Abstract
STUDY QUESTION
In women undergoing IVF, are urinary bisphenol A (BPA) concentrations associated with ovarian response and early reproductive outcomes, including oocyte maturation and fertilization, Day 3 embryo quality and blastocyst formation?
SUMMARY ANSWER
Higher urinary BPA concentrations were found to be associated with decreased ovarian response, number of fertilized oocytes and decreased blastocyst formation.
WHAT IS KNOWN ALREADY
Experimental animal and in vitro studies have reported associations between BPA exposure and adverse reproductive outcomes. We previously reported an association between urinary BPA and decreased ovarian response [peak serum estradiol (E2) and oocyte count at the time of retrieval] in women undergoing IVF; however, there are limited human data on reproductive health outcomes, such as fertilization and embryo development.
STUDY DESIGN, SIZE AND DURATION
Prospective preconception cohort study. One hundred and seventy-four women aged 18–45 years and undergoing 237 IVF cycles were recruited at the Massachusetts General Hospital Fertility Center, Boston, MA, USA, between November 2004 and August 2010. These women were followed until they either had a live birth or discontinued treatment. Cryothaw and donor egg cycles were not included in the analysis.
PARTICIPANTS/MATERIALS, SETTINGAND METHODS
Urinary BPA concentrations were measured by online solid-phase extraction-high-performance liquid chromatography-isotope dilution-tandem mass spectrometry. Mixed effect models, poisson regression and multivariate logistic regression models were used wherever appropriate to evaluate the association between cycle-specific urinary BPA concentrations and measures of ovarian response, oocyte maturation (metaphase II), fertilization, embryo quality and cleavage rate. We accounted for correlation among multiple IVF cycles in the same woman using generalized estimating equations.
MAIN RESULTS AND THE ROLE OF CHANCE
The geometric mean (SD) for urinary BPA concentrations was 1.50 (2.22) µg/l. After adjustment for age and other potential confounders (Day 3 serum FSH, smoking, BMI), there was a significant linear dose–response association between increased urinary BPA concentrations and decreased number of oocytes (overall and mature), decreased number of normally fertilized oocytes and decreased E2 levels (mean decreases of 40, 253 and 471 pg/ml for urinary BPA quartiles 2, 3 and 4, when compared with the lowest quartile, respectively; P-value for trend = 0.001). The mean number of oocytes and normally fertilized oocytes decreased by 24 and 27%, respectively, for the highest versus the lowest quartile of urinary BPA (trend test P < 0.001 and 0.002, respectively). Women with urinary BPA above the lowest quartile had decreased blastocyst formation (trend test P-value = 0.08).
LIMITATIONS AND REASONS FOR CAUTION
Potential limitations include exposure misclassification due to the very short half-life of BPA and its high variability over time; uncertainty about the generalizability of the results to the general population of women conceiving naturally and limited sample.
WIDER IMPLICATIONS OF THE FINDINGS
The results from this extended study, using IVF as a model to study early reproductive health outcomes in humans, indicate a negative dose–response association between urinary BPA concentrations and serum peak E2 and oocyte yield, confirming our previous findings. In addition, we found significantly decreased metaphase II oocyte count and number of normally fertilizing oocytes and a suggestive association between BPA urinary concentrations and decreased blastocyst formation, thus indicating that BPA may alter reproductive function in susceptible women undergoing IVF.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by grants ES009718 and ES000002 from the National Institute of Environmental Health Sciences and grant OH008578 from the National Institute for Occupational Safety and Health. None of the authors has actual or potential competing financial interests. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Keywords: bisphenol A, oocyte, fertility, human, women, reproduction
Introduction
Bisphenol A (BPA) is a ubiquitous chemical widely used in the manufacture of polycarbonate plastics (Brede et al., 2003; Carwile et al., 2009), epoxy resins and lacquer lining of food and beverage cans (Bae et al., 2002; Carwile et al., 2011). BPA is also used in the manufacture of some dental sealants and composites (Sasaki et al., 2005; Joskow et al., 2006) and has been found in thermal receipt paper (vom Saal and Myers, 2008; Biedermann et al., 2010). The widespread use of BPA-containing consumer products has led to ubiquitous exposure to BPA in the general population (Vandenberg et al., 2007). For example, in the 2003–2004 National Health and Nutrition Examination Survey (NHANES), BPA was detected in over 90% of urine samples obtained from a representative sample of US residents (Calafat et al., 2008). Detectable concentrations of BPA have also been measured in human follicular fluid (1–2 µg/l) and amniotic fluid (1–9 µg/l) (Ikezuki et al., 2002), suggesting that exposure may occur as early as the peri-conception period.
BPA, an estrogenic chemical with a molecular structure similar to that of diethylstilbestrol (Dodds and Lawson, 1936), has been shown to be a developmental and reproductive toxicant in experimental animal studies. A landmark study by Hunt et al. (2003) showed that in vivo exposure of mice to environmentally relevant doses of BPA results in a significant dose-related increase in oocyte aneuploidy. This finding was later investigated in in vitro studies, which showed adverse effects of BPA on meiotic spindle formation, centrosome dynamics and chromosome alignment and segregation (Can et al., 2005; Lenie et al., 2008; Machtinger et al., (2011)). Other in vitro studies using porcine and rat ovarian cells showed that exposure to environmentally relevant doses of BPA resulted in significant concentration-dependent inhibition of estradiol (E2) production (Mlynarcikova et al., 2005; Zhou et al., 2008; Peretz et al., 2011). Experimental animal studies also suggest that BPA exposure adversely impacts female fertility (Tsutsui et al., 1998; Takai et al., 2001; Berger et al., 2007, 2008; Berger et al., 2010).
According to the findings of in vitro and animal studies, the potential human health effects of BPA exposure may include decreased fertility due to disrupted oocyte maturation, E2 suppression and early pregnancy loss caused by chromosomal abnormalities. However, few studies of BPA exposure have focused on human reproduction. In our earlier publication on women undergoing IVF, we examined the association between urinary BPA and measures of ovarian response and found significant negative associations between increased urinary BPA concentrations and decreased peak E2 and oocyte yield on the day of egg retrieval (Mok-Lin et al., 2010). These findings for reduced peak E2 were replicated a year later in another IVF cohort at the University of San Francisco (UCSF) affiliated fertility center in which serum unconjugated BPA was used as a biomarker of exposure instead of urine total BPA: they also found a non-significant decrease in oocyte yield with increased serum unconjugated BPA (Bloom et al., 2011a,b). One concern over serum-free, unconjugated BPA as a biomarker of exposure for BPA during oocyte retrieval is the possibility of contamination from some medical equipment, e.g. during intravenous fluid administration (Calafat et al., 2009; Vandentorren et al., 2011). The comparability of our earlier results (Mok-Lin et al., 2010) with those from UCSF is therefore uncertain. On the basis of these previous studies, in this study we evaluated the association between urinary BPA concentrations and ovarian response and early reproductive outcomes, including oocyte maturation and fertilization, Day 3 embryo quality and cleavage rate and blastocyst formation. These sensitive early reproductive outcomes, all of which are critical to human reproduction, rely on very carefully balanced hormonal triggers and have been shown to be susceptible to the endocrine disrupting properties of BPA in animal and in vitro studies. Our unique study design, using IVF as a model of early reproductive health end-points, provided us with an opportunity to study these potential associations in humans.
Materials and Methods
Study participants and data collection
The present analysis included women undergoing IVF as part of a larger prospective cohort study of the impact of environmental chemicals on fertility and pregnancy outcomes. The study participants were female partners of couples seeking infertility evaluation and treatment at the Massachusetts General Hospital (MGH) Fertility Center Boston, MA USA. Couples were recruited between November 2004 and August 2010 and followed in IVF cycles through to November 2010. During this period of recruitment, the IVF protocols and success rates at the clinic remained unchanged. Women between the ages of 18–45 years who used their own oocytes for IVF were eligible. In this sub-study, only women who had an oocyte retrieval procedure were included in our analyses. Cryothaw and donor egg cycles were not included in this analysis. Women were followed from study entry through each of their IVF cycles until either a live birth or the discontinuation of treatment at the MGH fertility Center. The study was approved by the Institutional Review Boards of the Massachusetts General Hospital, Harvard School of Public Health and the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
At recruitment, following informed consent, a nurse administered a brief questionnaire to collect data on demographics, medical history and lifestyle. Women also completed a detailed take-home questionnaire with additional questions on lifestyle factors, occupation and medical history. Clinical information was obtained from the electronic medical record and infertility diagnoses were assigned according to the Society for Assisted Reproductive Technology (SART) definitions.
Treatment protocols and clinical IVF measures
Measurements of serum FSH on cycle day 3, peak E2 and details of oocyte retrieval have been previously described (Mok-Lin et al., 2010). The subjects underwent one of three IVF treatment protocols, as described in detail previously (Mok-Lin et al., 2010). Briefly, the IVF treatment protocols included: (i) luteal phase GnRH agonist protocol using low-, regular- and high-dose leuprolide (Lupron), (ii) follicular phase GnRH agonist/flare protocol and (iii) GnRH antagonist protocol. The antagonist and flare protocols were primarily used for women who had responded poorly in past IVF cycles; the flare protocol being indicated for women over age 40 years, while the antagonist protocol was assigned to women under age 40 years with diminished ovarian reserve and poor ovarian response.
For patients undergoing ICSI, the number of mature oocytes at metaphase II (MII oocytes) was noted on the day of egg retrieval, prior to performing ICSI, immediately after cumulus cells were stripped from the oocytes with hyaluronic acid (ICSI Cumulase, Origio Medicult Media, Denmark). In conventional IVF cycles, the number of MII oocytes could only be evaluated the day after egg retrieval, ∼ 16–18 h after insemination when the oocytes were stripped and evaluated for fertilization status.
Oocyte maturation was evaluated as both the number of MII oocytes (a count) and the percentage of mature (MII) oocytes among the total number of oocytes retrieved in that cycle, referred to as maturation rate. Similarly, fertilization was evaluated both as the number of normally fertilized oocytes with two pronuclei (a count) and also as the proportion of normally fertilized oocytes (2PN) among the total number of mature (MII) oocytes, otherwise referred to as fertilization rate. Owing to the clinical relevance of the count measures in determining IVF success, we chose to present the model results for count measures rather than rates of maturation and fertilization.
Embryos were evaluated by an embryologist and selected for uterine transfer on Day 2, 3 or 5 of embryo maturation in culture. Normal cell cleavage embryos on Day 3 had 6–8 cells; slow cleavage cell embryos had less than 6 cells, and accelerated cleavage embryos had more than 8 cells. Embryos were graded using a scoring system based on prior work and standardized by the MGH embryology laboratory (Veeck and Zaninovic, 2005). A score of 1 or 2 was considered high quality, 3 was considered intermediate quality and 4 or 5 indicated poor-quality embryos. On Day 5 in culture, the embryos were evaluated and the number of blastocysts formed was determined.
Urine sample collection and urinary BPA concentrations
Women provided up to two spot urine samples per IVF cycle, with the first one (not necessarily a fasting sample) collected between Day 3 and Day 9 of the gonadotrophin phase, and the second one, always a fasting sample, collected on the day of oocyte retrieval, prior to the procedure or administration of intravenous fluids. Urine was collected in a sterile clean polypropylene specimen cup. BPA concentrations were corrected for urine dilution by specific gravity (SG), as described previously (Meeker et al., 2010). SG was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. The urine was divided into aliquots, and frozen and stored at −80°C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40°C until analysis.
The urinary concentrations of the sum of free and conjugated BPA species (total BPA) were measured using online solid-phase extraction (SPE) coupled with isotope dilution-high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS), as described before (Ye et al., 2005). First, 100 µl of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co, St. Louis, MO, USA) to hydrolyze the BPA-conjugated species. BPA was then retained and concentrated on a C18 reversed-phase size-exclusion SPE column (Merck KGaA, Germany), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion-atmospheric pressure chemical ionization-MS/MS. The limit of detection (LOD) for BPA was reported as a single fixed value of 0.4 µg/l. In addition to study samples, each analytical run included low-concentration and high-concentration quality control materials, prepared with spiked pooled human urine, and reagent blanks to assure the accuracy and reliability of the data (Ye et al., 2005). The geometric mean of the SG-adjusted BPA concentrations from two spot urine samples collected during each IVF cycle was used as a measure of cycle-specific urinary BPA concentration. We also fit models adjusting for SG as a covariate. BPA concentrations <LOD were assigned a value equal to the LOD divided by the square root of 2 (Hornung and Reed, 1990) prior to adjustment for urine dilution by SG as described previously (Meeker et al., 2010).
Statistical analysis
The characteristics of the women and the IVF cycles and the outcomes of interest were summarized using means (and SDs) and percentages, as appropriate. The distribution of these geometric mean BPA concentrations was summarized using percentiles.
Mixed effect models were applied to evaluate the association between the SG-adjusted cycle-specific urinary BPA concentration and peak serum E2 concentration. Poisson regression models using a generalized estimating equation (GEE) approach to account for the correlation between repeated cycle outcomes within women were used to evaluate the association between urinary BPA concentrations and the total number of oocytes retrieved, mature MII oocytes as a count and normally fertilized (2PN) oocytes as a count. Both the mixed effect and Poisson regression models used a compound symmetry correlation structure to account for correlation between repeated IVF cycles in the same woman, and adjusted for selected covariates, which could be confounders. To evaluate the association between BPA and rates of oocyte maturation, fertilization, good embryo quality and normally cleaved embryos, we used Poisson regression, implemented via GEE models, accounting for the appropriate denominator of number of oocytes or embryos as the offset. In order to account for later evaluation of oocytes for conventional IVF compared with ICSI oocytes, we explored stratified analyses for MII count, maturation rate and fertilization rate by cycle type (IVF versus ICSI). Since the trends were similar, we combined the two groups (IVF and ICSI) to increase statistical power.
BPA concentrations were first adjusted for SG and then modeled in quartiles. An a priori decision was made to retain age (≥37 versus <37 years) in the final model regardless of statistical significance due to its biological and clinical relevance, and potential risk factors were selected on the basis of evidence from prior literature and clinical practice at the MGH fertility clinic. Covariates were considered as potential confounders if associated with the outcome of interest with P-value < 0.2 in univariate models. Backward selection methods were used to identify variables for inclusion in the multivariate model. Potential confounders included IVF protocol type (flare/antagonist versus regular luteal phase protocol), Day 3 serum FSH level (continuous measure), ever versus never smoker and BMI (as a binary measure: overweight/obese ≥25 kg/m2 versus normal/underweight, BMI <25 kg/m2) (Kligman and Rosenwaks, 2001; Bancsi et al., 2002; Broekmans et al., 2009; Carwile and Michels, 2011; Peretz et al., 2012; Zhang et al., 2010). The characteristics represented by <5% of the cohort were not included as candidate predictors. Variables were retained in the final model if they had P-value ≤ 0.10. A test for trend was performed to determine whether there was a linear dose–response relationship between quartiles of urinary BPA (coded ordinally as 1–4) and the outcomes of interest.
We considered IVF protocol type as potentially being on the causal pathway between urinary BPA concentration and measures of ovarian response. The rationale for this assumption was based on the clinical practice at the fertility clinic where alternative/low responder IVF protocols were administered to patients who responded poorly to the regular luteal phase protocol in a previous IVF cycle (i.e. poor ovarian response). Thus, the alternative protocol is assigned to women with anticipated decreased probability of success in a subsequent treatment cycle. We can also reasonably assume that current urinary BPA concentrations may be associated with past urinary BPA concentrations, provided dietary and lifestyle habits have remained relatively constant. A covariate was considered an intermediate variable if there was a biologically plausible explanation for a temporal association (as evidenced from scientific literature/clinical practice) between the exposure (urinary BPA concentration), intermediate variable (protocol type) and outcome (decreased peak serum E2). Also, we would expect the association between urinary BPA and peak serum E2 to be attenuated when the intermediate variable was included in the multivariate analyses, because of overadjustment (Schisterman et al., 2009). Other methods of mediation analyses have also recently been described (Vanderweele, 2012).We therefore conducted analyses both adjusted and unadjusted for treatment protocol. All data analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). A P-value of < 0.05 was considered statistically significant.
Results
The 174 women included in this analysis were on average 35.6 years old at the time of recruitment into the study; 87% were Caucasian and fewer than 5% were current smokers (Table I). Approximately one-third of the couples had a primary SART diagnosis of female infertility, a third had male factor infertility and a third had unexplained infertility. Almost one-third of the patients were either overweight or obese (BMI ≥25 kg/m2). The women underwent a total of 237 IVF cycles and the primary treatment protocol was the luteal phase GnRH agonist protocol for 60% of cycles (Table II).
Table I.
Demographics and infertility diagnoses among 174 women undergoing IVF.
| Characteristic | Total n (%) |
|---|---|
| Age (years) mean ± SD (range) | 35.6 ± 4.0 (21–44) |
| Age ≥37 (years) | 69 (40) |
| BMIa (kg/m2) mean ± SD (range) | 24.5 ± 4.7 (16.5–42.3) |
| BMI ≥25 | 56 (32) |
| Race | |
| White | 152 (87) |
| Black/African American | 5 (3) |
| Asian | 10 (6) |
| Hispanic/other | 7 (4) |
| Smoking | |
| Never-smoker | 127 (73) |
| Ever-smoker | 47 (27) |
| Current smoker | 6 (4) |
| Former smoker | 41 (24) |
| SART diagnosisb | |
| Female factor | 56 (33) |
| Diminished ovarian reserve | 13 (8) |
| Ovulation disorders | 18 (11) |
| Endometriosis | 12 (7) |
| Uterine disorders | 2 (1) |
| Tubal factor | 11 (6) |
| Male infertility | 63 (37) |
| Unexplained | 48 (28) |
| Other | 4 (2) |
SART, Society for Assisted Reproductive Technology.
aBMI missing for one woman.
bPrimary diagnosis of infertility (three missing values).
Table II.
Treatment protocols, cycle characteristics and pregnancy outcomes for 237 IVF cycles among 174 women.
| Cycle-specific characteristics | Among 237 IVF cycles, n (%) |
|---|---|
| IVF protocola | |
| Luteal phase | 143 (60) |
| LDLL | 135 (57) |
| RDLL | 8 (3) |
| Flare | 58 (25) |
| Antagonist | 35 (15) |
| Mean ± SD (range) | |
| Day 3 FSH (IU/l) | 7.1 ± 2.3 (0.2–15.2) |
| Peak E2 (pg/ml) | 2083 ± 902.1 (551–5263) |
| Oocytes retrieveda | 10.8 ± 5.3 (1–29) |
| Mature (MII) oocytes | 9.2 ± 4.5 (0–24) |
| Normal (2PN) fertilized oocytes | 6.5 ± 3.6 (0–21) |
| Mean (SD) | |
| Fertilization rate (2PN/MII oocytes)a | 0.71 (0.22) |
| Maturation rate (MII/oocytes retrieved)a | 0.87 (0.14) |
| Embryo-specific characteristics | Among 1449 embryos n (%) |
| Day 3 embryos (cleavage rate) | |
| No cleavage | 13 (1) |
| Slow (2–5 cells) | 426 (29) |
| Normal (6–8 cells) | 926 (64) |
| Accelerated (>8 cells) | 84 (6) |
| Day 3 embryo quality, among 1389 embryos | |
| Poor | 295 (21) |
| Intermediate | 322 (23) |
| High | 772 (56) |
| Blastocyst formation, among 1143 embryos | 361 (32) |
E2, estradiol; MII, metaphase II oocyte; LDLL, low-dose leuprolide lupron; RDLL, regular-dose leuprolide lupron.
a236 cycles; Owing to rounding, percentage totals may not sum to 100%.
Few women had their oocyte retrieval procedure cancelled (n = 9). The cycle-specific urine BPA concentration for these nine cycles was fairly evenly distributed: three of these cycles were in the lowest quartile of urine BPA concentration; one cycle was in quartile 2; two cycles were in quartile 3 and three cycles were in quartile 4. It, therefore, does not appear that selective loss of the most highly exposed women or those who did not have oocyte retrieval in relation to BPA quartile is relevant to this cohort.
Forty-four women (25%) contributed data from two or more IVF cycles. We collected two urine samples within an IVF cycle for 82% of cycles, yielding a total of 429 urine samples over the 237 IVF cycles. The cycle-specific SG-adjusted BPA ranged from below the LOD to 26.5 µg/l, with a geometric mean [nterquartile range (IQR) of 2.32 (1.60–3.77) µg/l]. The urinary BPA concentrations for the non-SG-adjusted BPA concentrations were slightly lower than that of the general US population concentrations with a geometric mean (IQR) of 1.50 (0.85–2.47) µg/l compared with 1.97 µg/l for females in NHANES 2007–2008 (CDC February 2011). It is possible that the NHANES female subgroup included girls that had higher urine BPA concentrations than the older women, thus explaining the slightly higher median BPA concentration for the general US women compared with our IVF cohort. Detectable concentrations of BPA were measured in 88% of individual urine samples analyzed, and 51 individual urine samples had BPA concentrations below the LOD (0.4 µg/l). All the results presented are for SG-standardized urinary BPA concentrations. However, in sensitivity analyses adjusting for SG by including it as a covariate rather than standardizing BPA by SG, similar results were obtained (see Supplementary data, Table SI).
We observed a significant decrease in peak serum E2 levels with increasing quartiles of urinary BPA concentrations (trend test P < 0.001), with a decrease of ∼470 pg/ml E2 for women in the highest quartile of urinary SG-adjusted BPA concentration compared with women in the lowest quartile, after adjusting for age, BMI and Day 3 FSH (Table III). This is the equivalent of a clinically and statistically significant average decrease of 22% in the serum peak E2 level (average serum E2 was 2083 pg/ml). This dose–response trend remained after further adjustment for treatment protocol, although the association with the fourth quartile of urinary BPA was mildly attenuated. There was a moderate correlation between peak serum E2 and number of oocytes retrieved (Spearman r = 0.60). Thus, not surprisingly, dose–response associations were observed between quartiles of urinary BPA concentrations and number of oocytes retrieved per cycle. After adjustment for age, BMI and serum FSH measured on cycle day 3 (Day 3 FSH), there were estimated mean percentage decreases in oocyte count of 1, 6 and 24% for urinary BPA quartiles 2, 3 and 4, respectively, compared with the lowest BPA quartile (see Table IV). An average of 12 oocytes were retrieved per cycle for women in the lowest BPA quartile, and thus women in the fourth quartile of urinary BPA concentration had on average three less oocytes retrieved per cycle than women in the lowest urinary BPA quartile. As part of a sensitivity analysis, we repeated the statistical models on the subset of women that were not included in our original publication, and the results were essentially the same as in the full set of women (see Supplementary data, Table SII). Similar magnitudes of decreased percentages of mature MII oocytes retrieved and normally fertilized oocytes were observed (Table IV), equivalent to approximately two fewer normally fertilized oocytes on average for those in the highest versus the lowest BPA quartile. When the analyses were restricted to the 152 Caucasian women who underwent 207 IVF cycles, the results were very similar to those of the entire study population (i.e. Caucasian and non-Caucasian).
Table III.
Estimated change in peak E2 (pg/ml) by urinary BPA quartiles among 174 women (237 IVF cycles).
| Covariates | Unadjusted |
Adjusteda (n = 173 women, 236 cycles) |
Protocol adjustedb (n = 172 women, 235 cycles) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Change in mean | 95% CI | P-value | Change in mean | (95% CI) | P-value | Change in mean | (95% CI) | P-value | |
| BPA quartiles (range µg/l) | |||||||||
| Q1 (≤1.60) | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 (1.61–2.32) | −64 | −370, 242 | 0.68 | −40 | −343, 262 | 0.79 | −38 | −340, 264 | 0.80 |
| Q3 (2.33–3.76) | −244 | −552, 64 | 0.12 | −253 | −556, 49 | 0.10 | −255 | −556, 47 | 0.10 |
| Q4 (≥3.77) | −441 | −753, −130 | 0.006 | −471 | −776, −165 | 0.003 | −408 | −719, −97 | 0.01 |
| Test for trend (P-value)c | 0.004 | 0.001 | 0.005 | ||||||
| Age (≥37 versus <37 years) | −201 | −454, 52 | 0.12 | −144 | −386, 98 | 0.24 | −25 | −284, 234 | 0.85 |
| BMI* (≥25 versus <25 kg/m2) | −246 | −506, 14 | 0.06 | −252 | −506, 0.8 | 0.05 | −244 | −493, 5.4 | 0.06 |
| Day 3 FSH (≥12 versus <12 IU/l) | −604 | −1304, 97 | 0.09 | −597 | −1272, 72 | 0.08 | −495 | −1167, 178 | 0.15 |
| IVF protocol (flare/ant. versus luteal) | −425 | −662, −188 | <0.001 | — | — | — | −302 | −564, −40 | 0.02 |
| Ever versus never smoker | −32 | −313, 249 | 0.82 | — | — | — | — | — | — |
BPA, Bisphenol A; Day 3 FSH, FSH on 3rd day of menses, Q1, first quartile; Q2, second quartile, Q3, third quartile; Q4, fourth quartile, 95% CI, 95% confidence intervals; flare/ant., flare or GnRH antagonist protocol *1 missing value.
aAdjusted for age, BMI, Day 3 FSH, using mixed models to account for correlated outcomes.
bAdjusted for age, BMI, Day 3 FSH, protocol (flare/antagonist versus regular luteal phase protocol).
cTest for linear trend based on ordinal quartiles.
Table IV.
Estimated percentage change in mean number of oocytes retrieved, mean number of mature oocytes (MII) and normally fertilized (2PN) oocytes by BPA quartiles and covariates (n = 174 women, 237 IVF cycles).
| Covariates | Percentage change in number of oocytes retrieved |
Percentage change in number of MII oocytes |
Percentage change in 2PN oocytes |
|||
|---|---|---|---|---|---|---|
| Unadjusted (95% CI) | Adjusteda (95% CI) | Unadjusted (95% CI) | Adjusteda (95% CI) | Unadjusted (95% CI) | Adjusteda (95% CI) | |
| BPA quartiles (range µg/l) | ||||||
| Q1 (≤1.60) | Ref | Ref | Ref | Ref | Ref | Ref |
| Q2 (1.61–2.32) | 1% (−13%, 17%) | −1% (−15%, 15%) | −6% (−20%, 9%) | −9% (−21%, 6%) | −7% (−23%, 12%) | −9% (−25%, 10%) |
| Q3 (2.33–3.76) | −4% (−18%, 13%) | −6% (−20%, 10%) | −8% (−22%, 8%) | −10% (−24%, 5%) | −10% (−26%, 8%) | −13% (−27%, 5%) |
| Q4 (≥3.77) | −21% (−32%, −8%) | −24% (−34%, −11%) | −27% (−38%, −14%) | −29% (−39%, −17%) | −27% (−39%, −11%) | −28% (−40%, −13%) |
| Test for trend (P-value) | 0.003 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 |
| Age (≥37 versus <37 year) | −18% (−29%, −6%) | −17% (−27%, −4%) | −16% (−27%, −4%) | −15% (−25%, −3%) | −19% (−30%, −5%) | −17% (−28%, −4%) |
| BMI (≥25 versus <25 kg/m2) | 3% (−11%, 18%) | 2% (−11%, 17%) | 3% (−10%, 18%) | 4% (−9%, 18%) | −2% (−16%, 15%) | −1% (−15%, 16%) |
| Day 3 FSH (≥12 versus <12 IU/l) | −49% (−61%, −33%) | −49% (−61%, −34%) | −46% (−63%, −23%) | −45% (−61%, −21%) | −49% (−64%, −25%) | −47% (−60%, −29%) |
| IVF protocol (flare/ant. versus luteal) | −36% (−44%, −27%) | — | −34% (−42%, −24%) | — | −35% (−43%, −25%) | — |
| Ever versus never smoker | −2% (−15%, 14%) | — | −6% (−19%, 8%) | — | 0% (−15%, 17%) | — |
Ref., reference group.
aAdjusted for age, BMI, Day 3 FSH.
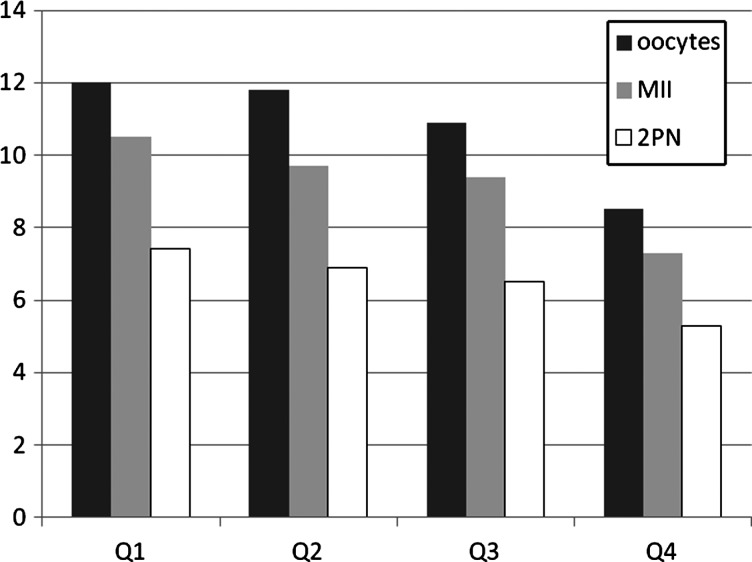
In models that considered oocyte maturation and fertilization as a proportion of total oocytes retrieved and of mature (MII) oocytes, respectively, no consistent dose–response associations were found. No significant change in fertilization rate was observed over the quartiles of urinary BPA concentration (see Fig. 1).
Figure 1.
Distribution of total number of oocytes retrieved, mature (MII) oocytes and normally fertilized (2PN) oocytes by quartiles of SG-adjusted urinary BPA concentrations. BPA, bisphenol A; SG, specific gravity; MII, mature oocytes at meiotic stage II; 2PN, fertilized egg with two pronuclei. Q1, lowest quartile (≤1.60 µg/l BPA); Q2, second quartile (1.61–2.32 µg/l BPA); Q3, third quartile (2.33–3.76 µg/l BPA); Q4, fourth quartile (≥3.77 µg/l BPA).
On Day 3 after oocyte retrieval, 1449 embryos from 169 women who underwent 222 IVF cycles were evaluated for cleavage rate and embryo quality. Oocytes that failed to fertilize had a Day-2 embryo transfer, and embryos with arrested development or with missing information because of difficulty in assessing the number of cells were excluded from the embryo analyses and in many cases discarded embryos were not graded (n = 1110 embryos/oocytes). After adjusting for age and Day-3 embryo transfer, a negative dose–response association was found for blastocyst formation on Day 5, with estimated mean decreases of 24–32% for those in the second, third or fourth quartile compared with those in the lowest quartile (trend test P-value = 0.08). There was no dose–response association between urinary BPA concentrations and Day 3 embryo quality, after adjusting for age. The incidence rate ratio of poor quality embryos in the second, third and fourth BPA quartiles compared with the lowest quartile, were = 1.43 [95% confidence interval (CI):1.01, 2.01], 0.89 (0.60, 1.32) and 1.05 (0.73, 1.51), respectively. Also, no significant associations were observed between urinary BPA quartiles and the cleavage rate for Day 3 embryos. None of the additional covariates (age, BMI, Day 3 FSH and protocol) included in our multivariate analyses acted as intermediate variables or confounders in this study cohort.
Alternative modeling approaches, such as non-linear mixed effect models, may be preferable as they better account for informative missingness. However, they could not be applied successfully given the limited sample size. Results based on only first cycle, however, were consistent with those presented.
Discussion
In the present study, urinary BPA concentrations were negatively associated with measures of ovarian response, which were defined as number of oocytes retrieved per cycle and peak serum E2 concentrations on the day of HCG administration. Serum E2 and oocyte yield during controlled ovarian hyperstimulation are important predictors of successful IVF outcomes (Orvieto et al., 2007; Scotchie et al., 2009). A decrease in both these parameters could impact the outcome of an IVF treatment cycle. Our current findings are consistent with those of previous analyses conducted on a smaller subset (n = 84 women, 112 IVF cycles) (Mok-lin et al., 2010) of our MGH IVF cohort. Previously, we reported an average decrease of 213 pg/ml in peak E2 per log unit increase in urine total BPA as well as an average 12% decrease in the number of oocytes retrieved per log unit increase in urine total BPA.
In a cohort of 44 women undergoing a first IVF or ICSI cycle at the UCSF-affiliated fertility center, Bloom et al. (2011a) reported similar findings for associations of BPA with some of the ovarian response results. For each log unit increase in serum unconjugated BPA, they found decreases of 106 and 50 pg/ml in peak E2 and peak E2 per mature-sized follicle, respectively, and a non-significant average decrease of 9% in number of oocytes retrieved. It is important to note that they measured serum unconjugated BPA, while we measured total urinary BPA concentration. Because it is unclear how well these two biomarkers of exposure correlate in terms of internal BPA concentrations at a given window of exposure, it is hard to draw direct dose–response comparisons between these two studies.
While there is a paucity of human data, experimental animal and in vitro studies have consistently observed decreased ovarian E2 synthesis with higher BPA levels (Mlynarcikova et al., 2005; Zhou et al., 2008; Peretz et al., 2011; Xu et al., 2002). An in vitro study in rat ovarian granulosa cells demonstrated a dose-dependent inhibitory effect of BPA (10−6–10−4 m) on E2 production as well as decreased expression of Cyp19 messenger RNA (mRNA), and cytochrome P450 aromatase (Zhou et al., 2008). More recently, Peretz et al. (2011) used an in vitro mouse follicle culture system and administered BPA concentrations of 44 and 440 μM. They found decreased E2 production in ovarian antral follicles with corresponding decreased mRNA expression of steroidogenic acute regulatory protein and cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc). Xu et al. (2002) showed that murine ovarian granulosa cells cultured with 100 μM BPA for 24–72 h had decreased viability and increased apoptosis in a dose- and time-dependent relationship. They demonstrated that BPA administration (100 μm) increased the protein expression and mRNA levels of Bcl-2 associated X protein (Bax/pro-apoptotic) and decreased those of Bc1-2 (anti-apoptotic) genes. An imbalance of these genes could result in increased cell death, resulting in granulosa cell apoptosis and follicular atresia (Hughes and Gorospe, 1991; Yu et al., 2004). These results suggest that BPA may antagonize the anti-apoptotic effect of endogenous estrogens synthesized by granulosa cells.
In addition to evaluating associations between urinary BPA and ovarian response, we examined associations between urinary BPA concentrations and oocyte maturation and fertilization. Significant decreases in both the average number of mature MII oocytes and number of normally fertilizing oocytes per IVF treatment cycle were observed in relation to urinary BPA concentrations. In conventional IVF cycles, the number of MII oocytes was evaluated the day after egg retrieval, ∼ 16–18 h after insemination, when the oocytes were stripped and evaluated for fertilization status. It is possible that in vitro maturation may occur in this time interval and consequently there may be an overestimate in the number of MII oocytes observed in the IVF relative to the ICSI cycles, leading to an underestimate in the fertilization rate in IVF cycles. However, when we stratified by IVF and ICSI cycles, the trends for the association between both urinary BPA concentrations and number of MII oocytes and that between urinary BPA concentrations and fertilization rates were similar for IVF and ICSI subgroups. We therefore, combined the two groups to increase our statistical power. Fujimoto et al. (2011) reported suggestive associations between higher serum BPA and decreased fertilization rate among 26 women who underwent IVF or ICSI; however, no association was found for oocyte maturation rate. Since they did not model these outcomes as counts, we cannot fully compare our results.
Other outcomes we examined were embryo cleavage rate, embryo quality on Day 3 and blastocyst formation on Day 5. Although we did not observe an association between urinary BPA concentrations and embryo quality, it is possible that there may have been bias to the null. For instance, if oocytes that did not reach embryo status due to failed fertilization or arrested development were associated with higher BPA concentration, this could have resulted in an underestimate of the BPA and embryo quality association. In the UCSF IVF cohort, no associations between female serum unconjugated BPA and similar parameters of embryo quality on Day 3 in culture were seen among 36 women undergoing IVF treatment (Bloom et al., 2011b). However, they did observe inverse associations for male BPA with embryo cell number and embryo fragmentation score, which they used as indicators of embryo quality.
One potential limitation in our study is the uncertainty regarding the generalizability of the results to the general population of women conceiving naturally. It is possible that women undergoing IVF may be more sensitive to BPA exposure for a variety of reasons, including their underlying infertility, the in vitro conditions of early embryonic development or the ovarian hyperstimulation protocols. At the same time, one strength of the study is the ability to use assisted reproduction technologies as a model to study BPA and early developmental outcomes in humans. Numerous animal and in vitro studies have shown these early end-points to be particularly susceptible to the endocrine disrupting properties of this very ubiquitous chemical (Takai et al., 2000; Hunt et al., 2003; Berger et al., 2007, 2008, 2010). It was therefore of interest to examine this potential association in humans. Furthermore, because ∼10–15% of the population in the USA and developed countries are infertile (Fritz and Speroff, 2010), the results of the present study are relevant to this substantial portion of the general population.
The open prospective cohort study design provided us with many challenges and strengths. We collected repeated urine samples to categorize exposure information prior to the outcome of interest, facilitating evaluation of temporal associations. However, when we collected two urine samples per treatment cycle, it is possible that we missed the relevant window of exposure because BPA is a short half-life chemical that is almost completely excreted in the urine within 24 h of exposure. Depending on the participant's diet and lifestyle habits, within-individual variability in urinary BPA concentrations can be high. Recently, BPA was shown to have a low intra-class correlation coefficient (ICC) of 0.23 for urines collected from women in this study cohort before pregnancy (ICC: 0.12 during pregnancy) (Braun et al., 2012). Thus, to minimize possible exposure misclassification (Braun et al., 2011; Ye et al., 2011), we calculated the geometric mean of two urine BPA concentrations collected over a period of ∼2 weeks. The exact timing of urine collection was dependent on the date of medical appointments and egg retrieval procedure. Therefore, the early cycle urine samples were not necessarily first morning or fasting urine samples. We assume that lifestyle habits will not change dramatically over short time intervals and the cycle-specific urinary BPA concentration is a good approximation of exposure. Ideally, daily urine samples would have been better but this is not financially or practically feasible. While our sample size was relatively small, to the best of our knowledge, this is the largest prospective IVF study designed to evaluate the associations between BPA and early reproductive health outcomes.
Conclusion
Women undergoing IVF who had higher urinary BPA concentrations had significantly lower serum peak E2, oocyte yield, MII oocyte count and number of normally fertilizing oocytes. There was a suggestive association between BPA urinary concentrations and decreased blastocyst formation. As recruitment continues and our sample size increases, we plan to extend these analyses to explore the contribution of the male partner's exposure to BPA on reproductive end-points from as early as fertilization through to blastocyst formation.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors’ roles
Substantial contributions to conception and design [R.H. (PI), S.E., P.L.W.)], or acquisition of data (J.C.P., D.W., A.M.C., X.Y., S.E. and R.H.) or analysis and interpretation of data (S.E., P.L.W., S.A.M., J.A.F. and R.H.), drafting the article or revising it critically for important intellectual content (all authors) and final approval of the version to be published (all authors).
Funding
This work was supported by grants ES009718 and ES000002 from the National Institute of Environmental Health Sciences and grant OH008578 from the National Institute for Occupational Safety and Health.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We thank the research team at the Hauser lab, MGH staff and Xiaoliu Zhou, Ryan Hennings and Lily Jia at the Centers for Disease Control and Prevention for technical assistance in measuring urinary BPA. Finally, special thanks to the patients who participated in the study while undergoing their IVF treatment cycles.
References
- Bae B, Jeong JH, Lee SJ. The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water Sci Technol. 2002;46:381–387. [PubMed] [Google Scholar]
- Bancsi LF, Broekmans FJ, Eijkemans MJ, de Jong FH, Habbema JD, te Velde ER. Predictors of poor ovarian response in in vitro fertilization: a prospective study comparing basal markers of ovarian reserve. Fertil Steril. 2002;77:328–336. doi: 10.1016/s0015-0282(01)02983-1. doi:10.1016/S0015-0282(01)02983-1. [DOI] [PubMed] [Google Scholar]
- Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod Toxicol. 2010;30:393–400. doi: 10.1016/j.reprotox.2010.06.006. doi:10.1016/j.reprotox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Berger RG, Hancock T, deCatanzaro D. Influence of oral and subcutaneous bisphenol-A on intrauterine implantation of fertilized ova in inseminated female mice. Reprod Toxicol. 2007;23:138–144. doi: 10.1016/j.reprotox.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Berger RG, Shaw J, deCatanzaro D. Impact of acute bisphenol-A exposure upon intrauterine implantation of fertilized ova and urinary levels of progesterone and 17beta-estradiol. Reprod Toxicol. 2008;26:94–99. doi: 10.1016/j.reprotox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–576. doi: 10.1007/s00216-010-3936-9. doi:10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Bloom MS, Kim D, vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril. 2011a;96:672–677. doi: 10.1016/j.fertnstert.2011.06.063. doi:10.1016/j.fertnstert.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, vom Saal FS, Kim D, Taylor JA, Lamb JD, Fujimoto VY. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environ Toxicol Pharmacol. 2011b;32:319–323. doi: 10.1016/j.etap.2011.06.003. doi:10.1016/j.etap.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–137. doi: 10.1289/ehp.1002366. doi:10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. doi:10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20:684–689. doi: 10.1080/0265203031000119061. doi:10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. doi:10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. doi:10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod. 2005;11:389–396. doi: 10.1093/molehr/gah179. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117:1368–1372. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. J Am Med Assoc. 2011;306:2218–2220. doi: 10.1001/jama.2011.1721. doi:10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ Res. 2011;111:825–830. doi: 10.1016/j.envres.2011.05.014. Epub 2011 Jun 14 doi:10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals- Updated tables. 2012. http://www.cdc.gov/exposurereport/ (5 March 2012, date last accessed) [PubMed]
- Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. doi:10.1038/137996a0. [Google Scholar]
- Fritz MA, Speroff L. Clinical Gynecologic Endocrinology and Infertility. Philadelphia: Lippincott Wilkins and Williams; 2011. [Google Scholar]
- Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril. 2011;95:1816–1819. doi: 10.1016/j.fertnstert.2010.11.008. doi:10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. doi:10.1080/1047322X.1990.10389587. [Google Scholar]
- Hughes FM, Jr, Gorospe WC. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology. 1991;129:2415–2422. doi: 10.1210/endo-129-5-2415. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. doi:10.1016/S0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. doi:10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J Am Dent Assoc. 2006;137:353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- Kligman I, Rosenwaks Z. Differentiating clinical profiles: predicting good responders, poor responders, and hyperresponders. Fertil Steril. 2001;76:1185–1190. doi: 10.1016/s0015-0282(01)02893-x. doi:10.1016/S0015-0282(01)02893-X. [DOI] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Hauser R, Combelles C, Racowsky C. The impact of bisphenol A (BPA) on human oocyte meiotic maturation. Fertil Steril. 2011;96:S3. [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, Ye X, Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. doi:10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. doi:10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto R, Zohav E, Scharf S, Rabinson J, Meltcer S, Anteby EY, Homburg R. The influence of estradiol/follicle and estradiol/oocyte ratios on the outcome of controlled ovarian stimulation for in vitro fertilization. Gynecol Endocrinol. 2007;23:72–75. doi: 10.1080/09513590601137137. [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernández-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–217. doi: 10.1093/toxsci/kfq319. doi:10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Craig ZR, Flaws JA. Bisphenol A. Inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.101899. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med. 2005;16:297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. doi:10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotchie JG, Will M, Fritz MA, Steiner AZ. Predictors and implications of estradiol rise after gonadotropin-releasing hormone antagonist initiation during in vitro fertilization cycles. J Reprod Med. 2009;54:211–217. [PubMed] [Google Scholar]
- Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, Yano T, Taketani Y. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000;270:918–921. doi: 10.1006/bbrc.2000.2548. doi:10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- Takai Y, Tsutsumi O, Ikezuki Y, Kamei Y, Osuga Y, Yano T. Preimplantation exposure to bisphenol A advances postnatal development. Reprod Toxicol. 2001;15:71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, Yamaguchi F, Barrett JC. Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer. 1998;75:290–294. doi: 10.1002/(sici)1097-0215(19980119)75:2<290::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. doi:10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandentorren S, Zeman F, Morin L, Sarter H, Bidondo ML, Oleko A, Leridon H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: implications for large-scale biomonitoring studies. Environ Res. 2011;111:761–764. doi: 10.1016/j.envres.2011.05.018. doi:10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Vanderweele TJ. Mediation analysis with multiple versions of the mediator. Epidemiology. 2012;23:454–463. doi: 10.1097/EDE.0b013e31824d5fe7. doi:10.1097/EDE.0b013e31824d5fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeck L, Zaninovic N. An Atlas of Human Blastocysts. Boca Raton: Parthenon Publishing Group; 2003. [Google Scholar]
- Vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. J Am Med Assoc. 2008;300:1353–1355. doi: 10.1001/jama.300.11.1353. doi:10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, et al. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun. 2002;29:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. doi:10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. Variability of urinary concentrations of bisphenol a in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect. 2011;119:983–988. doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YS, Sui HS, Han ZB, Li W, Luo MJ, Tan JH. Apoptosis in Granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Research. 2004;14:341–346. doi: 10.1038/sj.cr.7290234. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhu Y, Gao H, Zhou B, Zhang R, Wang T, Ding G, Qu F, Huang H, Lu X. Overweight and obesity negatively affect the outcomes of ovarian stimulation and in vitro fertilisation: a cohort study of 2628 Chinese women. Gynecol Endocrinol. 2010;26:325–332. doi: 10.3109/09513591003632100. doi:10.3109/09513591003632100. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.