Abstract
STUDY QUESTION
What is the effect of glucose ingestion on leukocytic reactive oxygen species (ROS) generation in normal-weight women with polycystic ovary syndrome (PCOS) with and without excess abdominal adiposity (AA)?
SUMMARY ANSWER
Normal-weight women with PCOS exhibit an increase in leukocytic ROS generation in response to glucose ingestion, and this increase is independent of excess AA.
WHAT IS KNOWN ALREADY
Excess adipose tissue is a source of oxidative stress. Normal-weight women with PCOS exhibit oxidative stress and can have excess AA.
STUDY DESIGN AND SIZE
This is a cross-sectional study involving 30 reproductive-age women.
PARTICIPANTS/MATERIALS, SETTING AND METHODS
Fourteen normal-weight women with PCOS (6 normal AA, 8 excess AA) and 16 body composition-matched controls (8 normal AA, 8 excess AA) underwent body composition assessment by dual-energy absorptiometry and an oral glucose tolerance test (OGTT) at a university medical center. Insulin sensitivity was derived from the OGTT (ISOGTT). Blood was drawn while fasting and 2 h after glucose ingestion to measure leukocytic ROS generation and p47phox protein content and plasma thiobarbituric acid-reactive substances (TBARS) and C-reactive protein (CRP).
MAIN RESULTS AND THE ROLE OF CHANCE
Compared with controls, both PCOS groups exhibited lower ISOGTT (43–54%) and greater percentage change (% change) in ROS generation (96–140%), p47phox protein (18–28%) and TBARS (17–48%). Compared with women with PCOS with excess AA, those with normal AA exhibited higher testosterone levels (29%) and lower CRP levels (70%). For the combined groups, ISOGTT was negatively correlated with the % change in ROS generation and p47phox protein. CRP was positively correlated with abdominal fat. The % change in p47phox protein was positively correlated with CRP and androgens.
LIMITATIONS, REASONS FOR CAUTION
Although this study is adequately powered to assess differences in ROS generation between the women with PCOS and control participants, the modest sample size merits caution when interpreting the corroborative results of the additional measures of oxidative stress and inflammation.
WIDER IMPLICATIONS OF THE FINDINGS
This study highlights the unique pro-oxidant contribution of circulating leukocytes in the development of insulin resistance and hyperandrogenism in PCOS.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by NIH grant HD-048535 to F.G. The authors have nothing to disclose.
Keywords: polycystic ovary syndrome, abdominal adiposity, oxidative stress, hyperglycemia
Introduction
Women with polycystic ovary syndrome (PCOS) exhibit oxidative stress, chronic low grade inflammation and insulin resistance (Dunaif et al., 1989; Kelly et al., 2001; González et al., 2006a). This is evidenced by increased circulating protein carbonyls and tumor necrosis factor-α (TNF-α) and decreased insulin signaling as a result of serine phosphorylation-induced suppression of insulin-stimulated insulin receptor substrate-1 (IRS-1) activation (González et al., 1999; Fenkci et al., 2003; Corbould et al., 2005). These same phenomena occur in obesity-related diabetic syndromes and have been linked in a causal fashion to adipose tissue hypoxia (Yin et al., 2009).
Hypoxia-related cell death in expanded adipose tissue of the obese leads to migration of circulating mononuclear cells (MNCs) into the adipose stromal-vascular compartment (Weisberg et al., 2003; Cinti et al., 2005). Phagocytic activity by MNC-derived macrophages induces membrane-bound NADPH oxidase following translocation of a cytosol component known as p47phox to the cell membrane (Groemping and Rittenger, 2005). Oxidation of NADPH by NADPH oxidase generates superoxide, a reactive oxygen species (ROS) that induces oxidative stress (Chanock et al., 1994). This in turn, activates the transcription factor, nuclear factor κB (NFκB) that promotes TNF-α transcription (Barnes and Karin, 1997). Adipose tissue TNF-α provides positive feedback to up-regulate the aforementioned molecular events and mediates insulin resistance by stimulation of serine phosphorylation to decrease insulin-stimulated IRS-1 activation (Barnes and Karin, 1997; Rui et al., 2001).
We have previously shown that circulating leukocytes of normal-weight women with PCOS are pre-activated in the fasting state and that glucose ingestion in this population promotes increased leukocytic ROS generation and altered TNF-α release (González et al., 2005, 2006a, 2007a). However, our previous studies did not take into account the 30% prevalence of excess abdominal adiposity (AA) in normal-weight women with PCOS (Carmina et al., 2007). Thus, it remains unclear whether the pro-oxidant, pro-inflammatory influence of excess AA in these individuals is the primary cause of glucose-stimulated oxidative stress.
We embarked on a study to examine the effect of glucose ingestion on ROS generation from leukocytes in normal-weight women with PCOS in whom AA was either normal or in excess. We also examined this effect on the protein content of p47phox, the key component of NADPH oxidase and on plasma thiobarbituric acid-reactive substances (TBARS), a commonly used index of lipid peroxidation. We hypothesized that in response to glucose ingestion, ROS generation, p47phox protein content and TBARS are increased in normal-weight women with PCOS regardless of AA status compared with age- and body composition-matched controls; and that these markers of oxidative stress are related to AA, insulin sensitivity and circulating androgens.
Materials and Methods
Participants
Fourteen normal-weight women with PCOS (6 normal AA and 8 excess AA) and 16 body composition-matched control subjects (8 normal AA and 8 excess AA) volunteered to participate in the study. Normal weight was defined as a BMI between 18 and 25 kg/m2. Excess AA was defined as the percentage ratio of truncal fat to total body fat (% TF/TBF) measured by dual-energy X-ray absorptiometry (DEXA) that was >42% which was 2 SDs above the mean of controls with normal AA. This definition was adopted from a previous study in a relatively lean Mediterranean population (Carmina et al., 2007). However, the cut-off value is based on DEXA findings in an American normal weight control population from our own report of greater TF in normal-weight women with PCOS (González et al., 2005). The diagnosis of PCOS was based on NIH criteria in that the presence of oligo-amenorrhea and hyperandrogenemia was required after excluding non-classic congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinemia and thyroid disease. However, all participants with PCOS also exhibited polycystic ovaries on ultrasound and, thus, met the European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine and Androgen Excess and PCOS Society diagnostic criteria for PCOS as well. All control participants had regular menses lasting 25–35 days and a luteal range serum progesterone level consistent with ovulation (>5 ng/ml). All control participants also had normal serum androgen concentrations and did not have any skin manifestations of androgen excess or polycystic ovaries on ultrasound.
Diabetes and inflammatory illnesses (e.g. asthma, upper respiratory infection, recent surgery) were excluded in all participants. None of them smoked tobacco or used medications that could impact carbohydrate metabolism or immune function (e.g. metformin, oral contraceptives, salicylates) for a minimum of 6 weeks before beginning the study. No participants exercised regularly during the 6 months before study participation. Written informed consent was obtained in all participants according to Institutional Review Board guidelines for the protection of human subjects.
Study design
An oral glucose tolerance test (OGTT) was administered to all participants between Days 5 and 8 after the onset of menses and an overnight fast of ∼12 h. A healthy diet consisting of 50% carbohydrate, 35% fat and 15% protein was given to all participants for three consecutive days before the OGTT. Body composition was assessed immediately before the OGTT.
Oral glucose tolerance test
All participants ingested a 75 g glucose beverage. Blood samples were drawn while fasting and at 30, 60, 90, 120 and 180 min after glucose ingestion. For each blood sample, plasma glucose was measured immediately while insulin was measured later from plasma stored at −80°C. Additional plasma was isolated from the fasting and 120 min (2 h) blood samples and stored at −80°C until assayed for fasting C-reactive protein (CRP) and pre- and post-glucose ingestion TBARS. Glucose tolerance was determined by the World Health Organization criteria (Modan et al., 1989). Insulin sensitivity was derived from the OGTT (ISOGTT) using the following formula: 10 000 divided by the square root of (fasting glucose × fasting insulin) × (mean glucose × mean insulin) (Matsuda and DeFronzo, 1999).
Body composition assessment
Height without shoes was measured to the nearest 1.0 cm. Body weight was measured to the nearest 0.1 kg. Waist circumference was measured at the level of the umbilicus and used to estimate AA (Kohrt et al., 1993). All participants also underwent DEXA to determine TF and TBF using the QDR 4500 Elite model scanner (Hologic Inc., Waltham, MA, USA). TF content was defined as the area between the dome of the diaphragm (cephalad limit) and the top of the great trochanter (caudal limit) (Taylor et al., 1998).
ROS generation and western blotting
MNC and polymorphonuclear cells (PMN) were isolated from blood samples obtained during the OGTT at 0 and 120 min (2 h). ROS generation was measured by chemiluminescence as previously described (González et al., 2007b). The protein content of p47phox and actin in MNC was quantified by western blotting as previously described using a monoclonal antibody against p47phox subunit (Transduction Laboratories Inc., San Diego, CA, USA) at a dilution of 1:500 or actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1000 (Aljada et al., 2002). Densitometry was performed on scanned films using Carestream Molecular Imaging software version 5.0.2.30 (Rochester, NY, USA), and all values for p47phox were corrected for loading using those obtained for actin.
Plasma and serum measurements
Plasma glucose, insulin, CRP and TBARS along with serum testosterone, androstenedione and dehydroepiandrosterone-sulfate (DHEA-S) were measured as previously described (González et al., 2006a). Participant samples were all measured in duplicate in the same assay upon study completion. The inter-assay and intra-assay coefficients of variation for all assays were no >7.4 and 12%, respectively.
Statistics
The StatView software package (SAS Institute, Cary, NC, USA) was used to perform the statistical analysis. The primary end-point was change from baseline of ROS generation in response to glucose ingestion across the four study groups. Sample size was calculated based on an expected difference of at least 40% between groups in ROS generation with an SD of 20% and desired power of 80% using our previous study as a reference (González et al., 2006a). Descriptive data and change from baseline in oxidative stress markers were compared using analysis of variance for multiple group comparisons followed by a post hoc analysis using Tukey's honestly significant difference test to identify the source of significance. Data are presented as mean ± SE. Treatment effects on oxidative stress markers were determined by calculating percentage change (% change) for each participant in view of inter-individual variability. Correlation analyses were performed by Pearson linear regression using the method of least squares. Results were considered significant at a two-tailed α-level of 0.05.
Results
Age, body composition and serum hormone levels
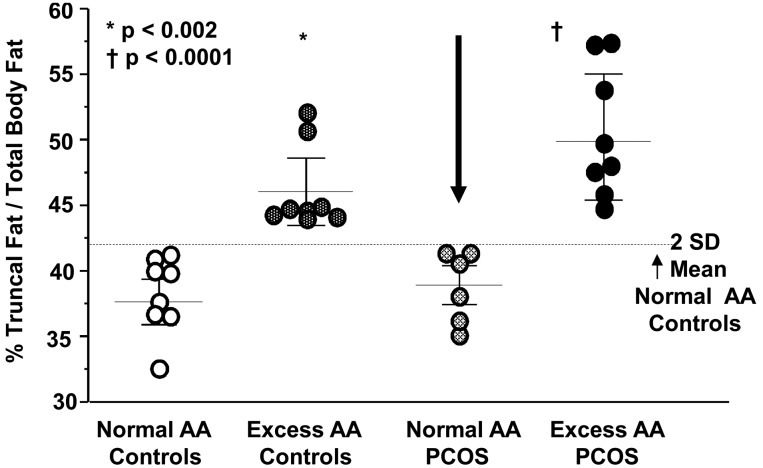
Age, height, body weight, BMI, waist circumference and TBF were similar among groups (Table I). By design, TF and % TF/TBF were significantly (P < 0.002) greater in individuals with excess AA compared with those with normal AA whether or not they had PCOS (Fig. 1).
Table I.
Age, body composition, endocrine and metabolic parameters and CRP levels.
| Controls |
PCOS |
|||
|---|---|---|---|---|
| Normal AA | Excess AA | Normal AA | Excess AA | |
| Age, year | 32 ± 2 | 30 ± 2 | 25 ± 2 | 29 ± 1 |
| Height, cm | 165.4 ± 1.4 | 165.4 ± 1.7 | 166.6 ± 2.6 | 160.7 ± 3.8 |
| Body weight, kg | 60.0 ± 1.8 | 63.3 ± 1.2 | 62.1 ± 2.6 | 62.8 ± 2.4 |
| BMI, kg/m2 | 22.0 ± 0.8 | 23.1 ± 0.7 | 22.4 ± 1.0 | 24.3 ± 0.4 |
| Waist circumference, cm | 70.8 ± 3.1 | 79.4 ± 4.1 | 73.7 ± 2.2 | 80.6 ± 2.4 |
| Total body fat, kg | 17.0 ± 1.5 | 21.1 ± 1.1 | 17.0 ± 1.7 | 20.5 ± 1.1 |
| Truncal fat, kg | 6.7 ± 0.7 | 9.7 ± 0.6a,b | 6.7 ± 0.8 | 10.4 ± 0.7c,d |
| LH, mIU/ml | 4.2 ± 0.6 | 2.7 ± 0.6b | 13.2 ± 1.6e | 13.0 ± 1.3d,f |
| Testosterone, ng/dl | 40.0 ± 5.9 | 41.6 ± 3.7b | 85.7 ± 8.4e | 60.8 ± 5.7c,d,f |
| Androstendione, ng/ml | 1.6 ± 0.2 | 1.2 ± 0.1b | 3.8 ± 0.2e | 3.2 ± 0.4d,f |
| DHEA-S, μg/dl | 185 ± 30 | 190 ± 29 | 294 ± 40e | 346 ± 38d,f |
| Fasting glucose, mg/dl | 86 ± 3 | 88 ± 1 | 83 ± 4 | 88 ± 2 |
| 2 h glucose, mg/dl | 96 ± 8.0 | 112 ± 5 | 92 ± 7 | 111 ± 6 |
| Fasting insulin, μIU/ml | 7.6 ± 1.2 | 5.2 ± 0.8b | 9.1 ± 0.5 | 11.7 ± 1.7d,f |
| ISOGTT | 9.8 ± 1.4 | 8.4 ± 0.9b | 4.9 ± 0.4e | 4.5 ± 0.6d,f |
| C-reactive protein, mg/l | 0.37 ± 0.09 | 0.69 ± 0.33 | 0.37 ± 0.09 | 1.23 ± 0.27c,d |
AA, abdominal adiposity; ISOGTT, insulin sensitivity derived by oral glucose tolerance testing; PCOS, polycystic ovary syndrome. Values are expressed as means ± SE; Conversion factors to SI units: Testosterone × 3.467 (nmol/l), androstenedione × 3.492 (nmol/l), dehydroepiandrosterone-sulfate (DHEA-S) × 0.002714 (µmol/l), glucose × 0.0551 (mmol/l), insulin × 7.175 (pmol/l), C-reactive protein × 9.524 (nmol/l).
aExcess AA controls versus normal AA controls, P < 0.004.
bExcess AA controls versus normal AA PCOS, P < 0.05.
cExcess AA PCOS versus normal AA PCOS, P < 0.008.
dExcess AA PCOS versus normal AA controls, P < 0.03.
eNormal AA PCOS versus normal AA controls, P < 0.003.
fExcess AA PCOS versus excess AA controls, P < 0.03.
Figure 1.
Scatter plots of the percentage ratio of truncal fat to total body fat (% TF/TBF) representing AA in normal-weight women with PCOS and normal weight controls. Excess AA was defined as the % TF/TBF that was 2 SDs above the mean of controls with normal AA.24 The arrow points to the select group of women with PCOS with normal AA. *Indicates the % TF/TBF of controls with excess AA was significantly greater compared with that of controls with normal AA and women with PCOS with normal AA, P < 0.002. † Indicates the % TF/TBF of women with PCOS with excess AA was significantly greater compared with that of controls with normal AA and women with PCOS with normal AA, P < 0.0001. Individual scatter points represent one data point. Horizontal lines indicate the mean; error bars represent the SEM; PCOS, polycystic ovary syndrome.
Serum levels of LH, testosterone and androstenedione were significantly (P < 0.03) higher in women with PCOS compared with control participants regardless of body composition status (Table I). In women with PCOS, testosterone levels were significantly (P < 0.008) higher in those with normal AA compared with those with excess AA. Serum DHEA-S levels were significantly (P < 0.03) higher in both groups of women with PCOS compared with controls with normal AA. DHEA-S levels were also significantly (P < 0.03) higher in women with PCOS with excess AA, and approached significance (P = 0.05) in women with PCOS with normal AA compared with controls with excess AA.
Glycemic status and insulin sensitivity
Glucose levels while fasting and 2 h post-glucose ingestion were similar in women with PCOS compared with controls regardless of body composition status (Table I). All participants had a normal glucose response during the OGTT with fasting glucose levels <100 mg/dl and 2 h glucose levels ranging between 75 and 134 mg/dl. Fasting insulin levels were significantly (P < 0.05) higher in both groups of women with PCOS compared with controls with excess AA and in women with PCOS with excess AA compared with controls with normal AA. ISOGTT was significantly (P < 0.05) lower in both groups of women with PCOS compared with control participants regardless of body composition status.
Markers of oxidative stress in leukocytes and plasma
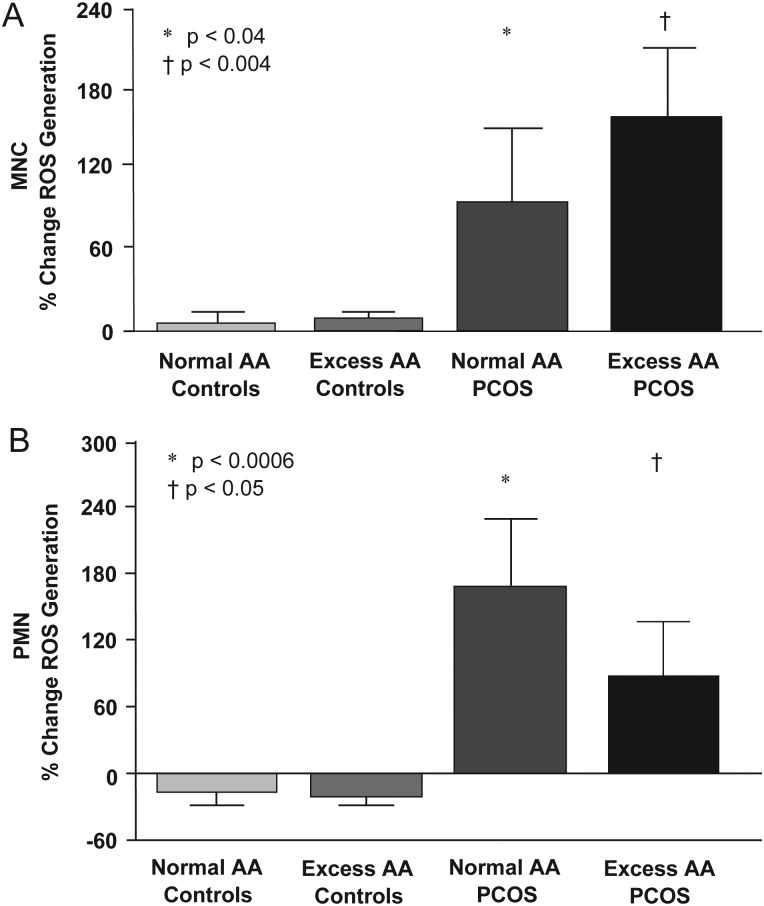
In response to glucose ingestion, the % change in ROS generation from MNC and PMN was minimal in both control groups, but increased in women with PCOS with both normal AA and excess AA and was significantly (P < 0.05) different compared with control participants regardless of body composition status (Fig. 2).
Figure 2.
Change from baseline (%) in ROS generation from leukocytes when fasting samples (pre) were compared with the samples collected 2 h after glucose ingestion (post). (A) *Denotes response in MNCs of women with PCOS with normal AA was significantly greater compared with that of either control group, P < 0.04. †Denotes response in the MNC of women with PCOS with excess AA was significantly greater compared with that of either control group P < 0.004. (B) *Represents response in PMN cells of women with PCOS with normal AA was significantly greater compared with that of either control group, P < 0.0006. †Iindicates response in PMN of women with PCOS with excess AA was significantly greater compared with that of either control group, P < 0.05. Columns represent the mean; error bars represent the SEM.
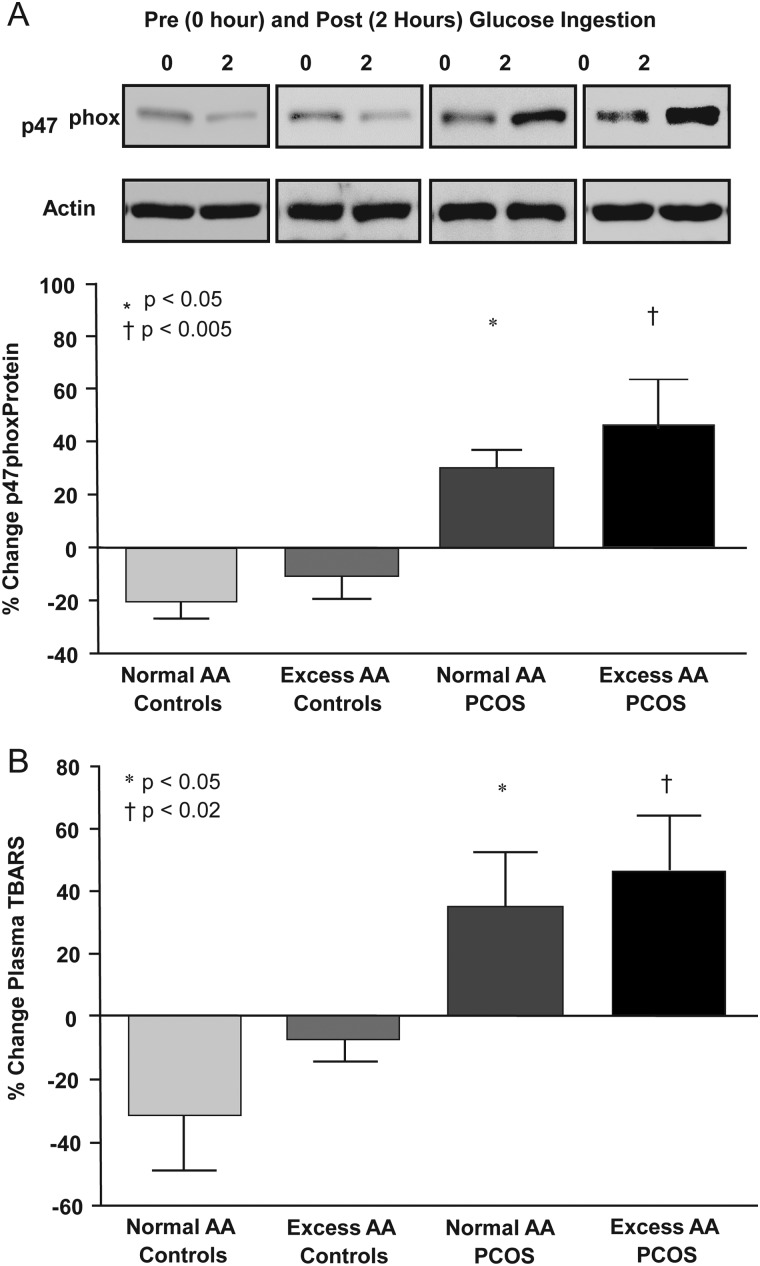
The % change in p47phox protein content and plasma TBARS decreased in both control groups, but increased in women with PCOS with both normal AA and excess AA, and was significantly (P < 0.05) different compared with control participants regardless of body composition status (Fig. 3). Fasting plasma CRP concentrations were significantly (P < 0.03) higher in women with PCOS with excess AA compared with women with PCOS with normal AA or controls with normal AA (Table I).
Figure 3.
(A) Representative western blots from the four study groups showing the change in quantity of p47phox and actin in MNC homogenates. The densitometric quantitative analysis depicted in the histograms below the blots show the change from baseline (%) in p47phox protein content in MNC when fasting samples (pre) were compared with the samples collected 2 h after glucose ingestion (post). The samples used to quantify proteins from all four study groups were run on the same gel. *Indicates response of p47phox in women with PCOS with normal AA was significantly greater compared with that of either control group, P < 0.05. †Denotes response of p47phox in women with PCOS with excess AA was significantly greater compared with that of either control group, P < 0.005. (B) Change from baseline (%) in plasma TBARS when fasting samples (pre) were compared with the samples collected 2 h after glucose ingestion (post). *Represents response in plasma TBARS of women with PCOS with normal AA was significantly greater compared with that of either control group, P < 0.005. †Represents response in plasma TBARS of women with PCOS with excess AA was significantly greater compared with that of either control group, P < 0.02. Columns in A and B represent the mean value with error bars representing the standard error of the mean.
Correlations
The % TF/TBF was positively correlated with the % change in p47phox protein content and TBARS and with fasting CRP for the combined groups (Table II). ISOGTT was negatively correlated with the % change in ROS generation from MNC and PMN as well as the % change in p47phox protein content and TBARS. The % change in p47phox protein content was positively correlated with the % change in plasma TBARS and with fasting CRP. None of the body composition markers including % TF/TBF were correlated with ISOGTT. BMI and waist circumference were also not correlated with any markers of oxidative stress (data not shown).
Table II.
Pearson correlations for the combined groups of oxidative stress and inflammation markers with AA, insulin sensitivity and each other.
| ROS generation (% change) |
p47phox (% change) | TBARS (% change) | CRP (mg/l) | ||
|---|---|---|---|---|---|
| MNC | PMN | ||||
| % TF/TBF | |||||
| r | 0.230 | 0.045 | 0.455 | 0.493 | 0.509 |
| P-value | 0.239 | 0.822 | 0.013* | 0.006* | 0.004* |
| ISOGTT | |||||
| r | −0.411 | −0.398 | −0.638 | −0.498 | 0.198 |
| P-value | 0.029* | 0.039* | 0.0002* | 0.005* | 0.294 |
| CRP (mg/l) | |||||
| r | 0.062 | 0.057 | 0.521 | 0.347 | — |
| P | 0.754 | 0.779 | 0.004* | 0.061 | — |
| TBARS (% change) | |||||
| r | 0.462 | 0.250 | 0.508 | — | — |
| P-value | 0.013* | 0.208 | 0.005* | — | — |
ROS generation, reactive oxygen species generation; p47phox, p47phox protein content; TBARS, thiobarbituric acid reactive substances; CRP, C-reactive protein; % TF/TBF, percent ratio of truncal fat to total body fat; ISOGTT,insulin sensitivity index derived from an oral glucose tolerance test.
*P < 0.05.
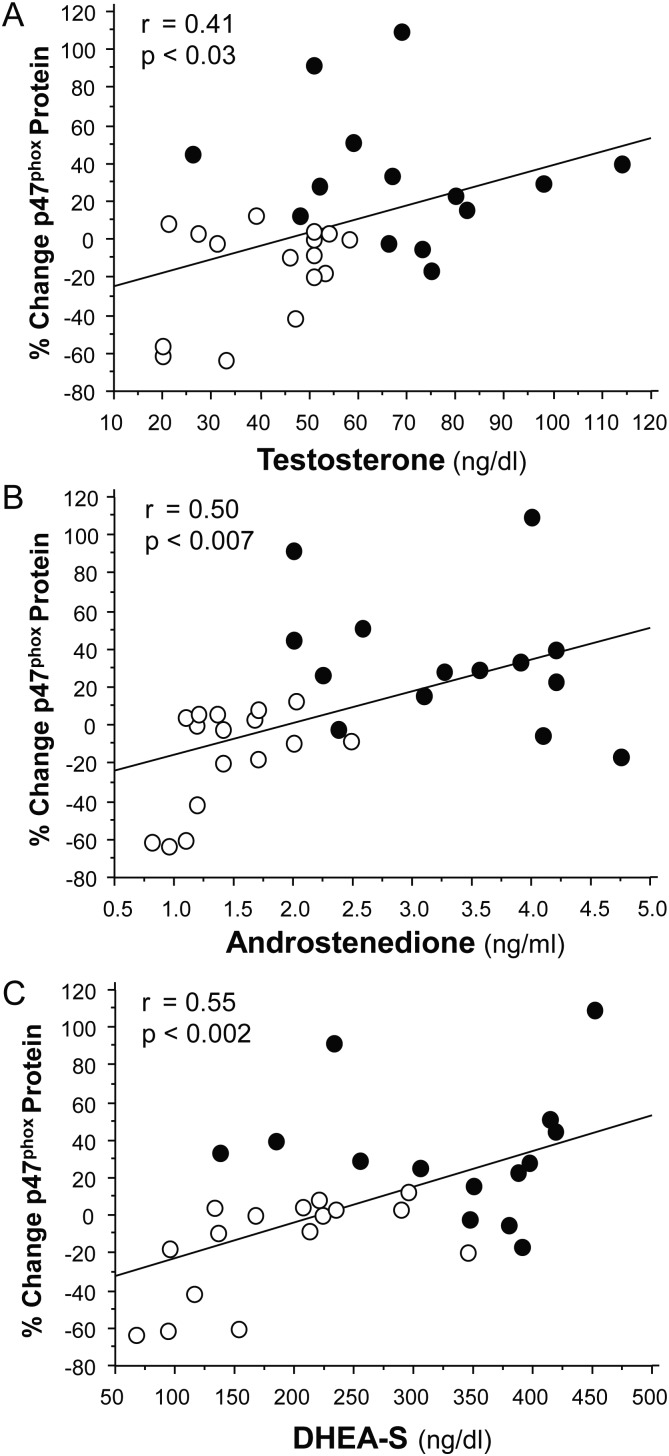
LH was positively correlated with the % change in ROS generation from MNC (r = 0.55, P < 0.02) and PMN (r = 0.49, P < 0.03), and the % change in p47phox protein content (r = 0.64, P < 0.002) and TBARS (r = 0.45, P < 0.03) for the combined groups. Testosterone was positively correlated with the % change in ROS generation from PMN (r = 0.71, P < 0.0002). Androstenedione was positively correlated with the % change in ROS generation from MNC (r = 0.39, P < 0.05) and PMN (r = 0.77, P < 0.0002) and the % change in TBARS (r = 0.39, P < 0.04). DHEA-S was positively correlated with CRP (r = 0.37, P < 0.05). All three androgen levels were positively correlated with the % change in p47phox protein content (Fig. 4).
Figure 4.
Pearson correlations between the change in baseline (%) in p47phox protein content from MNCs and serum levels of (A) testosterone, (B) androstenedione and (C) DHEA-S. Data are shown for the 16 control subjects and the 14 women with PCOS. Open circles, control subjects; closed circles, women with PCOS. Change from baseline was calculated following densitometric quantitative analysis of p47phox protein content from MNC when fasting samples (pre) were compared with the samples collected 2 h after glucose ingestion (post). DHEA-S, dehydroepiandrosterone-sulfate; PCOS, polycystic ovary syndrome.
In women with PCOS, testosterone was negatively correlated with the % TF/TBF (r = −0.55, P < 0.05), and CRP was positively correlated with the % TF/TBF (r = 0.49, P < 0.05) and p47phox protein content (r = 0.60, P < 0.03).
Discussion
Our data clearly show for the first time that in PCOS, oxidative stress emanating from circulating leukocytes in response to glucose ingestion occurs in the absence of excess AA. Normal-weight women with PCOS with normal AA exhibit increases in glucose-stimulated markers of oxidative stress from leukocytes compared with normal weight controls with normal AA. Markers of oxidative stress are inversely related to insulin sensitivity and directly related to LH and androgens, lending support to the concept that hyperglycemia-induced oxidative stress from leukocytes is involved in promoting insulin resistance and hyperandrogenism in PCOS. Furthermore, the direct relationship between AA and markers of oxidative stress suggests that excess AA contributes to the oxidative load in PCOS.
Containment of leukocytic oxidative stress following glucose ingestion appears to be the norm in normal-weight reproductive-age women regardless of body composition status. Leukocytic ROS generation is negligible, and p47phox protein content and circulating TBARS are suppressed in controls with both normal and excess AA. These findings are in keeping with our previous reports that MNC of normal-weight ovulatory women are inactive in the fasting state and leukocytic markers of oxidative stress and inflammation, including TNF-α release from MNC, are suppressed following glucose ingestion (González et al., 2006a, 2007a). This is important because ROS-induced oxidative stress from MNC-derived macrophages present in excess adipose tissue and muscle triggers NFκB activation and subsequent TNF-α release. This in turn can inhibit insulin signaling through a paracrine interaction that impairs glucose uptake (Hotamisligil et al., 1994; Weisberg et al., 2003; Nguyen et al., 2007; Varma et al., 2009). Improvement of insulin sensitivity following ablation of MNC-derived macrophages in muscle of insulin resistant animals corroborates this concept (Patsouris et al., 2008). Thus, suppression of postprandial NADPH oxidase activity to limit ROS generation may constitute a protective effect to optimize insulin signaling during glucose disposal in normal young women.
In contrast, leukocytes of normal-weight women with PCOS demonstrate increased sensitivity to glucose ingestion regardless of body composition status. Leukocytic ROS generation, p47phox protein content and plasma TBARS increase following glucose challenge in normal-weight women with PCOS with both normal AA and excess AA compared with either control group. These results corroborate our previous reports of leukocyte pre-activation in the fasting state and hyperglycemia-induced leukocytic ROS generation in women with PCOS (González et al., 2006a, 2007a). More importantly, they showcase the separate role of circulating leukocytes in establishing a pro-oxidant state that may be the impetus for increases in MNC-derived NFκB activation and altered TNF-α release following glucose ingestion in normal-weight women with PCOS (González et al., 2005, 2006b). Lipid and protein ingestion also provokes a pro-oxidant, pro-inflammatory response in MNC (Mohanty et al., 2002; Aljada et al., 2004). Thus, feeding alone in the absence of excess adiposity can stimulate oxidative stress in PCOS and culminates in an acute inflammatory response that may mediate insulin resistance. Reductions in oxidative stress and inflammation in normal individuals undergoing a 2-day fast and the inverse relationship between insulin sensitivity and oxidative stress markers in the current study provide support for this concept (Dandona et al., 2001).
In PCOS, hyperglycemia-induced oxidative stress may directly stimulate hyperandrogenism. LH, testosterone, androstenedione and DHEA-S are all directly related to glucose-stimulated ROS generation and p47phox protein content. This reaffirms similar findings in our previous studies (González et al., 2006a, 2007a). Although the association with LH suggests a central impact of oxidative stress on androgen production, local effects are well described. MNC-derived macrophages are present in the ovary (Best et al., 1996). CYP17, the steroidogenic enzyme responsible for androgen production is up-regulated in theca cells by pro-oxidant stimuli and inhibited by antioxidants such as resveratrol and statins (Piotrowski et al., 2005; Wong et al., 2011). Proliferation of theca cells from rat and human polycystic ovaries is stimulated by TNF-α and suppressed by statins (Spazynsky et al., 1999; Sokalska et al., 2012). Furthermore, CRP is unaltered in normal-weight women with PCOS following chronic androgen suppression (González et al., 2012). Thus, glucose-stimulated oxidative stress from leukocytes recruited into the polycystic ovary may cause a local inflammatory response that stimulates ovarian androgen production in women with PCOS.
Aside from the diet-induced pro-oxidant response of circulating leukocytes, oxidative stress and inflammation can also originate from excess AA in normal-weight women with PCOS. There is a trend towards higher markers of oxidative stress, and CRP is significantly elevated in normal-weight women with PCOS with excess AA compared with those with normal AA. Oxidative stress markers are also directly related to AA. Hypoxia-related cell death in excess adipose tissue promotes migration of MNC into the stromal-vascular compartment which incites oxidative stress (Weisberg et al., 2003; Furukawa et al., 2004; Cinti et al., 2005). Subsequent TNF-α production in MNC-derived macrophages stimulates adipocyte TNF-α production through paracrine interaction (Fain et al., 2004). It is noteworthy that CRP elevations in normal-weight women with PCOS with excess AA are much lower in magnitude compared with those in obesity (Boulman et al., 2004). Conversely, CRP levels in women with PCOS with normal AA are similar to those in normal-weight controls with normal AA. This suggests that circulating CRP is normal in PCOS in the absence of excess AA but requires confirmation in a larger cohort. Thus, the inflammatory load resulting from oxidative stress in excess AA in normal-weight women with PCOS is manifested by modest CRP elevations.
In PCOS, the quantity of fat present in a particular individual dictates the impact of adiposity in the development of insulin resistance and hyperandrogenism. Evidence has emerged that in PCOS, adipose tissue molecular inflammation marker expression is comparable with the amount of adiposity and is not discordantly increased when compared with individuals without PCOS (Lindholm et al., 2011). Excess AA is a promoter of insulin resistance in obesity and is inversely related to insulin sensitivity in reports that include obese women regardless of PCOS status (McLaughlin et al., 2011; Penaforte et al., 2011). However, there is no association between AA and insulin sensitivity in the current study. This suggests that in PCOS, the degree of oxidative stress emanating from excess AA in normal-weight individuals may be insufficient to induce insulin resistance. There is also a positive association between excess AA and circulating androgens when obese women with PCOS are included in studies (Holte et al., 1994). This supports a common concept that in PCOS, insulin resistance promotes hyperandrogenism (Tosi et al., 2012). However, testosterone levels are inversely related to AA in our PCOS study population and are higher in normal-weight women with PCOS with normal AA compared with those with excess AA. As a known suppressor of lipolysis, testosterone at these higher levels may keep AA in check in normal-weight women with PCOS (Xu et al., 1990). Thus, excess AA contributes to the inflammatory load induced by oxidative stress in normal-weight women with PCOS, but may not play a significant role in the promotion of insulin resistance in normal-weight women with PCOS. In addition, the degree of hyperandrogenism in PCOS before the onset of weight gain may control the development of excess AA.
In conclusion, normal-weight women with PCOS exhibit increased leukocytic ROS generation, p47phox protein content and plasma TBARS in response glucose ingestion that is independent of excess AA. This pro-oxidant phenomenon highlights the circulating leukocyte as a unique contributor to insulin resistance and hyperandrogenism in PCOS. The association of AA with markers of oxidative stress and CRP suggests that excess AA is an additional source of oxidative stress in normal-weight women with PCOS.
Authors’ roles
F.G. conceived, contributed to the design and conducted the study, performed some of the laboratory measurements, analyzed and interpreted the data, and prepared the initial draft of the manuscript. C.L.S. and J.M. performed most of the laboratory measurements and the assay quality controls. M.K.S. conducted the study and contributed to manuscript preparation. N.S.R. contributed to the design of the study and edited the manuscript. All authors approved the final version of the manuscript.
Funding
This research was supported by NIH grant HD-048535 to F.G.
Conflict of interest
None declared.
References
- Aljada A, Ghanim H, Dandona P. Translocation of p47phox and activation of NADPH oxidase in mononuclear cells. Methods Molec Biol. 2002;196:99–103. doi: 10.1385/1-59259-274-0:99. [DOI] [PubMed] [Google Scholar]
- Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudari A, Dandona P. Increase in intranuclear nuclear factor kappa B and decrease in inhibitor kappa B in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79:682–690. doi: 10.1093/ajcn/79.4.682. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. doi:10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11:790–797. doi: 10.1093/oxfordjournals.humrep.a019256. doi:10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- Boulman N, Leiba LR, Shachar S, Linn R, Zinder O, Blumenfeld Z. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–2165. doi: 10.1210/jc.2003-031096. doi:10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- Carmina E, Bucchierri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of it relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500–2505. doi: 10.1210/jc.2006-2725. doi:10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24919–24922. [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. doi:10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–E1054. doi: 10.1152/ajpendo.00361.2004. doi:10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Hamouda W, Ghanim H, Aljada A, Garg R, Kumar V. Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and meta-tyrosine concentrations. J Clin Endocrinol Metab. 2001;862:899–2902. doi: 10.1210/jcem.86.6.7745. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. doi:10.2337/diabetes.38.9.1165. [DOI] [PubMed] [Google Scholar]
- Fain JN, Bahouth SW, Madan AK. TNFα release by nonfat cells of adipose tissue. Int J Obes. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. doi:10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–127. doi: 10.1016/s0015-0282(03)00571-5. doi:10.1016/S0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González F, Thusu K, Rahman EH, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor α in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–441. doi: 10.1016/s0026-0495(99)90100-2. doi:10.1016/S0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-α release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5336–5342. doi: 10.1210/jc.2005-0694. doi:10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006a;91:336–340. doi: 10.1210/jc.2005-1696. doi:10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor κB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006b;91:1508–1512. doi: 10.1210/jc.2005-2327. doi:10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- González F, Rote NS, Minium J, Kirwan JP. Hyperandrogenism is related to reactive oxygen species generation from pre-activated leukocytes in polycystic ovary syndrome. Reprod Sci. 2007a;14(2 Suppl):215A. [Google Scholar]
- González F, Rote NS, Minium J, O'Leary VB, Kirwan JP. Obese reproductive age women exhibit a proatherogenic inflammatory response during hyperglycemia. Obesity. 2007b;15:2436–2444. doi: 10.1038/oby.2007.289. doi:10.1038/oby.2007.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González F, Sia CL, Stanczyk FZ, Blair HE, Krupa ME. Hyperandrogenism exerts an anti-inflammatory effect in obese women with polycystic ovary syndrome. Endocrine. 2012 doi: 10.1007/s12020-012-9728-6. July 1 (Epub ahead of print), doi 10.1007/s12020-012-9728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groemping Y, Rittenger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. doi:10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte J, Bergh T, Gennarelli G, Wide L. The independent effects of polycystic ovary syndrome and obesity on serum concentrations of gonadotrophins and sex steroids in premenopausal women. Clin Endocrinol. 1994;41:473–481. doi: 10.1111/j.1365-2265.1994.tb02578.x. doi:10.1111/j.1365-2265.1994.tb02578.x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. doi:10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JMC, Sattar N. Low grade chronic inflammation in women in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2001;86:2453–2455. doi: 10.1210/jcem.86.6.7580. doi:10.1210/jc.86.6.2453. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Kirwan JP, King DS, Staten MA, Holloszy JO. Insulin resistance of aging is related to abdominal obesity. Diabetes. 1993;42:273–281. doi:10.2337/diabetes.42.2.273. [PubMed] [Google Scholar]
- Lindholm A, Blomquist C, Bixo M, Dahlbom I, Hansson T, Sundström Poromaa I, Burén J. No difference in markers of adipose tissue inflammation between overweight women with polycystic ovary syndrome and weight-matched controls. Hum Reprod. 2011;26:1478–1485. doi: 10.1093/humrep/der096. doi:10.1093/humrep/der096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo R. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. doi:10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. doi:10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38:1630–1635. doi: 10.2337/diab.38.12.1630. doi:10.2337/diabetes.38.12.1630. [DOI] [PubMed] [Google Scholar]
- Mohanty P, Ghanim H, Hamouda W, Aljada A, Garg R, Dandona P. Both lipid and protein intakes stimulate increased generation of reactive oxygen species by polymorphonuclear leukocytes and mononuclear cells. Am J Clin Nutr. 2002;75:767–772. doi: 10.1093/ajcn/75.4.767. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A Subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. doi:10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalize insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. doi:10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaforte FR, Japur CC, Diez-Garcia RW, Chiarello PG. Upper trunk fat assessment and its relationship with metabolic and biochemical variables and body fat in polycystic ovary syndrome. J Hum Nutr Diet. 2011;24:39–46. doi: 10.1111/j.1365-277X.2010.01130.x. doi:10.1111/j.1365-277X.2010.01130.x. [DOI] [PubMed] [Google Scholar]
- Piotrowski PC, Rzepczynska IJ, Kwintkiewicz J, Duleba AJ. Oxidative stress induces expression of CYP11A, CYP17, STAR and 3βHSD in rat theca-interstitial cells. J Soc Gynecol Invest. 2005;12(2 Suppl):319A. [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. doi:10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokalska A, Piotrowski PC, Rzepczynska IJ, Cress A, Duleba AJ. Statins inhibit growth of human theca-interstitial cells in PCOS and non-PCOS tissues independently of cholesterol availability. J Clin Endocrinol Metab. 2012;95:5390–5394. doi: 10.1210/jc.2010-0770. doi:10.1210/jc.2010-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spazynsky RZ, Arici A, Duleba AJ. Tumor necrosis factor alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–998. doi: 10.1095/biolreprod61.4.993. doi:10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist to hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. Am J Clin Nutr. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- Tosi F, Negri C, Perrone F, Dorizzi R, Castello R, Bonora E, Moghetti P. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97:1712–1719. doi: 10.1210/jc.2011-2939. doi:10.1210/jc.2011-2939. [DOI] [PubMed] [Google Scholar]
- Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE, Jr, Kern PA, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296:E1300–E1310. doi: 10.1152/ajpendo.90885.2008. doi:10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil Steril. 2011;96:1252–1258. doi: 10.1016/j.fertnstert.2011.08.010. doi:10.1016/j.fertnstert.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Xu X, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–1234. doi: 10.1210/endo-126-2-1229. doi:10.1210/endo-126-2-1229. [DOI] [PubMed] [Google Scholar]
- Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333–E342. doi: 10.1152/ajpendo.90760.2008. doi:10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]