Abstract
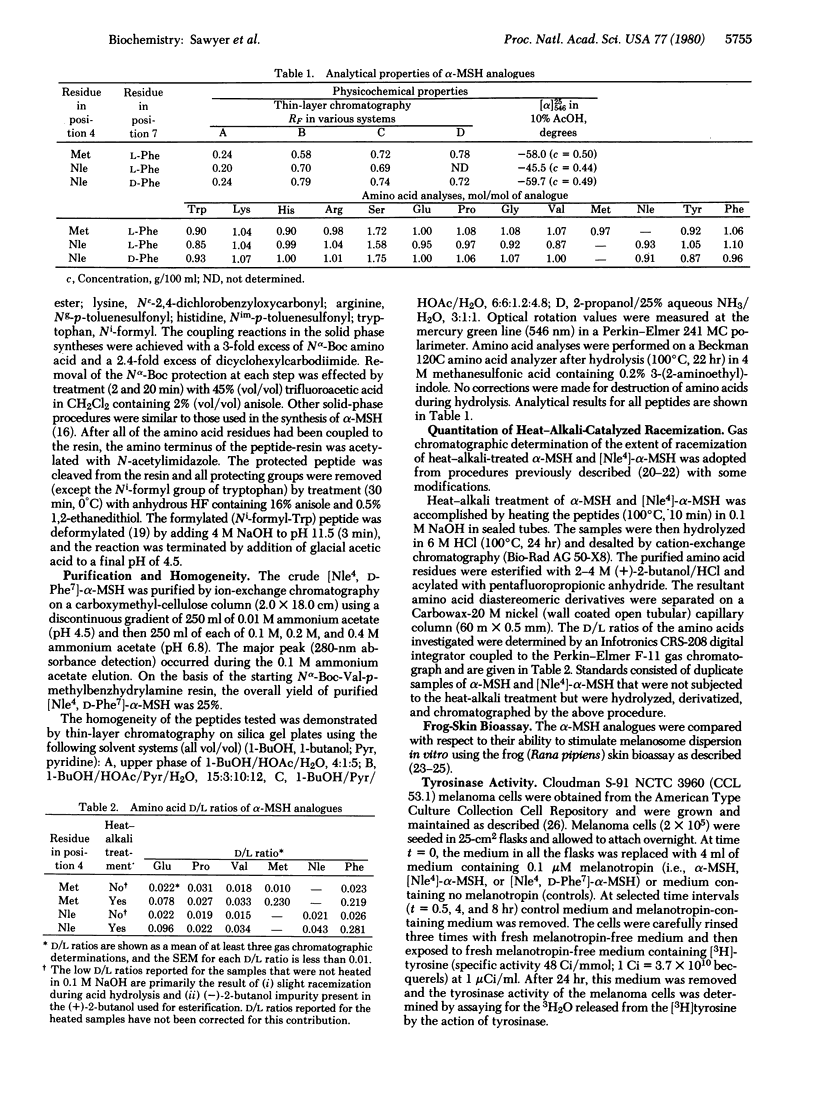
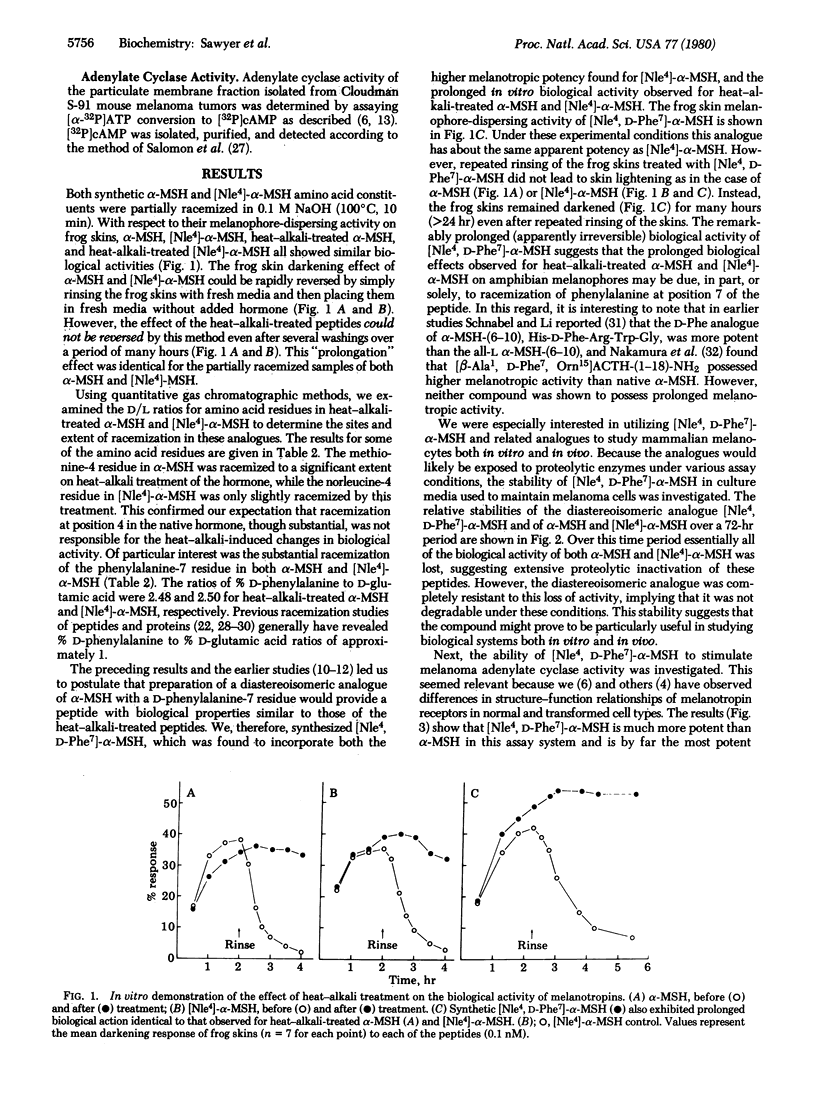
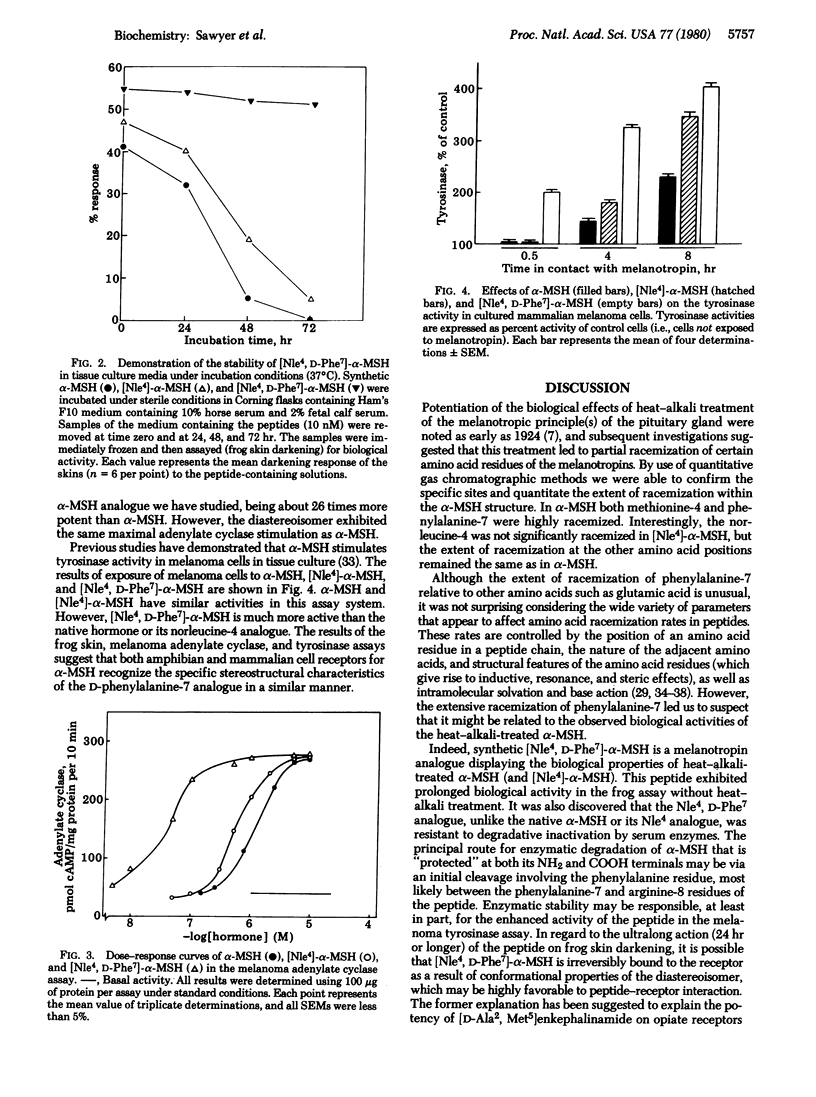
alpha-Melanocyte-stimulating hormone (alpha-MSH) reversibly darkens frog skins by stimulating melanosome movement (dispersion) within melanophores. Heat-alkali treatment of alpha-MSH results in prolonged biological activity of the hormone. Quantitative gas chromatographic analysis of the hydrolyzed heat-alkali-treated peptide revealed partial racemization particularly at the 4(methionine) and 7(phenylalanine) positions. [Nle4]-alpha-MSH, a synthetic analogue of alpha-MSH, reversibly darkens frog skins and also exhibits prolonged activity after heat-alkali treatment. Synthesis of [Nle4, D-Phe7]-alpha-MSH provided an analogue with prolonged biological activity identical to that observed with heat-alkali-treated alpha-MSH or [Nle4]-alpha-MSH. [Nle4, D-Phe7]-alpha-MSH was resistant to enzymatic degradation by serum enzymes. In addition, this peptide exhibited dramatically increased biological activity as determined by frog skin bioassay, activation of mouse melanoma adenylate cyclase, and stimulation of mouse melanoma cell tyrosinase activity. This Nle4, D-Phe7 synthetic analogue of alpha-MSH is a very porent melanotropin, 26 times as potent as alpha-MSH in the adenylate cyclase assay. The resistance of the peptide to enzymatic degradation and its extraordinarily potent and prolonged biological activity should make this analogue of alpha-MSH an important molecular probe for studying the melanotropin receptors of both normal and abnormal (melanoma) melanocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bregman M. D., Sawyer T. K., Hadley M. E., Hruby V. J. Adenosine and divalent cation effects on S-91 melanoma adenylate cyclase. Arch Biochem Biophys. 1980 Apr 15;201(1):1–7. doi: 10.1016/0003-9861(80)90480-4. [DOI] [PubMed] [Google Scholar]
- Engel M. H., Zumberge J. E., Nagy B. Kinetics of amino acid racemization in Sequoiadendron giganteum heartwood. Anal Biochem. 1977 Oct;82(2):415–422. doi: 10.1016/0003-2697(77)90179-8. [DOI] [PubMed] [Google Scholar]
- Fuller B. B., Viskochil D. H. The role of RNA and protein synthesis in mediating the action of MSH on mouse melanoma cells. Life Sci. 1979 Jun 25;24(26):2405–2415. doi: 10.1016/0024-3205(79)90448-x. [DOI] [PubMed] [Google Scholar]
- Heward C. B., Yang Y. C., Ormberg J. F., Hadley M. E., Hruby V. J. Effects of chloramine T and iodination on the biological activity of melanotropin. Hoppe Seylers Z Physiol Chem. 1979 Dec;360(12):1851–1859. doi: 10.1515/bchm2.1979.360.2.1851. [DOI] [PubMed] [Google Scholar]
- Heward C. B., Yang Y. C., Sawyer T. K., Bregman M. D., Fuller B. B., Hruby V. J., Hadley M. E. Iodination associated inactivation of beta-melanocyte stimulating hormone. Biochem Biophys Res Commun. 1979 May 14;88(1):266–273. doi: 10.1016/0006-291x(79)91725-x. [DOI] [PubMed] [Google Scholar]
- Huntington T., Hadley M. E. Evidence against mass action direct feedback control of melanophore-stimulating hormone (MSH) release. Endocrinology. 1974 Aug;95(2):472–479. doi: 10.1210/endo-95-2-472. [DOI] [PubMed] [Google Scholar]
- Kriausakul N., Mitterer R. M. Isoleucine epimerization in peptides and proteins: kinetic factors and application to fossil proteins. Science. 1978 Sep 15;201(4360):1011–1014. doi: 10.1126/science.201.4360.1011. [DOI] [PubMed] [Google Scholar]
- LEE T. H., BUETTNER-JANUSCH V. On the mechanism of sodium hydroxide modification of alpha-melanocyte-stimulating hormone. J Biol Chem. 1963 Jun;238:2012–2015. [PubMed] [Google Scholar]
- Lande S., Lerner A. B. Racemization of alpha-melanotropin. Biochim Biophys Acta. 1971 Nov 19;251(2):246–253. doi: 10.1016/0005-2795(71)90108-5. [DOI] [PubMed] [Google Scholar]
- Lemaire S., Yamashiro D., Li C. H. Synthesis and biological activity of camel and bovine beta-melanotropins. J Med Chem. 1976 Mar;19(3):373–376. doi: 10.1021/jm00225a006. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Tanaka A., Hirata M., Inoue S. Structure-melanotropic activity relationship in synthetic polypeptides related to ACTH. Endocrinol Jpn. 1972 Aug;19(4):383–388. doi: 10.1507/endocrj1954.19.383. [DOI] [PubMed] [Google Scholar]
- Noll B. W., Jarboe C. J., Hass L. F. Kinetic studies on the alkali-catalyzed hydrolysis and epimerization of model alkyl and hydroxyalkyl di- and tripeptides. Biochemistry. 1974 Dec 3;13(25):5164–5169. doi: 10.1021/bi00722a018. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Pert A., Chang J. K., Fong B. T. (D-Ala2)-Met-enkephalinamide: a potent, long-lasting synthetic pentapeptide analgesic. Science. 1976 Oct 15;194(4262):330–332. doi: 10.1126/science.968485. [DOI] [PubMed] [Google Scholar]
- Pollock G. E., Frommhagen L. H. The extent of racemization of some amino acids in dilute alkali-treated protein and soil humic and fulvic acid. Anal Biochem. 1968 Jul;24(1):18–26. doi: 10.1016/0003-2697(68)90055-9. [DOI] [PubMed] [Google Scholar]
- SHIZUME K., LERNER A. B., FITZPATRICK T. B. In vitro bioassay for the melanocyte stimulating hormone. Endocrinology. 1954 May;54(5):553–560. doi: 10.1210/endo-54-5-553. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Silman R. E., Chard T., Landon J., Lowry P. J., Smith I., Young I. M. ACTH and MSH peptides in the human adult and fetal pituitary gland. Front Horm Res. 1977;4:179–187. doi: 10.1159/000400364. [DOI] [PubMed] [Google Scholar]
- Smith G. G., DE Sol B. S. Racemization of Amino Acids in Dipeptides Shows COOH > NH2 for Non-Sterically Hindered Residues. Science. 1980 Feb 15;207(4432):765–767. doi: 10.1126/science.207.4432.765. [DOI] [PubMed] [Google Scholar]
- WRIGHT M. R., LERNER A. B. On the movement of pigment granules in frog melanocytes. Endocrinology. 1960 Apr;66:599–609. doi: 10.1210/endo-66-4-599. [DOI] [PubMed] [Google Scholar]
- Williams K. M., Smith G. G. A critical evaluation of the application of amino acid racemization to geochronology and geothermometry. Orig Life. 1977 Aug;8(2):91–144. doi: 10.1007/BF00927978. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Hruby V. J., Heward C. B., Hadley M. E. Synthesis of alpha- and beta-melanocyte stimulating hormones. Int J Pept Protein Res. 1980 Feb;15(2):130–138. [PubMed] [Google Scholar]
- van der Gugten A. A., Waters M. J., Murthy G. S., Friesen H. G. Studies on the irreversible nature of prolactin binding to receptors. Endocrinology. 1980 Jan;106(1):402–411. doi: 10.1210/endo-106-1-402. [DOI] [PubMed] [Google Scholar]


