R package for KEGG pathway analysis, which uses a graph-theoretical model to dissect graphs.14 This program parses KEGG XML files into a graph model. The selected subgraph of nodes and the edges were merged into a new graph and visualized. The most important nodes were computed and visualized by measuring the centrality in which the number of outgoing edges reflects the regulatory role, and the number of “ingoing” edges reflects the degree to which the protein is subject to intermolecular regulation.
is the average of the gene scores, −log(k-th best p-value) in a gene set, m and σ are the mean and the standard deviation (SD) of all the gene scores, and n is the number of genes in the gene set. The k-th best p-value means the k-th smallest value of the association p-values from GWA analysis. The present study used a k number of 2. The current authors also considered two sample permutation-based methods, which also incorporated into GSA-SNP. One of these is the recently developed restandardizated GSA and the other is a modified version of the GSEA. Both algorithms use the maxmean statistic (nonnegative mean) rather than the Kolmogorov-Smirnov statistic originally used in GSEA to summarize gene sets. Integrative analysis of pathways was carried out using KEGGgraph—specifically, the R package for KEGG pathway analysis, which uses a graph-theoretical model to dissect graphs.14 This program parses KEGG XML files into a graph model. The selected subgraph of nodes and the edges were merged into a new graph and visualized. The most important nodes were computed and visualized by measuring the centrality in which the number of outgoing edges reflects the regulatory role, and the number of “ingoing” edges reflects the degree to which the protein is subject to intermolecular regulation.
Dataset
The KEGG pathway database (www.genome.jp/kegg/) was used. It is provided as a curated .gmt file format in MSigDB (www.broadinstitute.org/gsea/msigdb/). SNP data were obtained from dbSNP (www.ncbi.nlm.nih.gov/snp).
Statistical analysis
All statistical analyses, including an analysis of variance (ANOVA), were performed using R software (version 2.12.0). p-Values of <0.05 were considered statistically significant.
Results
Subjects
The 1222 subjects were ultimately included in the present study after careful examination of the quality of the genotypes of individual subjects. The clinical distribution and pathophysiologic association of SC types reported previously8 are provided in Table 1. Five hundred and twelve (512) subjects were classified into TE, 302 into SE, 389 into SY, and 19 into TY. The TE type showed significant increases in body weight, total cholesterol level, and low-density lipoprotein level (LDL) in both genders. The TY type was excluded for the following analysis because of the small sample size of TY subjects.
Table 1.
Clinical Implications of Sasang Constitution Typesa
| |
Male |
|
Female |
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | TE (n=239) | SE (n=103) | TY (n=8) | SY (n=130) | pb | TE (n=273) | SE (n=199) | TY (n=11) | SY (n=259) | pb |
| Age (year) | 46.1±15.4 | 41.5±16.5 | 51.7±14.5 | 48.1±16.0 | 0.0094 | 50.3±14.7 | 43.9±14.8 | 41.7±10.4 | 45.1±14.5 | <0.0001 |
| Height (cm) | 169.9±7.1 | 169.6±7.6 | 169.5±4.4 | 168.6±5.8 | 0.39 | 157.0±5.6 | 158.3±6.0 | 161.7±3.3 | 157.3±6.0 | 0.0093 |
| Weight (kg) | 73.7±11.0 | 61.7±8.7 | 66.6±8.7 | 65.9±8.2 | <0.0001 | 62.1±9.4 | 52.7±6.5 | 56.1±9.6 | 55.1±6.9 | <0.0001 |
| Aspartate aminotransferase (IU/L) | 25.2±8.2 | 24.5±11.9 | 18.6±3.0 | 23.5±8.3 | 0.12 | 23.7±14.6 | 20.7±5.5 | 23.1±10.4 | 21.7±8.5 | 0.022 |
| Alanine aminotransferase (IU/L) | 29.3±14.7 | 23.8±23.2 | 16.4±6.0 | 25.9±15.7 | 0.011 | 22.7±25.8 | 16.8±7.8 | 20.8±16.2 | 19.9±19.0 | 0.018 |
| Alkaline phosphatase (IU/L) | 70.6±43.5 | 75.4±54.1 | 67.1±9.4 | 72.1±41.8 | 0.83 | 62.7±27.6 | 58.4±22.2 | 59.0±19.5 | 58.9±23.8 | 0.21 |
| Total cholesterol (mg/dL) | 192.0±37.2 | 177.9±32.1 | 182.9±38.9 | 184.3±31.5 | 0.0061 | 194.9±37.87 | 181.5±33.3 | 173.5±28.2 | 188.5±33.5 | 0.00042 |
| Triglycerides (mg/dL) | 162.2±115.5 | 113.7±65.0 | 138.8±52.4 | 166.4±111.9 | 0.00043 | 132.7±83.5 | 91.3±46.0 | 106.9±62.1 | 109.3±62.9 | <0.0001 |
| High-density lipoprotein (mg/dL) | 39.8±9.4 | 43.8±9.1 | 36.8±6.5 | 40.6±10.6 | 0.0034 | 44.9±11.3 | 51.0±12.1 | 45.5±9.8 | 48.6±12.0 | <0.0001 |
| Low-density lipoprotein (mg/dL) | 111.9±31.6 | 100.3±26.1 | 109.5±30.4 | 103.1±27.1 | 0.0030 | 112.3±32.4 | 98.7±28.7 | 96.0±21.0 | 105.6±30.5 | <0.0001 |
| Bilirubin (mg/dL) | 0.82±0.29 | 0.86±0.42 | 0.70±0.24 | 0.82±0.37 | 0.51 | 0.70±0.25 | 0.74±0.27 | 0.84±0.31 | 0.78±0.34 | 0.024 |
| Blood urea nitrogen (mg/dL) | 15.4±4.0 | 14.5±3.7 | 16.0±4.9 | 14.9±4.3 | 0.20 | 14.2±4.0 | 13.5±3.8 | 13.3±4.0 | 13.6±3.4 | 0.15 |
This table was previously reported.8
Significance was measured by analysis of variance.
TE, Taeum; SE, Soeum; SY, Soyang; TY, Taeyang, IU, international units.
Identification of pathways involved in SC types: One-versus-all
Three different algorithms were used—namely the Z-static, a restandardized GSA, and a GSEA method, all incorporated into GSA-SNP software13—to evaluate the pathways involved in SC from a GWA study of the 1222 subjects. The detailed list of SNPs associated with constitution types and clinical information regarding constitution types was previously reported.8
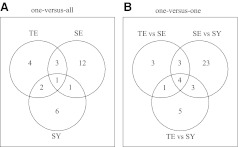
Initially, the pathways involved with each constitution type were identified by comparing one constitution type with the others, via a one-versus-all approach. Some significant pathways involved in each type of constitution are listed in Table 2 (p<0.05 for all algorithms). Pathways involved in different cellular functions were enriched in each constitution type. For example, the biosynthesis of unsaturated fatty acids, melanoma, and cardiac-muscle contraction–pathway were associated with the TE type. While pathways including phenylalanine metabolism, viral myocarditis, and tryptophan metabolism were associated with the SE type, long-term depression, arrhythmogenic right ventricular cardiomyopathy, and regulation of the actin cytoskeleton pathway were associated with the SY type. Table 2 shows that some pathways were commonly enriched across constitution types. A Venn diagram was constructed to illustrate the numbers of commonly significant pathways (p<0.05 for all algorithms) among constitution types (Fig. 1A). Table 2 shows that the cardiac-muscle contraction pathway was associated with all three constitution types. Axon guidance, adherens junction, and hypertrophic cardiomyopathy pathways were associated with the TE and SE types. Melanoma and regulation of the actin cytoskeleton pathway were associated with the TE and SY types, whereas tryptophan metabolism was associated with the SE and SY types.
Table 2.
Pathways Involved in Each Type of Sasang Constitution: One-Versus-All Approach
| |
|
|
|
|
Z-score |
Restandization |
GSEA |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Constitution | KEGG ID | Pathway | Gene counta | Pathway sizeb | p-Value | FDR | p-Value | FDR | p-Value | FDR |
| TE | hsa01040 | Biosynthesis of unsaturated fatty acids | 20 | 28 | 1.83×10−2 | 1.83×10−1 | 1.46×10−3 | 1.48×10−3 | 1.30×10−2 | 4.97×10−1 |
| hsa05218 | Melanoma | 65 | 77 | 1.94×10−2 | 1.83×10−1 | 3.77×10−3 | 4.35×10−3 | 5.08×10−3 | 5.13×10−1 | |
| hsa04260 | Cardiac muscle contraction | 64 | 86 | 7.50×10−3 | 9.00×10−2 | 3.09×10−3 | 3.41×10−3 | 5.13×10−3 | 4.29×10−1 | |
| hsa04810 | Regulation of actin cytoskeleton | 189 | 222 | 1.12×10−3 | 1.84×10−2 | 4.37×10−3 | 5.42×10−3 | 1.29×10−3 | 0.00 | |
| hsa04530 | Tight junction | 119 | 140 | 4.99×10−4 | 9.98×10−3 | 3.11×10−3 | 3.45×10−3 | 6.72×10−3 | 4.40×10−1 | |
| hsa04360 | Axon guidance | 118 | 135 | 1.37×10−8 | 2.48×10−6 | 1.25×10−3 | 1.26×10−3 | 3.24×10−3 | 4.56×10−1 | |
| hsa04510 | Focal adhesion | 181 | 207 | 7.30×10−7 | 4.38×10−5 | 2.51×10−3 | 2.69×10−3 | 3.13×10−3 | 5.86×10−1 | |
| hsa04520 | Adherens junction | 71 | 81 | 3.34×10−5 | 1.20×10−3 | 1.46×10−3 | 1.49×10−3 | 2.06×10−2 | 5.88×10−1 | |
| hsa05410 | Hypertrophic cardiomyopathy | 79 | 91 | 1.15×10−5 | 5.20×10−4 | 1.44×10−3 | 1.6×10−3 | 2.24×10−2 | 5.40×10−1 | |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 36 | 78 | 2.75×10−2 | 2.36×10−1 | 2.90×10−3 | 3.17×10−3 | 4.51×10−2 | 7.40×10−1 | |
| SE | hsa00360 | Phenylalanine metabolism | 17 | 24 | 8.63×10−4 | 1.55×10−2 | 1.05×10−3 | 1.05×10−3 | 7.10×10−6 | 0.00 |
| hsa05416 | Viral myocarditis | 67 | 79 | 4.78×10−4 | 9.56×10−3 | 3.20×10−3 | 3.33×10−3 | 6.80×10−4 | 2.30×10−1 | |
| hsa04260 | Cardiac muscle contraction | 64 | 86 | 1.36×10−3 | 2.05×10−2 | 3.48×10−3 | 3.70×10−3 | 2.33×10−4 | 2.53×10−1 | |
| hsa00380 | Tryptophan metabolism | 36 | 46 | 1.13×10−2 | 1.01×10−1 | 3.46×10−3 | 3.66×10−3 | 1.97×10−3 | 2.53×10−1 | |
| hsa04514 | Cell-adhesion molecules | 125 | 140 | 8.69×10−5 | 3.11×10−3 | 4.09×10−3 | 4.49×10−3 | 1.18×10−2 | 3.46×10−1 | |
| hsa04360 | Axon guidance | 118 | 135 | 7.62×10−6 | 6.86×10−4 | 3.24×10−3 | 3.39×10−3 | 3.79×10−2 | 4.43×10−1 | |
| hsa05410 | Hypertrophic cardiomyopathy | 79 | 91 | 4.82×10−5 | 2.89×10−3 | 2.83×10−3 | 2.91×10−3 | 4.10×10−2 | 4.17×10−1 | |
| hsa04520 | Adherens junction | 71 | 81 | 5.18×10−5 | 2.33×10−3 | 2.61×10−3 | 2.67×10−3 | 2.29×10−2 | 3.91×10−1 | |
| hsa04720 | Long-term potentiation | 64 | 76 | 9.45×10−5 | 2.83×10−3 | 2.48×10−3 | 2.52×10−3 | 1.11×10−2 | 3.52×10−1 | |
| hsa04270 | Vascular smooth-muscle contraction | 107 | 121 | 1.82×10−4 | 4.70×10−3 | 3.94×10−3 | 4.27×10−3 | 2.00×10−2 | 4.08×10−1 | |
| hsa04930 | Type II diabetes mellitus | 41 | 53 | 9.86×10−3 | 9.86×10−2 | 3.61×10−3 | 3.86×10−3 | 4.17×10−2 | 4.11×10−1 | |
| hsa05140 | Leishmaniasis | 57 | 78 | 2.14×10−2 | 1.60×10−1 | 5.51×10−3 | 6.49×10−3 | 1.16×10−3 | 2.52×10−1 | |
| hsa05332 | Graft-versus-host disease | 35 | 48 | 2.32×10−2 | 1.67×10−1 | 4.09×10−3 | 4.54×10−3 | 4.67×10−3 | 2.76×10−1 | |
| hsa05216 | Thyroid cancer | 27 | 35 | 2.33×10−2 | 1.61×10−1 | 3.44×10−3 | 3.62×10−3 | 7.65×10−3 | 3.09×10−1 | |
| hsa04144 | Endocytosis | 159 | 189 | 3.28×10−2 | 2.11×10−1 | 9.50×10−3 | 1.30×10−2 | 1.91×10−4 | 4.46×10−1 | |
| hsa00340 | Histidine metabolism | 28 | 35 | 3.97×10−2 | 2.38×10−1 | 4.14×10−3 | 4.63×10−3 | 5.03×10−3 | 2.62×10−1 | |
| hsa05330 | Allograft rejection | 34 | 44 | 4.56×10−2 | 2.56×10−1 | 5.12×10−3 | 5.91×10−3 | 3.34×10−2 | 4.33×10−1 | |
| SY | hsa04730 | Long-term depression | 63 | 76 | 1.44×10−6 | 1.30×10−4 | 1.33×10−3 | 1.34×10−3 | 6.73×10−3 | 1.26×10−1 |
| hsa05218 | Melanoma | 65 | 77 | 7.82×10−3 | 6.71×10−2 | 2.92×10−3 | 3.33×10−3 | 8.16×10−3 | 1.27×10−1 | |
| hsa05412 | Arrhythmogenic right-ventricular cardiomyopathy | 72 | 82 | 5.22×10−11 | 9.41×10−9 | 1.03×10−3 | 1.03×10−3 | 1.10×10−2 | 1.47×10−1 | |
| hsa04810 | Regulation of actin cytoskeleton | 189 | 222 | 4.82×10−4 | 7.88×10−3 | 3.61×10−3 | 4.48×10−3 | 1.29×10−2 | 1.51×10−1 | |
| hsa04916 | Melanogenesis | 93 | 108 | 7.61×10−3 | 6.85×10−2 | 3.51×10−3 | 4.33×10−3 | 3.02×10−2 | 2.19×10−1 | |
| hsa00380 | Tryptophan metabolism | 36 | 46 | 4.14×10−3 | 4.66×10−2 | 2.09×10−3 | 2.18×10−3 | 3.99×10−3 | 1.07×10−1 | |
| hsa05215 | Prostate cancer | 81 | 95 | 4.26×10−3 | 4.51×10−2 | 3.01×10−3 | 3.48×10−3 | 4.15×10−3 | 1.07×10−1 | |
| hsa04742 | Taste transduction | 44 | 58 | 1.20×10−2 | 8.36×10−2 | 2.59×10−3 | 2.84×10−3 | 3.04×10−2 | 2.19×10−1 | |
| hsa04260 | Cardiac-muscle contraction | 64 | 86 | 1.08×10−2 | 7.81×10−2 | 3.09×10−3 | 3.59×10−3 | 2.67×10−2 | 2.19×10−1 | |
| hsa04722 | Neurotrophin-signaling pathway | 118 | 132 | 2.87×10−2 | 1.66×10−1 | 4.59×10−3 | 6.12×10−3 | 2.62×10−2 | 2.19×10−1 | |
Number of genes analyzed in each pathway.
Number of total genes in each pathway.
FDR, false discovery rate; TE, Taeum; SE, Soeum; SY, Soyang.
FIG. 1.
Venn-diagram illustration of number of common significant pathways among constitution types. (A) Pathways were selected as significant (p<0.05 for all algorithms; Z-statistic, restandardized gene set assay, and gene set enrichment assay) via comparison of one constitution type with others. (B) Pathways were selected as significant (p<0.05 for all algorithms) between any two types of constitution, namely, TE-versus-SE, SE-versus-SY, and TE-versus-SY. TE, Taeum; SE, Soeum; SY, Soyang.
Identification of pathways involved in SC types: One-versus-one
In addition to the one-versus-all approach adopted herein, the current authors also measured the pathways associated with constitution types via a one-versus-one approach. Some significant pathways were enriched differently between two types of constitution—namely, TE versus SE, SE versus SY, and TE versus SY (Table 3). The Venn diagram for significant pathways shown in Figure 1B also confirmed the commonly identified pathways. Axon guidance, adherens junction, regulation of actin cytoskeleton, and the focal adhesion pathway were involved in all combinations of constitution type. Cardiac-muscle contraction, hypertrophic cardiomyopathy, and cell-adhesion molecule pathways were associated with both TE-SE and SE-SY combinations. While long-term depression, tight junction, and Fcɛ RI signaling pathways were involved in both SE–SY and TE–SY type combinations, aldosterone-regulated sodium reabsorption pathways were associated with both TE–SE and TE–SY combinations.
Table 3.
Pathways Involved in Each Type of Sasang Constitution: One-Versus-One Approach
| |
|
|
|
|
Z-score |
Restandization |
GSEA |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Constitution | KEGG ID | Pathway | Gene counta | Pathway sizeb | p-Value | FDR | p-Value | FDR | p-Value | FDR |
| TE vs. SE | hsa00360 | Phenylalanine metabolism | 17 | 24 | 6.34×10−3 | 7.61×10−2 | 1.74×10−3 | 1.74×10−3 | 1.44×10−4 | 0.00 |
| hsa04260 | Cardiac muscle contraction | 64 | 86 | 5.24×10−3 | 6.74×10−2 | 4.51×10−3 | 4.84×10−3 | 1.30×10−3 | 1.25×10−2 | |
| hsa05416 | Viral myocarditis | 67 | 79 | 3.79×10−3 | 5.26×10−2 | 4.31×10−3 | 4.59×10−3 | 5.00×10−3 | 1.61×10−2 | |
| hsa04930 | Type II diabetes mellitus | 41 | 53 | 7.80×10−3 | 8.77×10−2 | 3.48×10−3 | 3.62×10−3 | 2.60×10−2 | 2.95×10−2 | |
| hsa04360 | Axon guidance | 118 | 135 | 1.88×10−6 | 3.39×10−4 | 2.98×10−3 | 3.04×10−3 | 1.06×10−2 | 5.36×10−1 | |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 36 | 48 | 3.67×10−2 | 2.75×10−1 | 5.05×10−3 | 5.61×10−3 | 4.44×10−2 | 5.20×10−1 | |
| hsa04520 | Adherens junction | 71 | 81 | 8.29×10−5 | 3.73×10−3 | 2.75×10−3 | 2.79×10−3 | 2.60×10−2 | 5.03×10−1 | |
| hsa05410 | Hypertrophic cardiomyopathy | 79 | 91 | 4.73×10−5 | 2.84×10−3 | 2.87×10−3 | 2.91×10−3 | 3.27×10−2 | 4.83×10−1 | |
| hsa04514 | Cell adhesion molecules (CAMs) | 125 | 140 | 2.79×10−4 | 8.37×10−3 | 4.72×10−3 | 5.12×10−3 | 2.22×10−2 | 5.98×10−1 | |
| hsa04810 | Regulation of actin cytoskeleton | 189 | 222 | 2.61×10−2 | 2.23×10−1 | 9.83×10−3 | 1.34×10−2 | 2.65×10−2 | 4.65×10−1 | |
| hsa04510 | Focal adhesion | 181 | 207 | 2.04×10−4 | 7.36×10−3 | 5.75×10−3 | 6.68×10−3 | 4.98×10−2 | 5.14×10−1 | |
| SE vs. SY | hsa05211 | Renal cell carcinoma | 67 | 76 | 1.03×10−2 | 5.99×10−2 | 2.11×10−3 | 2.56×10−3 | 3.54×10–5 | 3.18×10−3 |
| hsa04360 | Axon guidance | 118 | 135 | 4.45×10−10 | 8.01×10−8 | 1.03×10−3 | 1.05×10−3 | 2.07×10–5 | 3.18×10−3 | |
| hsa04012 | ErbB signaling pathway | 80 | 93 | 1.17×10−4 | 1.75×10−3 | 1.35×10−3 | 1.48×10−3 | 1.84×10−4 | 1.10×10−2 | |
| hsa05223 | Non-small cell lung cancer | 50 | 60 | 3.90×10−3 | 3.34×10−2 | 1.42×10−3 | 1.60×10−3 | 1.37×10−3 | 2.75×10−2 | |
| hsa00532 | Glycosaminoglycan biosynthesis-chondroitin sulfate | 19 | 28 | 1.28×10−3 | 1.45×10−2 | 6.48×10−4 | 6.51×10−4 | 1.23×10−2 | 7.16×10−2 | |
| hsa04662 | B cell receptor signaling pathway | 67 | 81 | 9.98×10−3 | 6.19×10−2 | 2.11×10−3 | 2.55×10−3 | 2.14×10−3 | 2.24×10−1 | |
| hsa05214 | Glioma | 58 | 71 | 2.95×10−2 | 1.47×10−1 | 2.26×10−3 | 2.86×10−3 | 3.88×10−3 | 2.45×10−1 | |
| hsa04730 | Long-term depression | 63 | 76 | 2.41×10−5 | 5.42×10−4 | 1.19×10−3 | 1.25×10−3 | 4.76×10−3 | 2.29×10−1 | |
| hsa04722 | Neurotrophin signaling pathway | 118 | 132 | 3.23×10−2 | 1.57×10−1 | 2.81×10−3 | 4.15×10−3 | 5.88×10−4 | 2.21×10−1 | |
| hsa05410 | Hypertrophic cardiomyopathy | 79 | 91 | 2.43×10−6 | 8.76×10−5 | 1.17×10−3 | 1.22×10−3 | 3.12×10−3 | 2.53×10−1 | |
| hsa04320 | Dorso-ventral axis formation | 23 | 31 | 6.51×10−3 | 4.50×10−2 | 1.15×10−3 | 1.19×10−3 | 2.03×10−2 | 2.73×10−1 | |
| hsa00500 | Starch and sucrose metabolism | 47 | 58 | 2.37×10−3 | 2.24×10−2 | 1.35×10−3 | 1.47×10−3 | 3.18×10−2 | 2.85×10−1 | |
| hsa04020 | Calcium signaling pathway | 167 | 184 | 8.29×10−8 | 7.46×10−6 | 1.39×10−3 | 1.53×10−3 | 4.45×10−4 | 2.87×10−1 | |
| hsa00983 | Drug metabolism - other enzymes | 44 | 57 | 2.87×10−3 | 2.58×10−2 | 1.33×10−3 | 1.45×10−3 | 3.82×10−2 | 2.91×10−1 | |
| hsa04512 | ECM-receptor interaction | 80 | 90 | 6.06×10−7 | 2.73×10−5 | 1.09×10−3 | 1.12×10−3 | 3.81×10−3 | 2.64×10−1 | |
| hsa04520 | Adherens junction | 71 | 81 | 1.82×10−5 | 4.68×10−4 | 1.20×10−3 | 1.27×10−3 | 4.63×10−3 | 2.41×10−1 | |
| hsa00860 | Porphyrin and chlorophyll metabolism | 37 | 47 | 1.30×10−2 | 7.32×10−2 | 1.54×10−3 | 1.75×10−3 | 4.94×10−2 | 2.93×10−1 | |
| hsa00140 | Steroid hormone biosynthesis | 50 | 61 | 4.32×10−3 | 3.38×10−2 | 1.48×10−3 | 1.68×10−3 | 4.18×10−2 | 2.88×10−1 | |
| hsa04062 | Chemokine signaling pathway | 171 | 196 | 7.25×10−3 | 4.83×10−2 | 2.70×10−3 | 3.08×10−3 | 1.09×10−3 | 2.61×10−1 | |
| hsa04666 | Fc gamma R-mediated phagocytosis | 81 | 103 | 1.00×10−2 | 6.00×10−2 | 2.20×10−3 | 2.77×10−3 | 5.59×10−3 | 2.29×10−1 | |
| hsa00640 | Propanoate metabolism | 32 | 39 | 3.46×10−2 | 1.64×10−1 | 1.89×10−3 | 2.19×10−3 | 2.93×10−2 | 2.79×10−1 | |
| hsa04540 | Gap junction | 77 | 96 | 2.05×10−4 | 2.84×10−3 | 1.41×10−3 | 1.57×10−3 | 7.05×10−3 | 2.16×10−1 | |
| hsa04940 | Type I diabetes mellitus | 39 | 50 | 2.41×10−2 | 1.31×10−1 | 1.93×10−3 | 2.25×10−3 | 3.81×10−2 | 2.95×10−1 | |
| hsa04260 | Cardiac muscle contraction | 64 | 86 | 3.94×10−2 | 1.77×10−1 | 2.44×10−3 | 3.24×10−3 | 1.13×10−2 | 2.39×10−1 | |
| hsa04530 | Tight junction | 119 | 140 | 1.80×10−3 | 1.90×10−2 | 2.19×10−3 | 2.69×10−3 | 5.61×10−3 | 2.17×10−1 | |
| SY vs. TE | hsa03450 | Nonhomologous end-joining | 13 | 20 | 1.52×10−2 | 1.31×10−1 | 9.07×10−4 | 9.12×10−4 | 6.52×10−3 | 2.34×10−1 |
| hsa05218 | Melanoma | 65 | 77 | 9.80×10−3 | 1.10×10−1 | 2.33×10−3 | 2.67×10−3 | 5.48×10−3 | 2.34×10−1 | |
| hsa04810 | Regulation of actin cytoskeleton | 189 | 222 | 4.48×10−4 | 7.33×10−3 | 2.92×10−3 | 3.44×10−3 | 3.04×10−3 | 2.34×10−1 | |
| hsa04730 | Long-term depression | 63 | 76 | 2.62×10−6 | 1.18×10−4 | 9.25×10−4 | 9.36×10−4 | 8.02×10−3 | 2.40×10−1 | |
| hsa04530 | Tight junction | 119 | 140 | 2.41×10−4 | 4.35×10−3 | 2.05×10−3 | 2.26×10−3 | 1.23×10−2 | 3.03×10−1 | |
| hsa05215 | Prostate cancer | 81 | 95 | 1.51×10−2 | 1.35×10−1 | 2.93×10−3 | 3.46×10−3 | 1.35×10−2 | 6.48×10−1 | |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 36 | 48 | 1.10×10−2 | 1.16×10−1 | 1.57×10−3 | 1.69×10−3 | 3.05×10−2 | 7.07×10−1 | |
| hsa04520 | Adherens junction | 71 | 81 | 9.81×10−6 | 3.53×10−4 | 1.02×10−3 | 1.04×10−3 | 2.19×10−2 | 8.28×10−1 | |
| hsa05412 | Arrhythmogenic right ventricular cardiomyopathy | 72 | 82 | 2.13×10−9 | 3.84×10−7 | 6.85×10−4 | 6.85×10−4 | 2.61×10−2 | 8.31×10−1 | |
| hsa00380 | Tryptophan metabolism | 35 | 46 | 3.06×10−2 | 1.97×10−1 | 2.11×10−3 | 2.35×10−3 | 4.81×10−2 | 8.24×10−1 | |
| hsa04664 | Fc ɛ RI signaling pathway | 74 | 85 | 1.12×10−2 | 1.12×10−1 | 2.56×10−3 | 2.97×10−3 | 3.99×10−2 | 8.23×10−1 | |
| hsa04510 | Focal adhesion | 181 | 207 | 1.83×10−6 | 1.10×10−4 | 1.87×10−3 | 2.04×10−3 | 2.67×10−2 | 6.92×10−1 | |
| hsa04360 | Axon guidance | 118 | 135 | 1.26×10−7 | 1.13×10−5 | 1.13×10−3 | 1.16×10−3 | 4.33×10−2 | 8.19×10−1 | |
Number of genes analyzed in each pathway.
Number of total genes in each pathway.
FDR, false discovery rate; TE, Taeum; SE, Soeum; SY, Soyang.
Distribution of SNPs in pathways
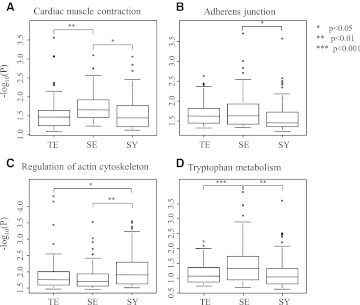
Tables 2 and 3 show that many pathways were identified as commonly significant using both approaches; namely, one-versus-all and one-versus-one approaches. Moreover, some pathways were commonly selected among three constitutional types, as shown in Fig. 1, thereby indicating that these pathways could be critical for discriminating among constitution types. The distribution of all commonly significant pathways is addressed in Table 4. The current authors measured the distribution of p-values of SNPs included in some commonly significant pathways in all or two constitution types (Fig. 2); specifically, the cardiac-muscle contraction, adherens junction, actin cytoskeleton regulation, and tryptophan metabolism pathways. From the distribution of the p-values of the most significant 100 SNPs in a pathway, at least one constitution type had a different p-value distribution, compared with those of other constitution types. These results indicated that these pathways were more significantly enriched in one type of constitution than in other types.
Table 4.
Distribution of Common Significant Pathways (*p<0.05 for all Algorithms)
| |
|
One-vs.-all |
One-vs.-one |
||||
|---|---|---|---|---|---|---|---|
| KEGG | Pathway | TE | SE | SY | TE vs. SE | SE vs. SY | TE vs. SY |
| hsa04520 | Adherens junction | * | * | * | * | * | |
| hsa04360 | Axon guidance | * | * | * | * | * | |
| hsa04260 | Cardiac-muscle contraction | * | * | * | * | * | |
| hsa04810 | Regulation of actin cytoskeleton | * | * | * | * | * | |
| hsa04510 | Focal adhesion | * | * | * | * | ||
| hsa05410 | Hypertrophic cardiomyopathy | * | * | * | * | ||
| hsa04960 | Aldosterone-regulated sodium reabsorption | * | * | * | |||
| hsa04514 | Cell-adhesion molecules | * | * | * | |||
| hsa04730 | Long-term depression | * | * | * | |||
| hsa05218 | Melanoma | * | * | * | |||
| hsa04530 | Tight junction | * | * | * | |||
| hsa00380 | Tryptophan metabolism | * | * | * | |||
| hsa05412 | Arrhythmogenic right-ventricular cardiomyopathy | * | * | ||||
| hsa04664 | Fcɛ RI signaling pathway | * | * | ||||
| hsa04720 | Long-term potentiation | * | * | ||||
| hsa04722 | Neurotrophin-signaling pathway | * | * | ||||
| hsa00360 | Phenylalanine metabolism | * | * | ||||
| hsa05215 | Prostate cancer | * | * | ||||
| hsa04930 | Type II diabetes mellitus | * | * | ||||
| hsa04270 | Vascular smooth-muscle contraction | * | * | ||||
| hsa05416 | Viral myocarditis | * | * | ||||
| hsa05330 | Allograft rejection | * | |||||
| hsa04662 | B-cell receptor–signaling pathway | * | |||||
| hsa01040 | Biosynthesis of unsaturated fatty acids | * | |||||
| hsa04020 | Calcium signaling pathway | * | |||||
| hsa04062 | Chemokine signaling pathway | * | |||||
| hsa00472 | d-Arginine and d-ornithine metabolism | * | |||||
| hsa04320 | Dorso-ventral axis formation | * | |||||
| hsa00983 | Drug metabolism—other enzymes | * | |||||
| hsa04512 | ECM-receptor interaction | * | |||||
| hsa04144 | Endocytosis | * | |||||
| hsa04012 | ErbB signaling pathway | * | |||||
| hsa04666 | Fc γ R-mediated phagocytosis | * | |||||
| hsa04540 | Gap junction | * | |||||
| hsa05214 | Glioma | * | |||||
| hsa00532 | Glycosaminoglycan biosynthesis-chondroitin sulfate | * | |||||
| hsa05332 | Graft-versus-host disease | * | |||||
| hsa00340 | Histidine metabolism | * | |||||
| hsa05140 | Leishmaniasis | * | |||||
| hsa04916 | Melanogenesis | * | |||||
| hsa03450 | Nonhomologous end-joining | * | |||||
| hsa05223 | Non–small cell lung cancer | * | |||||
| hsa00860 | Porphyrin and chlorophyll metabolism | * | |||||
| hsa00640 | Propanoate metabolism | * | |||||
| hsa05211 | Renal-cell carcinoma | * | |||||
| hsa05222 | Small-cell lung cancer | * | |||||
| hsa00500 | Starch and sucrose metabolism | * | |||||
| hsa00140 | Steroid hormone biosynthesis | * | |||||
| hsa04742 | Taste transduction | * | |||||
| hsa05216 | Thyroid cancer | * | |||||
| hsa04940 | Type I diabetes mellitus | * | |||||
TE, Taeum; SE, Soeum; SY, Soyang.
FIG. 2.
Distribution of single-nucleotide polymorphisms (SNPs) in common significant pathways among constitution types. In the cardiac muscle contraction pathway (A), adherens junction pathway (B), regulation of actin cytoskeleton pathway (C), and tryptophan metabolism pathway (D), the top 100 significant SNPs were selected from each constitution type. The differences in SNP distributions were measured using Tukey's test after an analysis of variance. TE, Taeum; SE, Soeum; SY, Soyang.
Identification of core elements in pathways
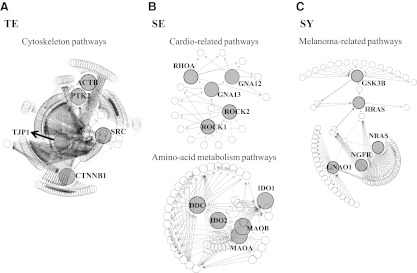
Interestingly, among the significant pathways, functionally related pathways were enriched in each constitution type, as shown in Tables 2 and 3. For example, cytoskeleton-related pathways—such as the focal adhesion, regulation of actin cytoskeleton, tight junction, and adherens junction pathways—were significantly associated with the TE type. Cardio-related pathways, such as hypertrophic cardiomyopathy, vascular smooth-muscle contraction, and cardiac-muscle contraction pathways, were enriched in the SE type. In addition, amino-acid metabolism pathways—including phenylalanine, tryptophan, and histidine metabolism pathways—were also selected in the SE type, whereas melanoma-related pathways—including the melanoma pathway, melanogenesis pathway, and neurotrophin-signaling pathway—were associated with the SY type. The presence of functionally related pathways in each constitution type indicates a possible implication of common regulators acting on multiple pathways. From the network analysis of functionally related pathways, the current authors identified CTNNB1, PTK2, ACTB, SRC, and TJP1 as the most important nodes in cytoskeleton-related pathways for the TE type (Fig. 3). In the SE type, GNA12, GNA13, RHOA, ROCK1, and ROCK2 were identified as central nodes in the cardio-related pathway and DDC, IDO1, IDO2, MAOA, and MAOB were central nodes in the in the amino-acid metabolism pathways. However, GNAO1, GSK3B, HRAS, NRAS, and NGFR were selected in melanoma-related pathways for the SY type. Finally, the current authors assessed if these identified core-node genes were associated with each constitution type. Table 5 shows the constitution-associated SNPs (p<0.05) located within the region encompassing±10 kb in either direction from the core-node genes. TJP1 (rs11073279), PTK2 (rs3639 and rs11991796), and SRC (rs747182) were associated with the TE type. RHOA (rs6997) and MAOA/MAOB (rs6609257 and rs3859959) were associated with the SE type. GNAO1 (rs9927506) was associated with the SY type. However, the roles of these constitution-associated core-node genes should be investigated in further detail at the molecular level to delineate the mechanism underlying the determination of constitution type.
FIG. 3.
Network analysis of pathways. Core-node genes were identified from functionally related multipathways. (A) For the TE type, cytoskeleton-related pathways, including focal adhesion, regulation of actin cytoskeleton, tight junction, and adherens junction pathways were analyzed. (B) For the SE type, cardio-related pathways, including hypertrophic cardiomyopathy, vascular smooth-muscle contraction, and cardiac-muscle contraction pathways were analyzed. In addition, amino-acid metabolism pathways including phenylalanine, tryptophan, and histidine metabolism pathways were also used. (C) For the SY type, melanoma-related pathways including melanoma, melanogenesis, and neurotrophin-signaling pathways, were analyzed. From the multipathways, core-node genes (big gray circles) were identified with a KEGGgraph. TE, Taeum; SE, Soeum; SY, Soyang.
Table 5.
Core-Node Genes and Related SNPs Identified with KEGGgraph
| |
|
|
|
|
|
Allele |
|
|
|---|---|---|---|---|---|---|---|---|
| Constitution | Symbol | SNP | Chromosome | Position | Association p-valuea | Major | Minor | Odds ratiob |
| TE | TJP1 | rs11073279 | 15 | 27893050 | 4.31×10–3 | A | G | 0.63 (0.46–0.86) |
| PTK2 | rs3639 | 8 | 141753352 | 1.09×10–2 | T | C | 0.64 (0.45–0.90) | |
| SRC | rs747182 | 20 | 35416303 | 4.15×10–2 | T | C | 1.25 (1.00–1.55) | |
| SE | RHOA | rs6997 | 3 | 49428838 | 3.65×10–2 | G | A | 1.34 (1.01–1.77) |
| MAOA/MAOB | rs6609257 | X | 43497652 | 1.24×10–4 | G | A | 1.48 (1.21–1.82) | |
| SY | GNAO1 | rs9927506 | 16 | 54896727 | 4.04×10–2 | A | G | 0.56 (0.32–0.98) |
χ2 analysis was performed for differences in allele frequencies between each constitution type and other constitution types.
Numbers in parenthesis represent a 95% confidence interval.
SNPs, single-nucleotide polymorphisms; TE, Taeum; SE, Soeum; SY, Soyang.
Discussion
Since the completion of the Human Genome Project, modern medicine has focused on searching for the genetic elements relevant to the differences between individuals.15 Despite the rapid progress made in recent years, most of this research is still conducted at the laboratory level. An alternative and practical approach to laboratory research would involve subgrouping human populations according to their homogeneous biologic characteristics. The classification of human populations based on individual constitution is a common feature of many traditional medicines, including Traditional Chinese Medicine and Ayurveda, an ancient Indian system of personalized medicine.16–18 In SC medicine, an individual is classified into one of four types based on physiologic characteristics.3 As shown in Supplementary Table S1 (online only at: www.liebertpub.com/acm), physical, psychologic, and clinical features differed among SC types.2,3,8 Although, these physiologic traits of an individual were measured to diagnose constitution type in the present study, as previously described,8,9 it will be necessary to apply more-objective criteria for determinations of constitution type. Therefore, the current authors have struggled to develop a diagnostic tool, using only objective physical measurements, such as facial features or body mass index to exclude subjective judgment.19,20
Considering the complex nature of the constitution, the whole-genome approach rather than the single-gene approach should prove to be an effective method for establishing a genetic basis for constitution.7,9 For example, genes and molecular functions correlated with the phenotypic class of constitution were also identified using the whole-genomic approach in Ayurveda.21 The current authors recently reported SNPs associated with SC types and biologic functions of these associated SNPs by using the same dataset used in the present study.8 However, only a small number of genes or SNPs via that method were obtained, and, thus, it was difficult to determine the biologic significance of selected elements in the context of the whole-genome level.22 Therefore, in this study, the current authors used information from whole genome-wide results and pathway information in an effort to elucidate the biologic functions associated with each constitutional type. As shown in Tables 2 and 3, diverse pathways were associated (p<0.05) with each constitutional type. The p-value distribution of SNPs included in these pathways confirmed that at least one constitutional type was highly enriched (Fig. 2). Among the various pathways identified, some functionally related pathways could be segregated in each constitutional type. Cytoskeleton-related pathways, cardio-related pathways, and melanoma-related pathways were associated with TE, SE, and SY types, respectively (Table 2).
Although the direct association of this pathway information with SC types is difficult to identify, because a pathway is not a clinically or quantitatively measurable variable, some pathways were indirectly related to the clinical characteristics of SC type. For example, cytoskeletal pathways regulating muscle contraction, which was associated with the TE type, has been shown to be a major cause of diseases such as hypertension and vasospasm of the coronary and cerebral arteries via Rho kinase.23 Actually, a recent study showed that the prevalence of hypertension was highest in the TE type.24 Moreover, the pathophysiology of diabetes, which is also a highly risky disease in the TE type, has been associated with the loss of tight junction pathways, which regulate permeability in the intestinal membrane or blood–brain barrier.25,26 Considering that membrane-maintenance pathways, including the tight junction, focal adhesion, and adherens junction, were associated with the TE type, it can be speculated that the TE type would be more vulnerable to diseases such as hypertension and diabetes.3 In contrast with the TE type, the SE type is the least vulnerable to these diseases.27,28 This was also partially observed in the samples, in which the SE type had the lowest body weight, total cholesterol level, and LDL level (Table 1). Therefore, the cardio-related pathways identified in SE type may reflect the low susceptibility of the SE type to these diseases, although the current authors did not measure the direction of pathways, such as activation or suppression, in the present analysis. Taken together, the involvement of specific pathways with constitution types could provide some insights into the pathophysiologic differences among constitution types.
In addition, it is very important to isolate core-node genes that can control functionally related multipathways. In this study, core genes were identified with a high number of inward or outward connections with other genes in functionally related multiple pathways (Fig. 3). Furthermore, among core genes, TJP1 (rs11073279), PTK2 (rs3639, and rs11991796) and SRC (rs747182) were associated with the TE type, whereas RHOA (rs6997) and MAOA/MAOB (rs6609257 and rs3859959) were associated with the SE type, and GNAO1 (rs9927506) was associated with the SY type (p<0.05; Table 5). Because SNPs associated with core genes were located in the untranslated or intron regions of genes, the current authors are now measuring the cis-effects of these SNPs on the expression of core genes. Moreover, the activities of pathways based on gene expression levels also should be elucidated in detail. As core-node genes can regulate functionally related multiple pathways, these genes may play a crucial role in delineating the complex molecular physiology of constitution.
Conclusions
The current authors identified the biologic pathways and core-node genes associated with SC types from the GWA study; however, the biologic functions of the pathways identified herein should be evaluated further in association with each constitutional type.
Supplementary Material
Acknowledgments
This work was supported by National Research Foundation of Korea grant (NRF, No. 20110027739) and a Korea Institute of Oriental Medicine grant (KIOM, No. K11070) funded by the Korean government.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lee J. Seoul: Kyung Hee University Press; 1996. Longevity & Life Preservation in Oriental Medicine. [Google Scholar]
- 2.Shim EB. Lee S. Kim JY. Earm YE. Physiome and sasang constitutional medicine. J Physiol Sci. 2008;58:433–440. doi: 10.2170/physiolsci.RV004208. [DOI] [PubMed] [Google Scholar]
- 3.Kim BY. Cha S. Jin HJ. Jeong S. Genetic approach to elucidation of sasang constitutional medicine. Evid Based Complement Alternat Med. 2009;6(Sl):51–57. doi: 10.1093/ecam/nep058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha S. Koo I. Park BL, et al. Genetic Effects of FTO and MC4R polymorphisms on body mass in constitutional types. Evid Based Complement Alternat Med. 2011;2011:106390. doi: 10.1093/ecam/nep162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JH. Kwon YD. Hong SH, et al. Interleukin-1β gene polymorphism and traditional constitution in obese women. Int J Neurosci. 2008;118:793–805. doi: 10.1080/00207450701242883. [DOI] [PubMed] [Google Scholar]
- 6.Song JS. Jeong HJ. Kim SJ, et al. Interleukin-1α polymorphism-889C/T related to obesity in Korean Taeumin women. Am J Chin Med. 2008;36:71–80. doi: 10.1142/S0192415X0800559X. [DOI] [PubMed] [Google Scholar]
- 7.Yin CS. Park HJ. Chung JH, et al. Genome-wide association study of the Four-Constitution medicine. J Altern Complement Med. 2009;15:1327–1333. doi: 10.1089/acm.2009.0205. [DOI] [PubMed] [Google Scholar]
- 8.Kim BY. Jin HJ. Kim JY. Genome-wide association analysis of Sasang constitution in Korean population. J Altern Complement Med. 2012;18:262–269. doi: 10.1089/acm.2010.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Won HH. Lee S. Jang E, et al. A genome-wide scan for the Sasang constitution in a Korean family suggests significant linkage at chromosomes 8q11.22–23 and 11q22.1–3. J Altern Complement Med. 2009;15:765–769. doi: 10.1089/acm.2009.0067. [DOI] [PubMed] [Google Scholar]
- 10.Park SH. Kim MG. Lee SJ, et al. Temperament and character profiles of sasang typology in an adult clinical sample. Evid Based Complement Alternat Med. 2011;2011:794795. doi: 10.1093/ecam/nep034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ. Kim JY. A clinical report on the adverse reactions of sasangin by the prescriptions of soeumin, soyangin. J Sasang Constitutional Med. 2008;20:107–117. [Google Scholar]
- 12.Purcell S. Neale B. Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam D. Kim J. Kim SY. Kim S. GSA-SNP: A general approach for gene set analysis of polymorphisms. Nucleic Acids Res. 2010;38:W749–W754. doi: 10.1093/nar/gkq428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JD. Wiemann S. KEGGgraph: A graph approach to KEGG PATHWAY in R and bioconductor. Bioinformatics. 2009;25:1470–1471. doi: 10.1093/bioinformatics/btp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S. Lv F. Gao J, et al. HLA class II polymorphisms associated with the physiologic characteristics defined by Traditional Chinese Medicine: Linking modern genetics withan ancient medicine. J Altern Complement Med. 2007;13:231–239. doi: 10.1089/acm.2006.6126. [DOI] [PubMed] [Google Scholar]
- 17.Patwardhan B. Bodeker G. Ayurvedic genomics: Establishing a genetic basis for mind–body typologies. J Altern Complement Med. 2008;14:571–576. doi: 10.1089/acm.2007.0515. [DOI] [PubMed] [Google Scholar]
- 18.Zwickey H. Schiffke HC. Genetic correlates of Chinese medicine: In search of a common language. J Altern Complement Med. 2007;13:183–184. doi: 10.1089/acm.2007.7026. [DOI] [PubMed] [Google Scholar]
- 19.Koo I. Kim JY. Kim MG. Kim KH. Feature selection from a facial image for distinction of sasang constitution. Evid Based Complement Alternat Med. 2009;6(S1):65–71. doi: 10.1093/ecam/nep065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham DD. Do JH. Ku B, et al. Body mass index and facial cues in sasang typology for young and elderly persons. Evid Based Complement Alternat Med. 2011;2011:749209. doi: 10.1155/2011/749209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasher B. Negi S. Aggarwal S, et al. Whole genome expression and biochemical correlates of extreme constitutional types defined in Ayurveda. J Transl Med. 2008;6:48. doi: 10.1186/1479-5876-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng G. Luo L. Siu H, et al. Gene and pathway-based second-wave analysis of genome-wide association studies. Eur J Hum Genet. 2010;18:111–117. doi: 10.1038/ejhg.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukata Y. Amano M. Kaibuchi K. Rho–Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee J. Lee J. Lee E, et al. The sasang constitutional types can act as a risk factor for hypertension. Clin Exp Hypertens. 2011;33:525–532. doi: 10.3109/10641963.2011.561901. [DOI] [PubMed] [Google Scholar]
- 25.Visser J. Rozing J. Sapone A, et al. Tight junctions, intestinal permeability, and autoimmunity: Celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195–205. doi: 10.1111/j.1749-6632.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins BT. Lundeen TF. Norwood KM, et al. Increased blood–brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 27.Choi K. Lee J. Yoo J, et al. Sasang constitutional types can act as a risk factor for insulin resistance. Diabetes Res Clin Pract. 2011;91:e57–e60. doi: 10.1016/j.diabres.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Lee TG. Koh B. Lee S. Sasang constitution as a risk factor for diabetes mellitus: a cross-sectional study. Evid Based Complement Alternat Med. 2009;6(S1):99–103. doi: 10.1093/ecam/nep054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.