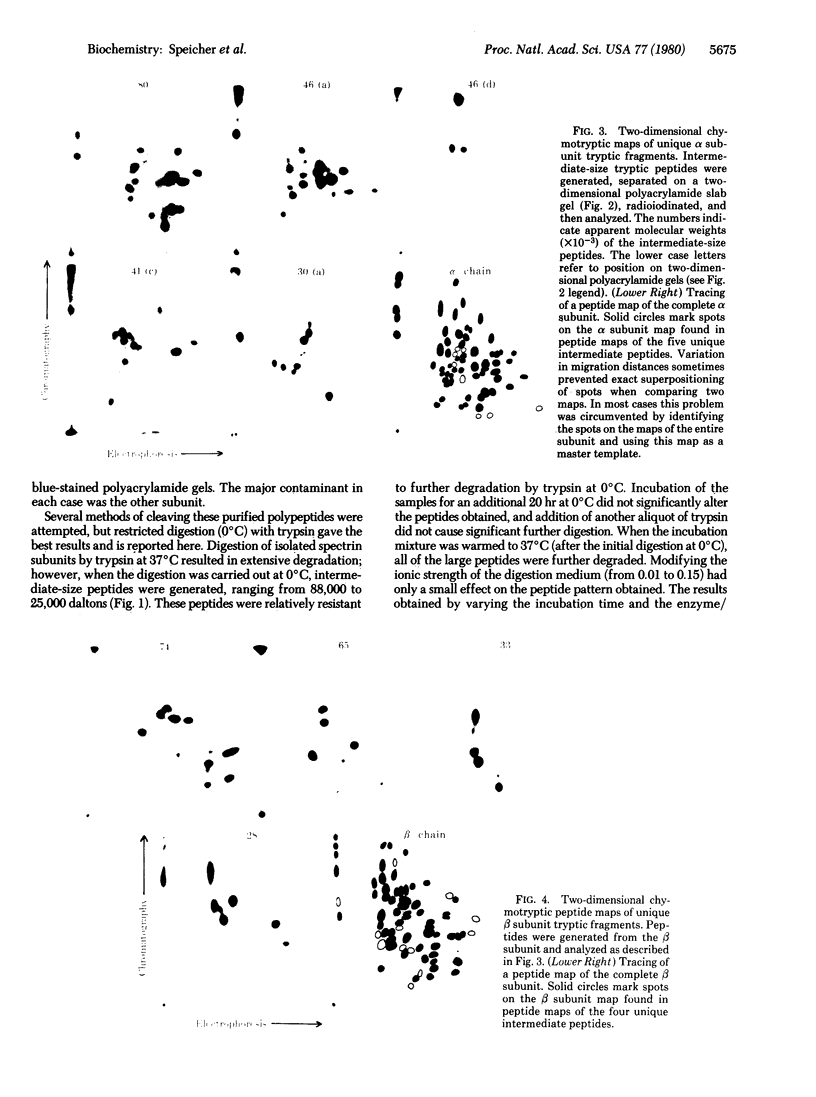
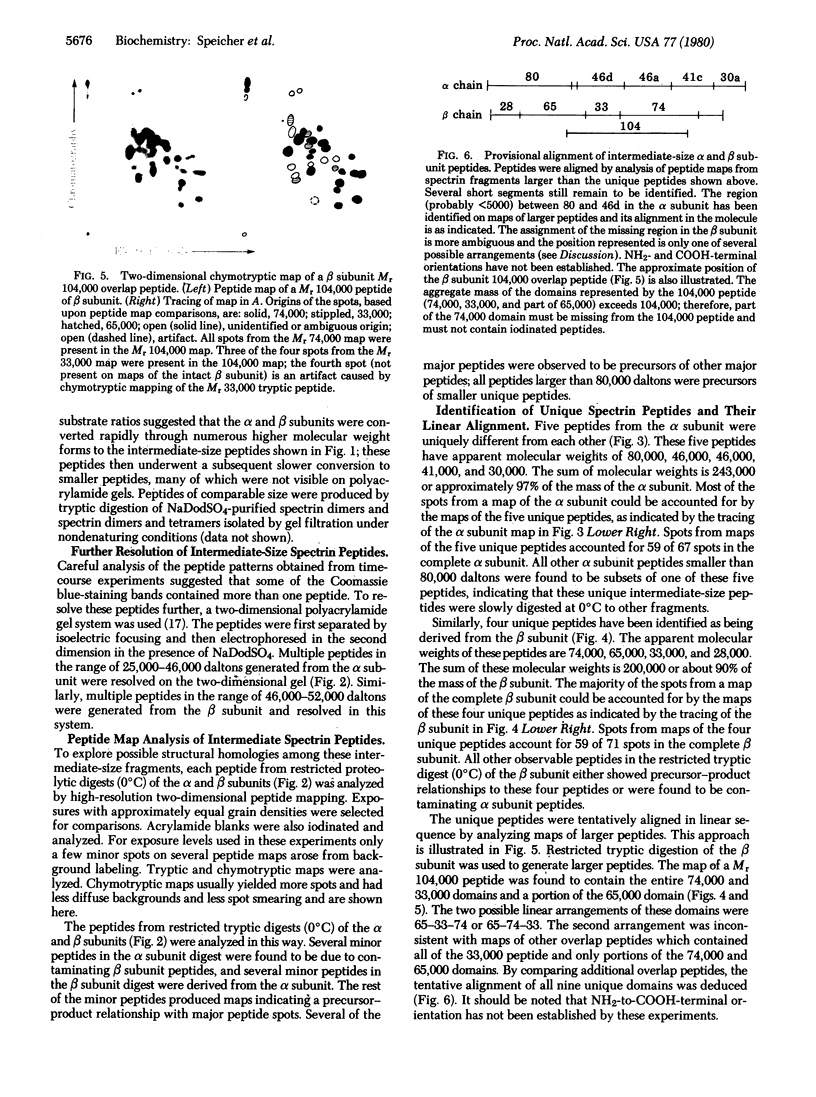
Abstract
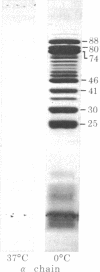
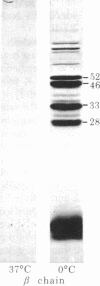
Digestion of purified human erthrocyte spectrin with proteolytic enzymes at 0 degrees C results in the production of intermediate-size peptides that resist further cleavage at 0 degrees C. By two-dimensional peptide analysis of these intermediate peptides it has been determined that five unique peptides are produced by tryptic cleavage of the alpha subunit of spectrin (band 1); these have apparent molecular weights of 80,000, 46,000, 46,000, 41,000, and 30,000 and account for 97% of the alpha subunit. Similarly, four unique peptides having apparent molecular weights of 74,000, 65,000, 33,000, and 38,000 account for 90% of the beta subunit (band 2). By examining larger peptide fragments, the linear alignment of the unique peptides along each of the spectrin subunits has been established. These results indicate that spectrin is composed of two nonidentical subunits, each containing multiple proteolytically resistant domains. These domains, which may be largely alpha-helical, seem to be connected by small protease-sensitive segments. The proteolytic resistance of these domains is not influenced by the multimeric state of the spectrin molecule.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Structural studies on human spectrin. Comparison of subunits and fragmentation of native spectrin. J Biol Chem. 1979 Feb 10;254(3):939–944. [PubMed] [Google Scholar]
- Dunn M. J., Maddy A. H. The molecular status of the large polypeptides of erythrocyte membranes. FEBS Lett. 1973 Oct 1;36(1):79–82. doi: 10.1016/0014-5793(73)80341-2. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fuller G. M., Boughter J. M., Morazzani M. Evidence for multiple polypeptide chains in the membrane protein spectrin. Biochemistry. 1974 Jul 16;13(15):3036–3041. doi: 10.1021/bi00712a006. [DOI] [PubMed] [Google Scholar]
- Hsu C. J., Lemay A., Eshdat Y., Marchesi V. T. Substructure of human erythrocyte spectrin. J Supramol Struct. 1979;10(2):227–239. doi: 10.1002/jss.400100212. [DOI] [PubMed] [Google Scholar]
- Hudson J. R., Ralston G. B. Quantitative analysis of the amino-terminal residues of spectrin by use of the transamination reaction. Biochim Biophys Acta. 1978 Aug 21;535(2):169–177. doi: 10.1016/0005-2795(78)90082-x. [DOI] [PubMed] [Google Scholar]
- Knufermann H., Bhakdi S., Schmidt-Ullrich R., Wallach D. F. N-terminal amino acid analysis reveal peptide heterogeneity in major electrophoretic protein components of erythrocyte ghosts. Biochim Biophys Acta. 1973 Dec 22;330(3):356–361. doi: 10.1016/0005-2736(73)90246-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Kidd G. H., Branton D. Identification by peptide analysis of the spectrin-binding protein in human erythrocytes. J Biol Chem. 1979 Apr 10;254(7):2526–2532. [PubMed] [Google Scholar]
- Maddy A. H., Dunn M. J. Analysis of the major polypeptides of spectrin by tryptic digestion. J Supramol Struct. 1978;8(4):465–471. doi: 10.1002/jss.400080409. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Spectrin: present status of a putative cyto-skeletal protein of the red cell membrane. J Membr Biol. 1979 Dec 14;51(2):101–131. doi: 10.1007/BF01869164. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Yu J. Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct. 1973;1(3):220–232. doi: 10.1002/jss.400010307. [DOI] [PubMed] [Google Scholar]
- Zweig S. E., Singer S. J. The two components of spectrin, filamin, and the heavy chain of smooth muscle myosin show no detectable homologies to one another by two-dimensional mapping of iodinated tryptic peptides. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1147–1152. doi: 10.1016/0006-291x(79)91528-6. [DOI] [PubMed] [Google Scholar]