Abstract
Background:
Both obstructive sleep apnea (OSA) and prolonged QRS duration are associated with hypertension, heart failure, and sudden cardiac death. However, possible links between QRS duration and OSA have not been explored.
Methods:
Cross-sectional study of 221 patients who underwent polysomnography at our center. Demographics, cardiovascular risk factors and ECG were collected to explore a relationship between OSA and QRS duration.
Results:
The apnea-hypopnea index (AHI) was positively correlated with QRS duration (r = 0.141, p = 0.03). Patients were divided into 3 groups: AHI < 5 (61), AHI 5-29 (104), and AHI > 30 (55). The mean QRS duration prolonged significantly as OSA worsened (AHI < 5, 85 ± 9.5; AHI 5-29, 89 ± 11.9; and AHI > 30, 95 ± 19.9 ms, p = 0.001). QRS ≥ 100 ms was present in 12.7% of patients with severe OSA compared with 0% in the rest of the sample (p < 0.0001). After adjustment for age, race, and cardiovascular risk factors, this association remained significant in women but not in men.
Conclusion:
QRS duration and OSA were significantly associated. Severity of OSA independently predicted prolonged QRS in women but not men. Nevertheless, prolongation of QRS duration in either sex may potentiate arrhythmic risks associated with OSA.
Citation:
Gupta S; Cepeda-Valery B; Romero-Corral A; Shamsuzzaman A; Somers VK; Pressman GS. Association between QRS duration and obstructive sleep apnea. J Clin Sleep Med 2012;8(6):649-654.
Keywords: Obstructive sleep apnea, electrocardiogram, QRS duration
Obstructive sleep apnea (OSA) has been associated with several cardiovascular conditions1,2 including hypertension,3 congestive heart failure,4 and sudden cardiac death.5 In fact, OSA has been associated with a 60% to 70% increased risk of cardiovascular morbidity and mortality.6
OSA is highly prevalent and underdiagnosed7 in the general population. This problem may be worse in subjects with coronary artery disease, as recent studies have shown a 50% to 70% prevalence of OSA in such patients.7,8 This high prevalence suggests that OSA may play a key role in the development of cardiovascular events.
Numerous studies have shown that OSA is associated with changes in cardiac structure, including left ventricular hypertrophy,9–12 which can widen the QRS complex on the electrocardiogram (ECG).13 QRS duration is an independent predictor of sudden cardiac death15,16 and mortality16,17 in conditions commonly associated with OSA, such as hypertension and heart failure. However, the relationship between OSA and QRS duration is unknown.
In this study we hypothesized that OSA severity, measured by the apnea-hypopnea index (AHI), is independently associated with QRS prolongation. If so, this could help explain any increased risk of sudden cardiac death in OSA.
METHODS
We conducted a retrospective cross-sectional study of consecutive patients who had a clinically indicated polysomnogram (PSG) study from January 2007 through July 2009 at Albert Einstein Medical Center, Philadelphia, PA. Inclusion criteria were age over 18 years and availability of a resting 12-lead ECG within the 6 months preceding polysomnography. Patients with known sleep apnea were excluded as were those with classic right or left bundle branch block or a ventricular paced rhythm. Patients with atrial fibrillation were included but other non-sinus rhythms were excluded. The final study population consisted of 221 subjects. This study was approved by the Albert Einstein Institutional Review Board.
BRIEF SUMMARY
Current Knowledge/Study Rationale: QRS duration is an independent predictor of mortality. Obstructive sleep apnea produces structural changes of the heart which can lead to a widened QRS but this potential association has not been adequately explored.
Study Impact: QRS duration prolonged significantly as sleep apnea severity increased; however, on multivariate analysis the effect remained significant only in women. QRS widening could help explain associations between sleep apnea and cardiovascular events while the effect in women points to possible areas for future research.
Polysomnogram
All patients were initially evaluated by a certified sleep medicine certified physician. PSG recording and scoring were performed using the VIASYS SomnoStar Pro System. Electroencephalogram, electro-oculogram, and electromyogram were monitored for sleep staging. Nasal pressure monitoring, chest wall movement, abdominal movement, snoring, and oxygen saturation were monitored for respiratory assessment. Electrocardiogram and tibial electromyogram were monitored for cardiac arrhythmias and nocturnal limb movements, respectively. The AHI, defined as the sum of apneas and hypopneas per hour of sleep, was recorded from the PSG report. OSA severity was defined by AHI according to recommendations from the Sleep Task Force of the American Academy of Sleep Medicine (no OSA when AHI < 5, mild-moderate OSA with AHI 5-30, and severe OSA with AHI > 30).17
Electrocardiogram
The resting 12-lead ECGs of these patients were accessed from their electronic medical records. Heart rate, QRS duration, PR interval, and corrected QT interval (QTc) were abstracted from the computerized ECG interpretation (Marquette 12SL ECG Analysis Program run on the MUSE ECG system). The onset of QRS is detected first because it is the easiest; the slope change is usually very rapid and in great contrast to the other slopes in the median. This is followed by QRS offset. The onsets and offsets are determined by an analysis of simultaneous slopes in all 12 leads. Onsets are defined as the earliest deflection in any lead, and offsets as the latest deflection in any lead. Thus, the QRS duration is measured from the earliest onset in any lead to the latest deflection in any lead. Similarly, the QT interval is measured from the earliest detection of depolarization in any lead to the latest detection of repolarization in any lead. The PR interval is measured form the earliest detection of atrial depolarization in any lead to the earliest detection of ventricular depolarization in any lead (the QRS onset). The QT is corrected for heart rate using Bazett's formula. The values of these intervals were recorded in milliseconds (ms).
Data Collection and Cardiovascular Risk Factors
PSG, ECG, and demographic data including age, gender, and race were collected from electronic medical records. Cardiovascular risk factors—namely hypertension, diabetes, coronary artery disease, congestive heart failure, atrial fibrillation, obesity (BMI ≥ 30 kg/m2), and smoking status—were noted from the records. Recording of these variables were done blinded to the OSA status or severity.
Statistical Methods
Data were summarized by calculating mean ± SD for continuous variables, and numbers and percentages for categorical variables. Due to non-linearity of the variables analyzed we used Spearman correlation coefficients to explore the association as continuous variables between AHI, mean and minimum oxygen saturation during sleep, with QRS and other ECG intervals. We performed one-way ANOVA and independent t-tests to assess differences between cardiovascular risk factors and ECG intervals by presence or absence of OSA and by its severity (AHI < 5; no OSA, AHI 5-29; mild-to-moderate OSA and AHI ≥ 30; severe OSA) and by mean oxygen saturation < 88 % (cut-off used by Medicare for home oxygen) and minimum oxygen saturation < 94% (lowest quartile) during sleep. We then assessed whether severity of OSA was associated with a prolonged QRS (≥ 100 ms). Finally, we performed regression analyses to identify significant univariate predictors for QRS duration (p-value ≤ 0.10). To assess the independent effect of OSA on AHI and mean and minimum oxygen saturation during sleep, we performed multivariate analyses adjusting for age, sex, and cardiovascular risk factors (p-value < 0.05). Due to significant differences in QRS duration between men and women previously reported18,19 and noted in our study, we performed analyses stratified by gender, and therefore no adjustment for gender was needed. All analyses were performed using JMP 8.0 (SAS Institute; Cary, NC) and 2-tailed p values < 0.05 were considered significant in advance.
RESULTS
Baseline characteristics of the 221 participants (64.2% female) are shown in Table 1. Characteristics of the subjects and prevalence of cardiovascular risk factors according to the presence or absence of OSA and its severity are shown in Table 2. Overall, subjects with OSA tended to be older and male. Subjects with mild to moderate OSA had a higher prevalence of congestive heart failure and subjects with severe OSA tended to be more obese and hypertensive. Because there were no significant differences between the mild and moderate OSA groups, we decided to cluster them in a single group.
Table 1.
Baseline characteristics of the subjects included in the study
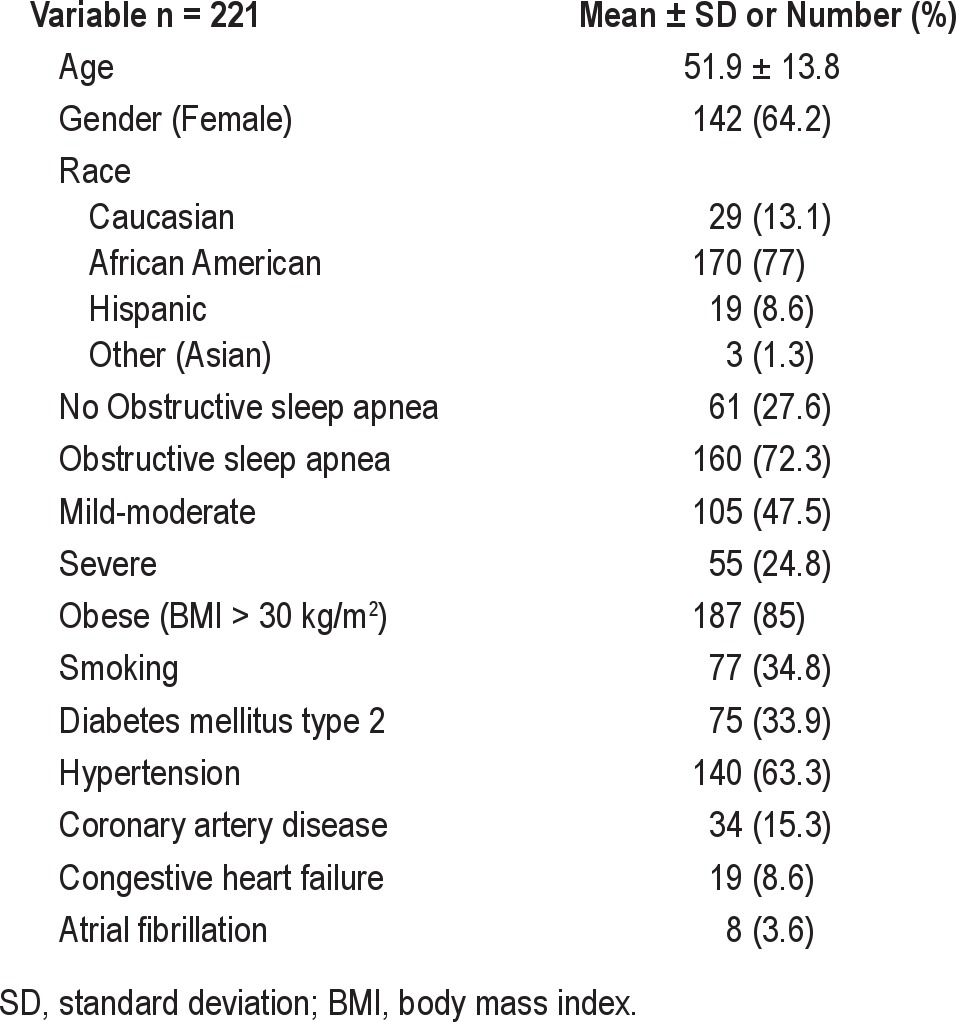
Table 2.
Characteristics by presence or absence of obstructive sleep apnea and by its severity
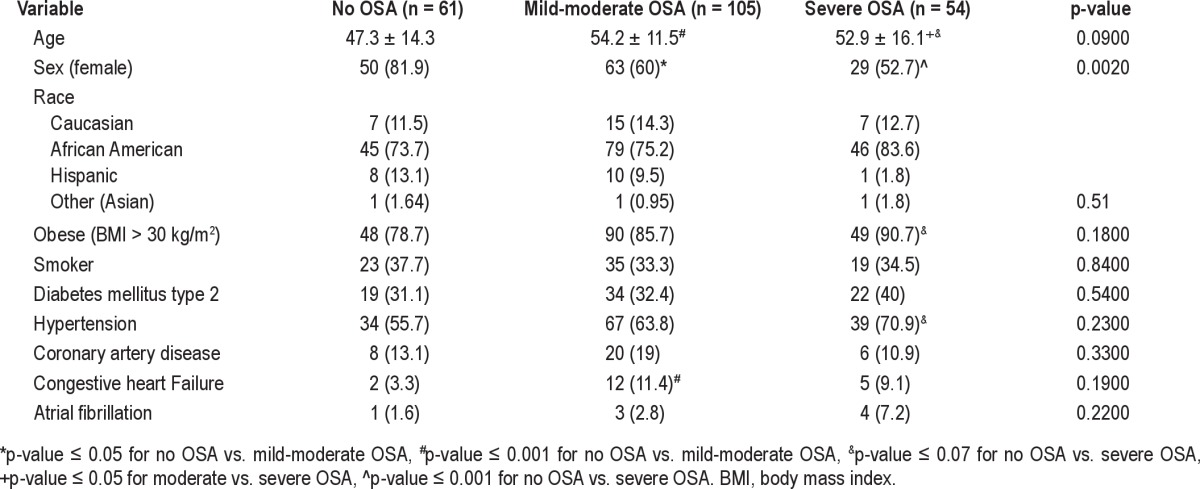
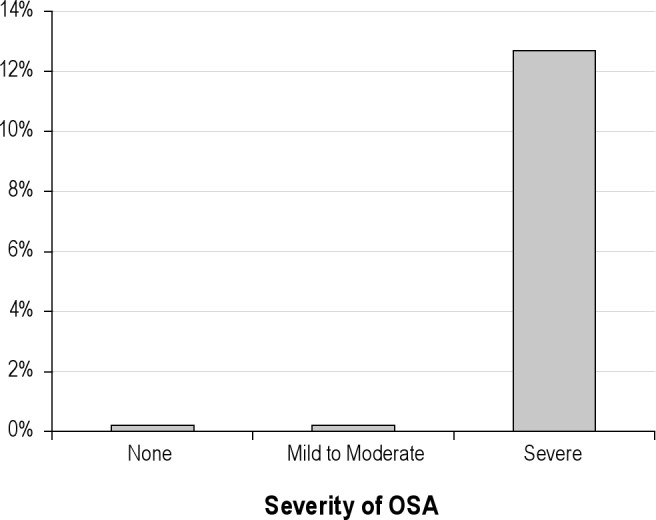
QRS and AHI showed a weak but significant and positive correlation (rho = 0.14, p = 0.03). Table 3 displays ECG intervals by presence or absence of OSA and by its severity. QRS was significantly wider as OSA severity increased (Figure 1). Furthermore, QRS ≥ 100 ms was present in 12.7% of those with severe OSA compared with 0% of patients in the mild to moderate OSA group, and 0% in those without OSA, p < 0.0001 (Figure 2). PR and QTc intervals were not significantly correlated with AHI (rho = −0.03, p = 0.58 and rho = −0.02, p = 0.75, respectively) and were not significantly different among groups.
Table 3.
ECG intervals by presence or absence of OSA and by its severity
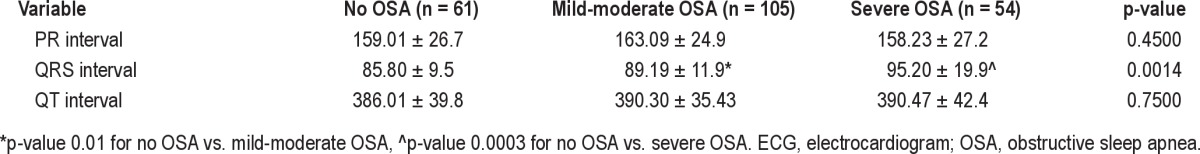
Figure 1. QRS duration by obstructive sleep apnea severity measured by apnea-hypopnea index.
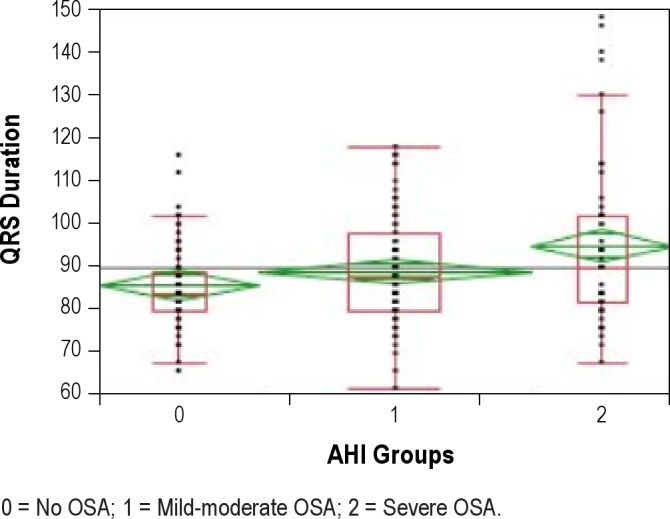
0 = No OSA; 1 = Mild-moderate OSA; 2 = Severe OSA.
Figure 2. QRS > 100 ms by presence or absence of OSA and by its severity.
To examine a possible relationship between nocturnal hypoxemia and QRS duration, we also looked at mean oxygen saturation and minimal oxygen saturation. Mean QRS duration was 93.4 ± 16.4 ms for the lowest quartile of mean oxygen saturation (< 94%) versus 88.9 ± 14.3 ms for those with mean oxygen saturation ≥ 94% (p = 0.049). We next divided patients into those with minimum oxygen saturation of ≤ 88% (current Medicare criteria for home oxygen prescription) and those with minimum oxygen saturation > 88%. Mean QRS duration for patients with nocturnal hypoxemia as defined above was 91.6 ± 16.0 ms compared to 86.1 ± 10.3 ms for those without (p = 0.005). After stratifying by sex, the difference in QRS duration remained significant in women (88.6 ± 14.3 ms vs. 83.5 ± 8.3 ms in patients with and without nocturnal hypoxemia), but not in men (96.1 ± 17.5 ms vs. 96.4 ± 11.5 ms in patients with and without nocturnal hypoxemia).
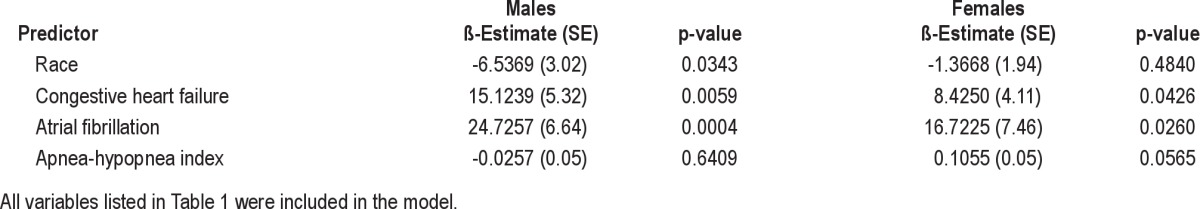
Sex, race, hypertension, coronary artery disease, congestive heart failure, and AHI were significant univariate predictors for QRS duration (Table 4). After stratifying by sex, women had a shorter QRS duration than men (86.8 ± 12.7 vs. 94.9 ± 15.1 ms), consistent with previous studies.18,19 In multivariate analyses, in men, atrial fibrillation, congestive heart failure, and race remained significant predictors of QRS duration after adjustments, while in women, atrial fibrillation, congestive heart failure, and AHI remained as significant predictors (Table 5). Minimal oxygen saturation was not a significant predictor of QRS duration on multivariate analyses.
Table 4.
Univariate predictors for QRS duration
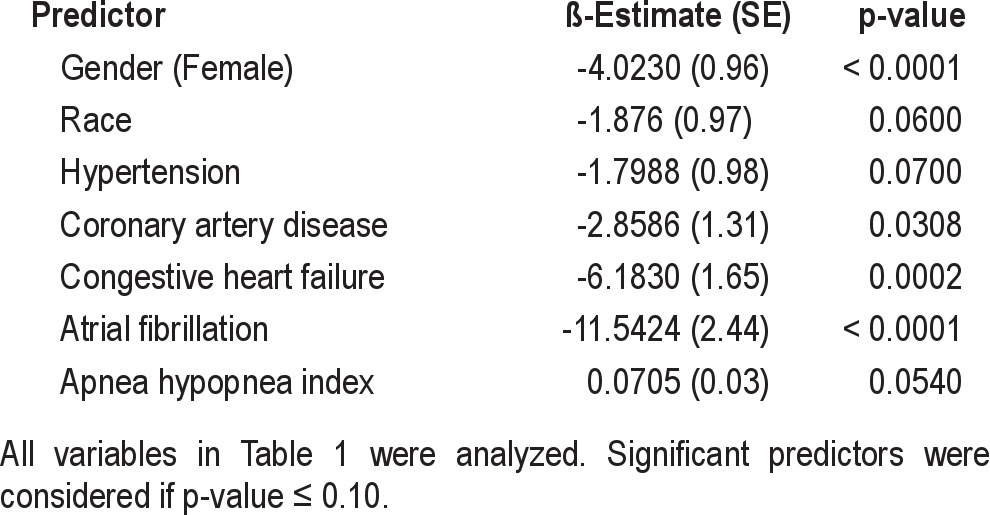
Table 5.
Multivariate predictors for QRS duration stratified by sex
DISCUSSION
Our major finding is that QRS duration and OSA are significantly associated. This was particularly true for patients with severe OSA (AHI > 30), where 12.7% had a QRS ≥ 100 ms compared to 0% of patients without OSA or with only mild to moderate OSA. After multivariate analyses, AHI remained a significant predictor of prolonged QRS in women but not in men.
Previous Studies
Previous small studies have looked for a possible association between QRS duration and OSA. Andreas et al.20 found a mean QRS duration of 96 ± 9 ms in OSA, similar to the 95.20 ± 19.9 ms found in our study. Their control subjects had similar QRS duration (96 ± 10 ms) and the authors concluded that OSA was not associated with QRS prolongation. In our study, subjects without OSA had a more normal QRS duration (85.8 ± 9.5 ms). This difference may relate to the fact that their controls were snorers with an AHI up to 10, while in our study controls had an AHI < 5 as suggested by the American Academy of Sleep Medicine Task Force.17 Furthermore, their sample size was small (n = 47). In another study, Steiner et al.21 studied 12 patients with congestive heart failure and compared ECG parameters by presence or absence of OSA. Although they noted no statistically significant changes in QRS duration between OSA groups, the mean QRS was 99 ± 13 ms in controls and 144 ± 43 ms in patients with OSA. This study is limited by small sample size (6 patients in each group) and by the mean AHI of the OSA group being only 17.8; therefore few conclusions can be drawn from these results.
In previous studies, QRS ≥ 120 ms has been independently associated with all-cause mortality15,16 and sudden death.14–16 Though mean QRS duration in our patients with severe OSA was < 120 ms, it is notable that only severe OSA subjects had a QRS ≥ 100 ms while this finding was absent in others. We can speculate that changes in QRS duration occur slowly and move in only one direction (wider). In addition OSA probably worsens over time. Therefore our patients might, over time, develop additional QRS widening. Further study is needed to investigate this possibility.
One of the interesting findings in this study is that AHI was independently associated with QRS duration in women but not in men. There are several possible ways to explain this. Prior studies have noted shorter QRS duration in women, as we also found. Any increase in QRS duration in response to OSA would produce a greater percentage change in women versus men, making it easier to detect statistically. On the other hand, there may be a true difference between men and women in response of the left ventricle to the afterload burden imposed by OSA. Animal studies have shown differences in gene expression between males and females in response to pressure overload.22 In addition, women appear to have greater wall thickening in response to aortic stenosis than do men.23 Among hypertensive patients followed by the LIFE study, women had reduced regression of electrocardiographic hypertrophy after 5 years of therapy versus men.24 Given these findings, it may be that women develop greater left ventricular hypertrophy in response to OSA, leading to greater QRS prolongation. Wall thickness tends to be slightly less in women than men; thus the female heart might experience greater wall stress from the high negative intrathoracic pressure produced by obstructive apneas.
Regarding the relationship between QT duration and OSA, previous studies have reported conflicting findings. Smith et al.25 measured QT and PR interval changes associated with spontaneous and respiratory-related arousals in OSA patients, finding a shortening of the QT during arousal. However, that study included only 20 OSA patients and lacked a control group. In another study, Lue et al.26 reported opposite findings with prolongation of the QT and corrected QT intervals in the OSA group compared to controls. In our study, we did not find an association between OSA and QTc interval. These conflicting results might be explained by the multiple factors that influence QT duration, and the fact that it can change very rapidly.
Mechanistic Considerations
Prolonged QRS and OSA can each be seen in various cardiovascular conditions with important clinical implications.1,2 Both have been associated with increased left ventricle mass, hypertension,3 congestive heart failure,4 and ventricular arrhythmias.27,28 Several pathophysiologic mechanisms can explain these associations. In OSA, breathing against a closed upper airway generates high negative intrathoracic pressure, which increases left ventricular transmural pressure and afterload, contributing to left ventricular hypertrophy.29 Furthermore, OSA is an accepted causal factor for the development of hypertension, which also contributes to left ventricular hypertrophy. Other possible mechanisms by which OSA might contribute to QRS widening include endothelial dysfunction,29 systemic inflammation, sympathetic activation, blood pressure surges, and oxidative stress, all of which contribute to increased arterial stiffness9 and increased afterload stress on the left ventricle.
Strengths and Limitations
Our study has a large sample size in comparison with most OSA studies. All of our patients underwent polysomnography study, the gold standard test to define this condition; this allowed us to assess OSA severity. We performed multivariate analyses to control for many of the clinical factors that could confound our results. Our study has some limitations. Given the cross-sectional nature of the study we cannot determine causality, as we cannot assess if prolonged QRS duration preceded or followed development of OSA. Our sample population was mainly African American, limiting generalizability of these results to other populations. In the multivariate analysis QRS prolongation was independently associated with OSA in women, but not in men, which could be due to overadjustment (example: hypertension and obesity, conditions commonly found in OSA) or insufficient power, as the majority of our population sample were women. Lead time bias is likely present in our study, as the onset of OSA in our population is not well established. However, in theory the longer the disease has been present, the greater the impact on cardiovascular disease, in our case the greater the QRS prolongation.
CONCLUSIONS
This is the first study linking increased QRS duration with OSA and may help explain the increased risk of sudden cardiac death in both conditions. For the overall sample, increased QRS duration was significantly associated with OSA, particularly in those with severe OSA (AHI > 30). After multivariate analyses severe OSA remained a significant independent predictor of prolonged QRS in women but not in men, while congestive heart failure and atrial fibrillation remained independent predictors in both men and women. Nevertheless, prolongation of QRS duration in either sex may potentiate arrhythmic risk associated with OSA. Mechanisms underlying the gender effects of the QRS-OSA interaction remain to be determined.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Somers serves as a consultant for Apnex Medical, Inc., Johnson & Johnson, Sova Pharmaceuticals, Respicardia, ResMed, and Neupro. He has received research support from Philips Respironics, Inc. Dr. Pressman has received a research grant from Philips Respironics, Inc. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Palomaki H, Partinen M, Juvela S, Kaste M. Snoring as a risk factor for sleep-related brain infarction. Stroke. 1989;20:1311–15. doi: 10.1161/01.str.20.10.1311. [DOI] [PubMed] [Google Scholar]
- 4.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovac Electrophysiol. 2008;19:997–03. doi: 10.1111/j.1540-8167.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 6.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 7.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 8.Sert Kuiyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drager L, Bortolotto LA, Figueiredo LA, Caldin B, Krieger E, Lorenzi-Filho E. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 10.Noda A, Okada T, Yasuma F, Nakashima N, Yokota Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest. 1995;107:1538–44. doi: 10.1378/chest.107.6.1538. [DOI] [PubMed] [Google Scholar]
- 11.Shivalkar B, Van de Heyning C, Kerremans M, Rinkevich D, Verbraecken J, De Backer W. Obstructive sleep apnea syndrome: more insights on structural and functional cardiac alterations, and the effects of treatment with continuous positive airway pressure. J Am Coll Cardiol. 2006;47:1433–9. doi: 10.1016/j.jacc.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 12.Usui K, Parker JD, Newton GE, Floras JS, Ryan CM, Bradley TD. Left ventricular structural adaptations to obstructive sleep apnea in dilated cardiomyopathy. Am J Respir Crit Care Med. 2006;173:1170–5. doi: 10.1164/rccm.200503-320OC. [DOI] [PubMed] [Google Scholar]
- 13.Dhingra R, Ho Nam B, Benjamin EJ, et al. Cross-sectional relations of electrocardiographic QRS duration to left ventricle dimensions. J Am Coll Cardiol. 2005;45:685–9. doi: 10.1016/j.jacc.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Olshansky B. Wide QRS, narrow QRS. J Am Coll Cardiol. 2005;46:317–9. doi: 10.1016/j.jacc.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Morin DP, Oikarinen L, Viitasalo M, et al. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: the LIFE study. Eur Heart J. 2009;30:2908–14. doi: 10.1093/eurheartj/ehp321. [DOI] [PubMed] [Google Scholar]
- 16.Ott P, Marcus FI. Electrocardiographic markers of sudden death. Cardiol Clin. 2006;24:453–69. doi: 10.1016/j.ccl.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 18.Macfarlane PW, McLaughlin SC, Devine B, Yang TF. Effects of age, sex, and race on ECG interval measurements. J Electrocardiol. 1994;27:14–9. doi: 10.1016/s0022-0736(94)80039-1. [DOI] [PubMed] [Google Scholar]
- 19.Okin PM, Roman MJ, Devereux RB, Kligfield P. Gender differences and the electrocardiogram in left ventricular hypertrophy. Hypertension. 1995;25:242–9. doi: 10.1161/01.hyp.25.2.242. [DOI] [PubMed] [Google Scholar]
- 20.Andreas S, Von Breska B, Schaumann A, Gonska BD, Kreuzer H. Obstructive sleep apnoea and signal averaged electrocardiogram. Eur Respir J. 1995;8:546–50. [PubMed] [Google Scholar]
- 21.Steiner S, Schueller PO, Hennersdorf MG, Strauer BE. Obstructive sleep apnea in heart failure patients: evidence for persistent conduction disturbances or sinus node dysfunction. J Physiol Pharmacol. 2008;59:669–74. [PubMed] [Google Scholar]
- 22.Weinberg EO, Thienelt CD, Katz SE, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34:264–73. doi: 10.1016/s0735-1097(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 23.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–65. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 24.Okin P, Gerdts E, Kjeldsen SE, et al. Devereux for the Losartan Intervention for Endpoint Reduction in Hypertension Study Investigators. Gender differences in regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy. Hypertension. 2008;52:100–6. doi: 10.1161/HYPERTENSIONAHA.108.110064. [DOI] [PubMed] [Google Scholar]
- 25.Smith JH, Baumert M, Nalivaiko E, McEvoy RD, Catcheside PG. Arousal in obstructive sleep apnea with ECG RR and QT interval shortening and PR interval lengthening. J Sleep Res. 2009;18:188–95. doi: 10.1111/j.1365-2869.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 26.Luo YQ, Yang Y, Yu SF. Influence of obstructive sleep apnea-hypopnea syndrome on the Qtc interval. Zhong Nan Da Xue Bao Yi Xue Ban. 2004;29:97–8. [PubMed] [Google Scholar]
- 27.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 28.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Shewan LG and Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]


