Abstract
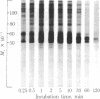
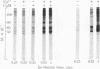
Calcium stimulated the phosphorylation of several specific synaptosomal cytosolic proteins. The effects of calcium were both concentration and time dependent and were most apparent for proteins with molecular weights of 50,000, 55,000, and 60,000. Exogenous calcium (1.0-100 microM) enhanced the net incorporation of phosphate into protein by as much as 23-fold. In the absence of added calcium, the calcium chelator [ethylenebis(oxyethylenenitriolo)]tetraacetic acid did not lower the phosphorylation of any protein below control levels. The antipsychotic, fluphenazine (1.0-100 microM), caused a concentration-dependent decrease in calcium-stimulated protein phosphorylation. When the heat-stable calcium-binding protein, calmodulin, was removed from synaptosomal cytosol by affinity chromatography on fluphenazine-Sepharose, calcium-stimulated protein phosphorylation was abolished. Responsiveness to calcium could be restored by the addition of calmodulin to the phosphorylation assay. These results indicate that calcium-dependent protein kinases are of major importance in regulating the phosphorylation of specific cytosolic proteins in neuronal tissue. Furthermore, it would appear that one of the three substrates under investigation is specific to synaptosomal cytosol whereas the other two are present in both the cytosol and membrane fractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames M. M., Lerner P., Lovenberg W. Tyrosine hydroxylase. Activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978 Jan 10;253(1):27–31. [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Kendrick N. K. The regulation of intracellular calcium in presynaptic nerve terminals. Ann N Y Acad Sci. 1978 Apr 28;307:195–212. doi: 10.1111/j.1749-6632.1978.tb41943.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Browning M., Bennett W., Lynch G. Phosphorylase kinase phosphorylates a brain protein which is influenced by repetitive synaptic activation. Nature. 1979 Mar 15;278(5701):273–275. doi: 10.1038/278273a0. [DOI] [PubMed] [Google Scholar]
- Bylund D. B., Krebs E. G. Effect of denaturation on the susceptibility of proteins to enzymic phosphorylation. J Biol Chem. 1975 Aug 25;250(16):6355–6361. [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Emple G. P., Glaser G. H. Regulation of the level of endogenous phosphorylation of specific brain proteins by diphenylhydantoin. J Neurochem. 1977 Jan;28(1):21–30. doi: 10.1111/j.1471-4159.1977.tb07704.x. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egrie J. C., Campbell J. A., Flangas A. L., Siegel F. L. Regional, cellular and subcellular distribution of calcium-activated cyclic nucleotide phosphodiesterase and calcium-dependent regulator in porcine brain. J Neurochem. 1977 Jun;28(6):1207–1213. doi: 10.1111/j.1471-4159.1977.tb12311.x. [DOI] [PubMed] [Google Scholar]
- Forn J., Greengard P. Depolarizing agents and cyclic nucleotides regulate the phosphorylation of specific neuronal proteins in rat cerebral cortex slices. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5195–5199. doi: 10.1073/pnas.75.10.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. H., Matus A. I. Isolation of synaptic plasma membrane from brain by combined flotation-sedimentation density gradient centrifugation. Biochim Biophys Acta. 1974 Aug 9;356(3):276–287. doi: 10.1016/0005-2736(74)90268-5. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Zinman S. M. Purification and properties of a heat-stable protein inhibitor of phosphoprotein phosphatase from rabbit liver. J Biol Chem. 1978 Jan 25;253(2):560–565. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. The informational role of calcium in the cytosol. Adv Cyclic Nucleotide Res. 1979;11:1–26. [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- Kuhn D. M., O'Callaghan J. P., Juskevich J., Lovenberg W. Activation of brain tryptophan hydroxylase by ATP-MG2+: dependence on calmodulin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4688–4691. doi: 10.1073/pnas.77.8.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D. M., Vogel R. L., Lovenberg W. Calcium-dependent activation of tryptophan hydroxylase by ATP and magnesium. Biochem Biophys Res Commun. 1978 May 30;82(2):759–766. doi: 10.1016/0006-291x(78)90940-3. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by "calcium-dependent regulator". Proc Natl Acad Sci U S A. 1978 Nov;75(11):5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W., Forn J., Greengard P. Ca2+ and cyclic AMP regulate phosphorylation of same two membrane-associated proteins specific to nerve tissue. Proc Natl Acad Sci U S A. 1979 May;76(5):2475–2479. doi: 10.1073/pnas.76.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Roth R. H. Striatal tyrosine hydroxylase: comparison of the activation produced by depolarization and dibutyryl-cAMP. Mol Pharmacol. 1979 Jul;16(1):224–233. [PubMed] [Google Scholar]
- Sundberg L., Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifuctional oxiranes. J Chromatogr. 1974 Mar 13;90(1):87–98. doi: 10.1016/s0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- Szmigielski A., Guidotti A., Costa E. Endogenous protein kinase inhibitors. Purification, characterization, and distribution in different tissues. J Biol Chem. 1977 Jun 10;252(11):3848–3853. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Inoue M., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977 Nov 10;252(21):7603–7609. [PubMed] [Google Scholar]
- Ueda T., Maeno H., Greengard P. Regulation of endogenous phosphorylation of specific proteins in synaptic membrane fractions from rat brain by adenosine 3':5'-monophosphate. J Biol Chem. 1973 Dec 10;248(23):8295–8305. [PubMed] [Google Scholar]
- Wolff D. J., Poirier P. G., Brostrom C. O., Brostrom M. A. Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. J Biol Chem. 1977 Jun 25;252(12):4108–4117. [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Activation of tryptophan 5-monooxygenase by calcium-dependent regulator protein. Biochem Biophys Res Commun. 1979 Sep 12;90(1):28–35. doi: 10.1016/0006-291x(79)91585-7. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Most of the Ca2+-dependent endogenous phosphorylation of rat brain cytosol proteins requires Ca2+-dependent regulation protein. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1172–1178. doi: 10.1016/0006-291x(79)91160-4. [DOI] [PubMed] [Google Scholar]