Abstract
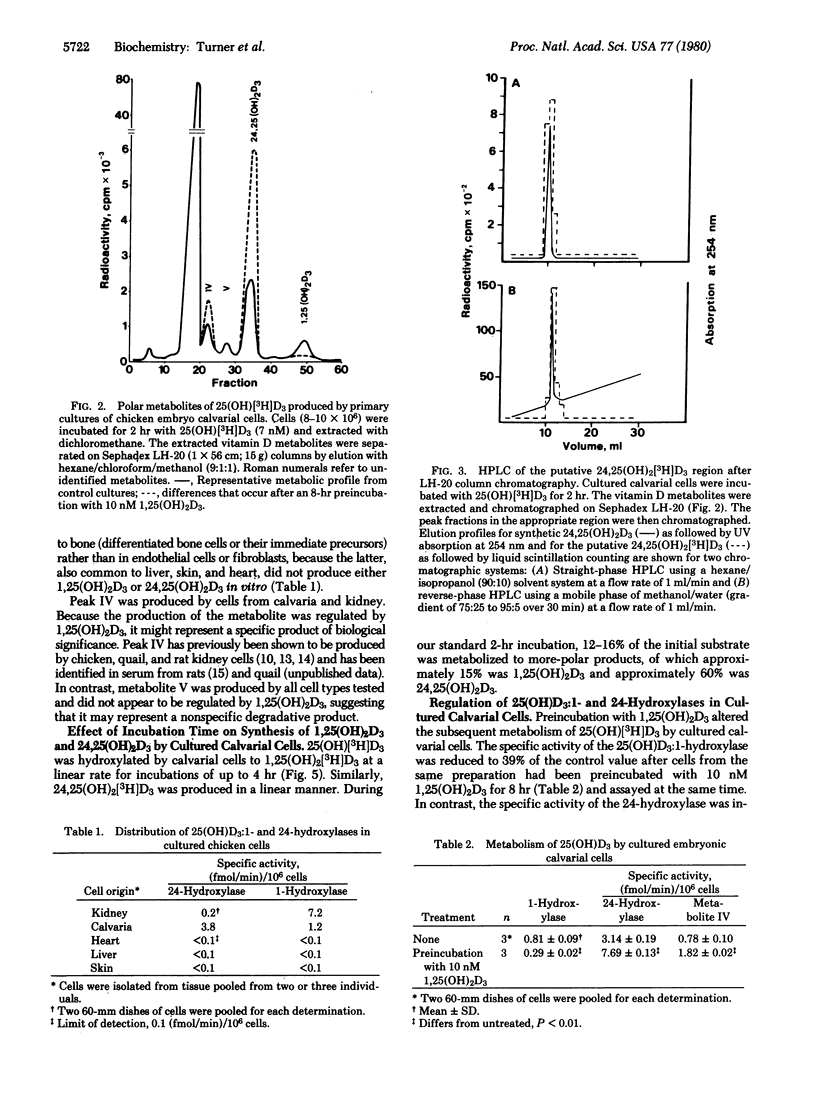
The question of whether the skeleton metabolizes 25-hydroxycholecalciferol [25(OH)D3] to more-polar products was studied. Calvarial cells were dispersed from 16-day old chicken embryos by using collagenase and then grown in culture in serum-free medium. Confluent cell cultures were incubated with 7 nM 25(OH)[3H]D3 for 2 hr, and the vitamin D metabolites were then extracted. At least four polar metabolites were produced. Based on separation by Sephadex LH-20 chromatography followed by high-pressure liquid chromatography, two of these metabolites were identified as 1,25-dihydroxycholecalciferol [1,25(OH)2D3] and 24,25-dihydroxycholecalciferol [24,25(OH)2D3]. These metabolites were also produced by cultured kidney cells but not by liver, heart muscle, or skin cells isolated from the same embryos. The specific activities of the calvarial 1- and 24-hydroxylases were similar in magnitude to those in isolated kidney cells. The specific activity of the calvarial 25(OH)D3:1-hydroxylase was inhibited by an 8-hr preincubation with 1,25(OH)2D3, whereas the 24-hydroxylase was enhanced. It is concluded that (i) vitamin D metabolism by isolated cells is organ-specific, (ii) calvarial cells produce active metabolites of vitamin D in significant amounts, (iii) vitamin D metabolism by calvarial cells is regulated by 1,25(OH)2D3, and (iv) locally produced, active metabolites could act locally, thereby adding a new dimension to the regulation of mineral metabolism by vitamin D metabolites.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGERS J. D., GWATKIN R. B., HEYNER S. Growth of embryonic avian and mammalian tibiae on a relatively simple chemically defined medium. Exp Cell Res. 1961 Oct;25:41–58. doi: 10.1016/0014-4827(61)90305-6. [DOI] [PubMed] [Google Scholar]
- Boyle I. T., Miravet L., Gray R. W., Holick M. F., Deluca H. F. The response of intestinal calcium transport to 25-hydroxy and 1,25-dihydroxy vitamin D in nephrectomized rats. Endocrinology. 1972 Mar;90(3):605–608. doi: 10.1210/endo-90-3-605. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Castillo L., Korycka-Dahl M., DeLuca H. F. Role of vitamin D metabolites in phosphate transport of rat intestine. J Nutr. 1974 Aug;104(8):1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Spelsberg T. C., MacIntyre I. Feedback regulation of vitamin D metabolism by 1,25-dihydroxycholecalciferol. Biochem J. 1977 Apr 15;164(1):83–89. doi: 10.1042/bj1640083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Lester G. E., Lorenc R. S. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 1979 Jun 22;204(4399):1311–1313. doi: 10.1126/science.451538. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Baylink D. J., Hughes M. R., Brumbaugh P. F., Wergedal J. E., Shen F. H., Nielsen R. L., Counts S. J., Bursac K. M., McCain T. A. The assay of 1alpha,25-dihydroxyvitamin D3: physiologic and pathologic modulation of circulating hormone levels. Clin Endocrinol (Oxf) 1976;5 (Suppl):151S–165S. doi: 10.1111/j.1365-2265.1976.tb03823.x. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 1,25-dihydroxycholecalciferol: metabolite of vitamin D3 active on bone in anephric rats. Science. 1972 Jun 9;176(4039):1146–1147. doi: 10.1126/science.176.4039.1146. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Turner R. T., Bottemiller B. L., Rader J. I. Serum-free culture of Japanese quail kidney cells. Regulation of vitamin D metabolism. Biochim Biophys Acta. 1979 Nov 1;587(4):495–506. doi: 10.1016/0304-4165(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Hughes M. R., Brumbaugh P. F., Hussler M. R., Wergedal J. E., Baylink D. J. Regulation of serum 1alpha,25-dihydroxyvitamin D3 by calcium and phosphate in the rat. Science. 1975 Nov 7;190(4214):578–580. doi: 10.1126/science.1188357. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., DeLuca H. F. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974 Mar 26;13(7):1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- Kumar R., Schnoes H. K., DeLuca H. F. Rat intestinal 25-hydroxyvitamin D3- and 1alpha,25-dihydroxyvitamin D3-24-hydroxylase. J Biol Chem. 1978 Jun 10;253(11):3804–3809. [PubMed] [Google Scholar]
- Puzas J. E., Vignery A., Rasmussen H. Isolation of specific bone cell types by free-flow electrophoresis. Calcif Tissue Int. 1979 Jul 3;27(3):263–268. doi: 10.1007/BF02441195. [DOI] [PubMed] [Google Scholar]
- Rader J. I., Howard G. A., Feist E., Turner R. T., Baylink D. J. Bone mineralization and metabolism of 3H-25-hydroxyvitamin D3 in thyroparathyroidectomized rats treated with parathyroid extract. Calcif Tissue Int. 1979 Nov;29(1):21–26. doi: 10.1007/BF02408051. [DOI] [PubMed] [Google Scholar]
- Shen F-H, Baylink D. J., Sherrard D. J., Shen L., Maloney N. A., Wergedal J. E. Serum immunoreactive parathyroid hormone and 25-hydroxyvitamin D in patients with uremic bone diseases. J Clin Endocrinol Metab. 1975 Jun;40(6):1009–1017. doi: 10.1210/jcem-40-6-1009. [DOI] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard D. J., Baylink D. J., Wergedal J. E., Maloney N. A. Quantitative histological studies on the pathogenesis of uremic bone disease. J Clin Endocrinol Metab. 1974 Jul;39(1):119–135. doi: 10.1210/jcem-39-1-119. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Halloran B., Schnoes H. K., DeLuca H. F. In vitro production of 1,25-dihydroxyvitamin D3 by rat placental tissue. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5033–5035. doi: 10.1073/pnas.76.10.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. R., Baylink D. J., Wergedal J. E. Increases in number and size of osteoclasts in response to calcium or phosphorus deficiency in the rat. Endocrinology. 1975 Aug;97(2):283–289. doi: 10.1210/endo-97-2-283. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Bottemiller B. L., Howard G. A., Baylink D. J. In vitro metabolism of 25-hydroxyvitamin D3 by isolated rat kidney cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1537–1540. doi: 10.1073/pnas.77.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. T., Rader J. I., Eliel L. P., Howard G. A. Metabolism of 25-hydroxyvitamin D3 during photo-induced reproductive development in female Japanese quail. Gen Comp Endocrinol. 1979 Feb;37(2):211–219. doi: 10.1016/0016-6480(79)90109-6. [DOI] [PubMed] [Google Scholar]
- Weisman Y., Harell A., Edelstein S., David M., Spirer Z., Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979 Sep 27;281(5729):317–319. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]




