Abstract
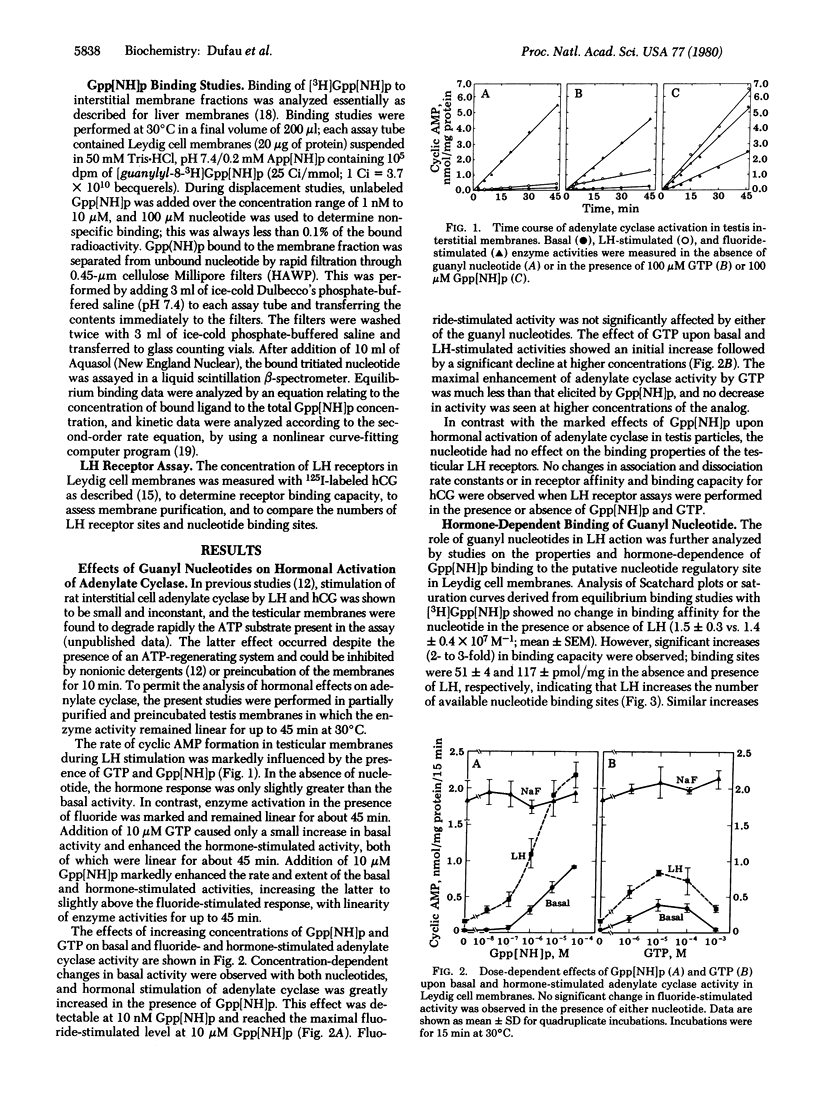
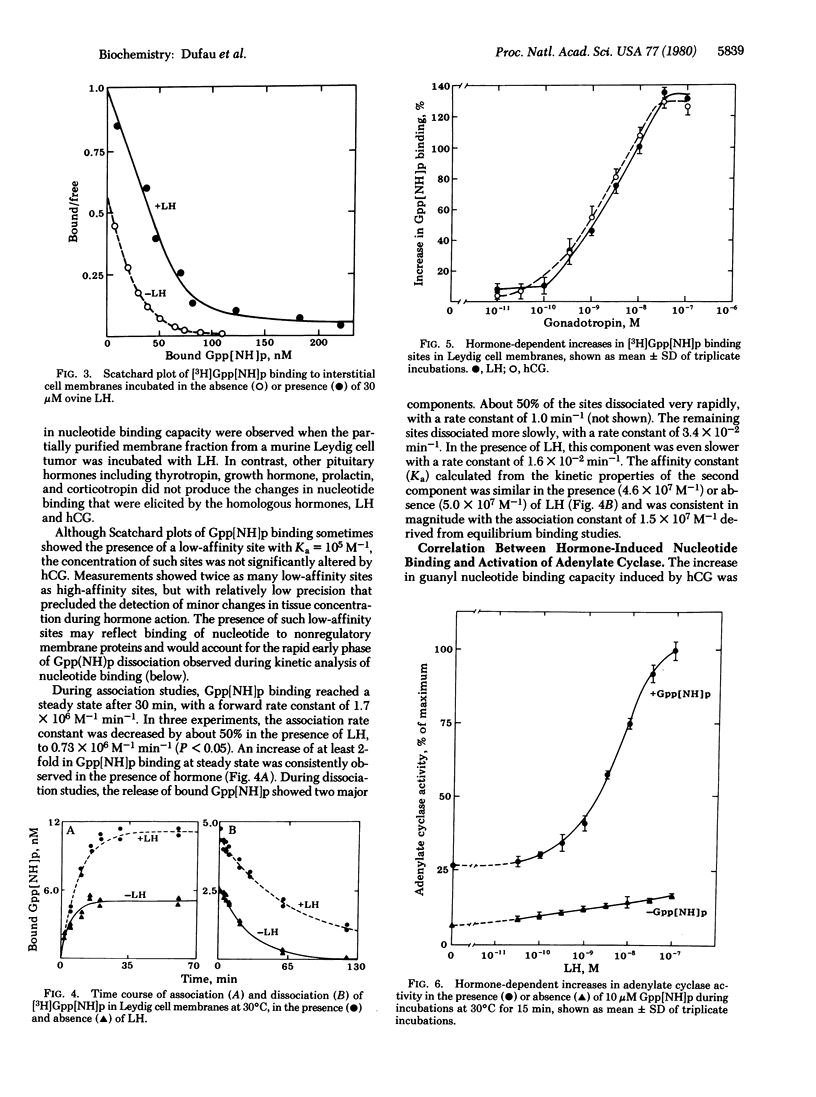
The adenylate cyclase activity of Leydig cell homogenates and membrane fractions is highly dependent on guanyl nucleotides, and enzyme responses to luteinizing hormone or human chorionic gonadotropin are small in the absence of guanyl nucleotides. However, in the presence of 10 microM guanosine 5'-[beta, gamma-imido]triphosphate Gpp[NH]p, both hormones consistently stimulated testicular adenylate cyclase activity by up to 200%. Leydig cell membranes bound [3H]Gpp[NH]p at 30 degrees C with high affinity (Ka = 1.5 X 10(7) M-1) and binding capacity of 60 pmol/mg of protein. During kinetic studies, the association rate constant was 1.7 X 10(6) M-1 min-1, and the dissociation constant was 0.038 min-1. In the presence of gonadotropin (10 pM to 10 nM), concentration-dependent increases of 40% to 100% in Gpp[NH]p binding were observed in Leydig cell membranes. Kinetic studies showed that gonadotropin decreased the association rate constant to 0.73 X 10(6) M-1 min-1 and the dissociation rate constant to 0.017 min-1, with no effect on the equilibrium binding constant. Thus, the increase in Gpp[NH]p binding was not due to a change in receptor affinity but was attributable to increased availability of nucleotide binding sites. The 50% effective dose for adenylate cyclase activation by gonadotropin in the presence of Gpp[NH]p was identical with that observed for gonadotropin-induced binding of the GTP analog (50 nM). Gonadotropin-induced binding of Gpp[NH]p in Leydig cell membranes may represent interaction with the guanyl nucleotide regulatory site during hormonal activation of adenylate cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Yang P. C., Hunzicker-Dunn M., Bockaert J., Duran J. M. Adenylyl cyclase activities in ovarian tissues. I. Homogenization and conditions of assay in graafian follicles and corpora lutea of rabbits, rats, and pigs: regulation by ATP, and some comparative properties. Endocrinology. 1976 Jul;99(1):163–184. doi: 10.1210/endo-99-1-163. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-induced release of [3H]-Gpp(NH)p from turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Feb;3(1):11–22. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catt K. J., Tsuruhara T., Dufau M. L. Gonadotrophin binding sites of the rat testis. Biochim Biophys Acta. 1972 Aug 18;279(1):194–201. doi: 10.1016/0304-4165(72)90254-1. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Schlegel W., Lin M. C., Rodbell M. The fat cell adenylate cyclase system. Characterization and manipulation of its bimodal regulation by GTP. J Biol Chem. 1979 Sep 25;254(18):8927–8931. [PubMed] [Google Scholar]
- Dufau M. L., Baukal A. J., Ryan D., Catt K. J. Properties of detergent-solubilized adenylate cyclase and gonadotropin receptors of testis and ovary. Mol Cell Endocrinol. 1977 Feb;6(4-5):253–269. doi: 10.1016/0303-7207(77)90100-9. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Hayashi K., Sala G., Baukal A., Catt K. J. Gonadal luteinizing hormone receptors and adenylate cyclase: transfer of functional ovarian luteinizing hormone receptors to adrenal fasciculata cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4769–4773. doi: 10.1073/pnas.75.10.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra E., Salomon Y. Mechanism of desensitization of adenylate cyclase in lutropin. GTP-dependent uncoupling of the receptor. J Biol Chem. 1980 Jan 25;255(2):653–658. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C., Sternweis P. C., Macik B. A., Van Arsdale P. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Association of a regulatory component of the enzyme with membranes containing the catalytic protein and beta-adrenergic receptors. J Biol Chem. 1979 Apr 10;254(7):2287–2295. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Ketelslegers J. M., Knott G. D., Catt K. J. Kinetics of gonadotropin binding by receptors of the rat testis. Analysis by a nonlinear curve-fitting method. Biochemistry. 1975 Jul 15;14(14):3075–3083. doi: 10.1021/bi00685a006. [DOI] [PubMed] [Google Scholar]
- Limbird L. E., Gill D. M., Lefkowitz R. J. Agonist-promoted coupling of the beta-adrenergic receptor with the guanine nucleotide regulatory protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1980 Feb;77(2):775–779. doi: 10.1073/pnas.77.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. C., Lin C., Whitlock J. P., Jr Reduction of GTP activation of adenylate cyclase system by its coupling to hormone receptor. J Biol Chem. 1979 Jun 10;254(11):4684–4688. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Mintz Y., Amir Y., Amsterdam A., Lindner H. R., Salomon Y. Properties of LH-sensitive adenylate cyclase in purified plasma membranes from rat ovary. Mol Cell Endocrinol. 1978 Sep;11(3):265–283. doi: 10.1016/0303-7207(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T. Guanine nucleotide-controlled interactions between components of adenylate cyclase. FEBS Lett. 1979 May 1;101(1):85–89. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Rodbell M. Evidence for specific binding sites for guanine nucleotides in adipocyte and hepatocyte plasma membranes. A difference in fate of GTP and guanosine 5'-(beta, gamma-imino) triphosphate. J Biol Chem. 1975 Sep 25;250(18):7245–7250. [PubMed] [Google Scholar]


