Summary
Purpose
Accumulating data have demonstrated that seizures induced by kainate (KA) or pilocarpine activate the mammalian target of rapamycin (mTOR) pathway and mTOR inhibitor rapamycin can inhibit mTOR activation which subsequently has potential anti-epileptic effects. However, a preliminary study showed a paradoxical exacerbation of increased mTOR pathway activity reflected by S6 phosphorylation when rapamycin was administrated within a short period before KA injection. In the present study, we examined this paradoxical effect of rapamycin in more detail, both in normal rats and KA-injected animals.
Methods
Normal Rats or KA-treated rats pretreated with rapamycin at different time interval were sacrificed at various time points (1h, 3h, 6h, 10h, 15h and 24h) after rapamycin administration or seizure onset for Western blotting analysis. Phosphorylation of mTOR signaling target of Akt, mTOR, Rictor, Raptor, S6K and S6 were analyzed. Seizure activity was monitored behaviorally and graded according to a modified Racine scale (n=6 for each time point). Neuronal cell death was detected by Fluoro-Jade B staining.
Key findings
In normal rats, we found that rapamycin showed the expected dose-dependent inhibition of S6 phosphorylation 3–24 h after injection, while a paradoxical elevation of S6 phosphorylation was observed 1 hour after rapamycin. Similarly, pretreatment with rapamycin over 10 h prior to KA inhibited the KA seizure induced mTOR activation. In contrast, rapamycin administered 1 to 6 hours before KA caused a paradoxical increase in the KA seizure-induced mTOR activation. Rats pretreated with rapamycin 1 h prior to KA exhibited an increase in severity and duration of seizures and more neuronal cell death as compared to vehicle treated groups. In contrast, rapamycin pretreated 10 h prior to KA had no effect on the seizures and decreased neuronal cell death. The paradoxical effect of rapamycin on S6 phosphorylation was correlated with upstream mTOR signaling and was reversed by pre-treatment of perifosine, an Akt inhibitor.
Significance
These data indicate the complexity of S6 regulation and its effect on epilepsy. Paradoxical effects of rapamycin need to be considered in clinical applications, such as for potential treatment for epilepsy and other neurological disorders.
Keywords: Rapamycin, mTOR signaling pathway, S6 phosphorylation, Kainate, paradoxical effect
Introduction
The mammalian target of rapamycin (mTOR) is a serine-threonine protein kinase, which forms two distinct cellular signaling complexes known as mTORC1 and mTORC2. mTOR signaling is regulated by a number of upstream pathways involved in responding to metabolic demands, growth signals, and other physiological stimuli. In turn, the mTOR pathway is an important regulator of cellular growth, proliferation, and survival, primarily via modulation of mechanisms mediating protein synthesis. Pathologically, dysregulation of the mTOR pathway may be involved in a variety of disease states, including cancer, diabetes, and neurological diseases (Zoncu et al., 2011). mTOR has been implicated in the pathophysiology of some types of epilepsy, such as in the genetic disease, tuberous sclerosis complex (TSC), as well as in animal models of acquired epilepsies (Wong, 2010). Furthermore, seizures in themselves activate increased mTOR activity (Zeng et al., 2009; Buckmaster and Lew, 2011; Zhang and Wong, 2012; Huang et al., 2010)
Rapamycin is a macrolide which was initially discovered as an anti-fungal agent with immunosuppresssive properties (Yatscoff et al., 1993). mTOR, specifically mTORC1, was subsequently found to be the primary mechanistic target of rapamycin. As a mTORC1 inhibitor, rapamycin also has antiproliferative properties and has been used as an anti-cancer agent. Rapamycin may have multiple downstream effects that mediate its anti-tumor actions, including direct inhibition of cell growth and proliferation, regulation of cell cycle, and modulation of cell death and survival (Jacinto et al., 2004; Manning & Cantley, 2007; Basso et al., 2011).
We have previously reported that the mTOR signaling pathway is activated during seizures triggered by the convulsant drug, kainate (KA), and that rapamycin pretreatment almost completely abolishes this seizure-induced mTOR activation and correspondingly has antiepileptogenic effects in inhibiting epilepsy (Zeng et al., 2009). In pilot studies to determine the effective timing and dose of rapamycin for inhibiting KA-induced mTOR activation, we initially observed a paradoxical exacerbation of increased mTOR pathway activity reflected by S6 phosphorylation when rapamycin was administrated within a short period before KA injection (Zeng et al., 2010). In the present study, we examined this paradoxical effect of rapamycin on S6 phosphorylation in more detail, including the critical time interval and dosage, both in normal rats and KA- induced seizure rats.
Materials and Methods
Animals and drug treatment
Male Sprague Dawley (SD) rats of 5–6 weeks of age were purchased from Shanghai Slac Laboratory Animal Corporation (certificate: SCXK 2007–0005). The rats were housed in a controlled environment with ad libitum access to food and water. All animal experiments were performed in accordance with guidelines approved by the Animal Studies Committee at Zhejiang University School of Medicine. Rapamycin was obtained from LC Laboratories (Woburn, MA, USA). It was initially dissolved in 100% ethanol, stored at 20°C, and diluted in a vehicle solution containing 5% Tween 80, 5% PEG 400 (low-molecular-weight grade of polyethylene glycol) (Sigma, St. Louis, MO, USA), and 4% ethanol immediately before injection, as described previously (Zeng et al., 2008). A couple of different rapamycin treatment paradigms were used. Some rats were treated only with rapamycin once by i.p. at different doses of 0.3, 1, 3, 10 mg/kg and sacrificed 1 h and 6 h later to observe the dose-dependent effects of rapamycin on S6 phosphorylation. For the time-course of rapamycin’s effect, rats were treated with 3 mg/kg rapamycin once and sacrificed at different time intervals. Other rats received rapamycin before KA injection (12 mg/kg, i.p.; Nanocs, New York, NY, USA) at predetermined time points, and sacrificed at different time points after seizure onset. Control rats received corresponding injections of vehicle in all experiments. Seizure activity was monitored behaviorally and graded according to a modified Racine scale (Racine, 1972) by two trained investigators blinded to the experimental groups: stage 1, behavioral arrest with mouth/facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling. The latency to first behavioral seizure activity, total seizure duration, and maximal stage severity were measured. Seizure scores were based on the stage of the most severe seizure recorded for each rat. Calculation of seizure duration was started as rats exhibited seizure stage 4, and ended when the rats moved freely around the cage. Rats that had stage 4 or 5 seizures were used for subsequent experiments.
Western blot analysis
Rapamycin treated normal rats and KA-induced seizure rats were killed for Western blot analysis of markers of mTOR activation at various time points (1 h, 3 h, 6 h, 15 h and 24 h) after rapamycin injection or seizure onset, respectively. Western blot analysis was performed using standard methods, as described previously (Zeng et al., 2008). In brief, proteins extracted from temporal neocortex and/or both whole hippocampi were separated by SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% skim milk, the membranes were incubated with the rabbit anti-phospho-S6 (Ser240/244), anti-phospho-Akt (Ser 473),anti-phospho-mTOR (Ser 2448),anti-phospho-Raptor (Ser 792),anti-phospho-Rictor (Thr1135) and anti-phospho-S6K (Thr 389) antibody (1:1000; Cell Signaling Technology, Beverly, MA, USA), followed by peroxidase conjugated anti-rabbit secondary antibody. After the signals were visualized with ECL reagent (Pierce, Rockford, IL, USA), the membranes were reprobed and incubated with the rabbit anti-S6, anti-Akt, anti-mTOR, anti-Raptor, anti-Rictor and anti-S6K antibody (1:1000; Cell Signaling Technology). Signals were quantitatively analyzed with NIH ImageJ software. Intensity of each lane in each blot was measured by ImageJ and ratio of p-S6 to total S6 was calculated. The ratio of p-S6/S6 of the control group was set as 1 and experimental groups were compared to the control group. Statistics were analyzed in at least 3 independent experiments.
Neuronal death assays
Rats treated with rapamycin 1h or 10 h prior to KA were killed for histological analysis of neuronal death by FJB (Histo-Chem, Jefferson, AR, USA) 7 d after KA-induced seizure. Rats were anesthetized with chloral hydrate (300mg/kg, i.p.) and transcardially perfused with PBS, followed by 4% paraformaldehyde. The brains were removed immediately and postfixed with 4% paraformaldehyde overnight at 4°C. After dehydrating in 30% sucrose for at least 24 h, the brains were sectioned coronally at a thickness of 20 µm with frozen microtome (Themo-Scientific). Three sections selected from a one-in-six series were collected from each animal at the same level of hippocampus and stained for FJB as described previously (Zeng et al., 2010; Schmued et al., 2000). Briefly, sections were first immersed in 1% NaOH/80% ethanol for 5 min and then sequentially in 70% ethanol, 50% ethanol and distilled water for 2 min each. After that the slices were transferred to 0.06% potassium permanganate solution for 10 min, rinsed gently, and stained with 0.0004% FJB and 0.1% acetate for 20 min in dark. The degree of neuronal death was assessed by counting FJB positive neurons in the hippocampal CA1 pyramidal layer under a Zeiss LSM confocal microscope with a 10×/ 0.3 NA objective to acquire images (920µm×920µm fields).
Statistics
All statistical analysis was performed using SPSS (SPSS 16.0, Chicago, IL, USA). Quantitative differences between groups were analyzed by one-way ANOVA or two-way ANOVA with Turkey’s post-hoc comparison when comparing more than two groups. Non-parametric comparison was conducted when comparing seizure stage. Quantitative data are expressed as mean ± SEM. A value of p < 0.05 was considered significant.
Results
Rapamycin treatment has paradoxical effects on S6 phosphorylation in normal rats
To examine the dose- and time-dependent effects of rapamycin on S6 phosphorylation, we first treated normal rats with rapamycin at the dose of 0.3, 1, 3 and 10 mg/kg and sacrificed 1 h (Fig. 1A) or 6 h (Fig. 1B) after rapamycin injection. In rats sacrificed 6 h after rapamycin administration, rapamycin inhibited S6 phosphorylation in a dose-dependent manner. This inhibitory effect was first observed at the dose of 1mg/kg (p<0.01 as compared with control group) and became more obvious when the dose increased to 3 and 10 mg/kg (p<0.001), whereas 0.3 mg/kg had no effect on S6 phosphorylation. To our surprise, in the rats sacrificed 1 h after rapamycin injection, S6 phosphorylation was increased at the dose of 1, 3 and 10 mg/kg. To clarify the time-dependent effects of rapamycin, normal SD rats were treated with rapamycin (3 mg/kg, i.p. once) and hippocampal and temporal neocortical total proteins were collected at different time points. As shown in Fig.1C, rapamycin showed a biphasic effect on S6 phosphorylation, which was first characterized by a paradoxical increase around 1 h after administration and then followed by the expected inhibition starting at 3h and extending to at least 24 h. Further study revealed that the increase in p-S6 started 10 min after rapamycin treatment, peaked at 1 h, and continued until 3 h (Fig. 5). To exclude the effect of vehicle on S6 activation, we treated rats with vehicle only and sacrificed them at different time points. No significant effect on S6 phosphorylation was observed in vehicle-treated groups (supplemental Fig. 1A). These results indicate that rapamycin induces a short-term paradoxical increase of p-S6 before its expected inhibition on S6 phosphorylation.
Figure 1.
Rapamycin had paradoxical effects on S6 phosphorylation in normal rats. Western blots of hippocampal and temporal neocortex homogenates were performed at various time points following rapamycin injection in adult rats. A: Rapamycin was administered i.p. in a dose of 0.3, 1, 3 and 10 mg/kg. Hippocampal homogenates were used for Western blotting 1 h after rapamycin injection. Rapamycin showed a paradoxical elevation of S6 phosphorylation at 1–10 mg/kg **p < 0.01, ***p < 0.001 (F=28.5) compared to the control group. B. Rapamycin injected 6 h prior to hippocampal homogenates exhibited the expected dose-dependent inhibition of S6 phosphorylation from 1 to 10 mg/kg. **p < 0.01 ***p < 0.001 (F=29.5) compared to the control group. C: Time-course of the paradoxical rapamycin effect on S6 phosphorylation. Rapamycin at the dose of 3 mg/kg caused a transient elevation for 1 hour, before longer term inhibition of S6 phosphorylation.*p < 0.01, #p < 0.01 (F=29.5) compared to the 0 h group (n=6 rats per group). Control groups were injected with the same volume of vehicle and sacrificed 1 h, 6 h and 0 h after injection in A, B and C, respectively.
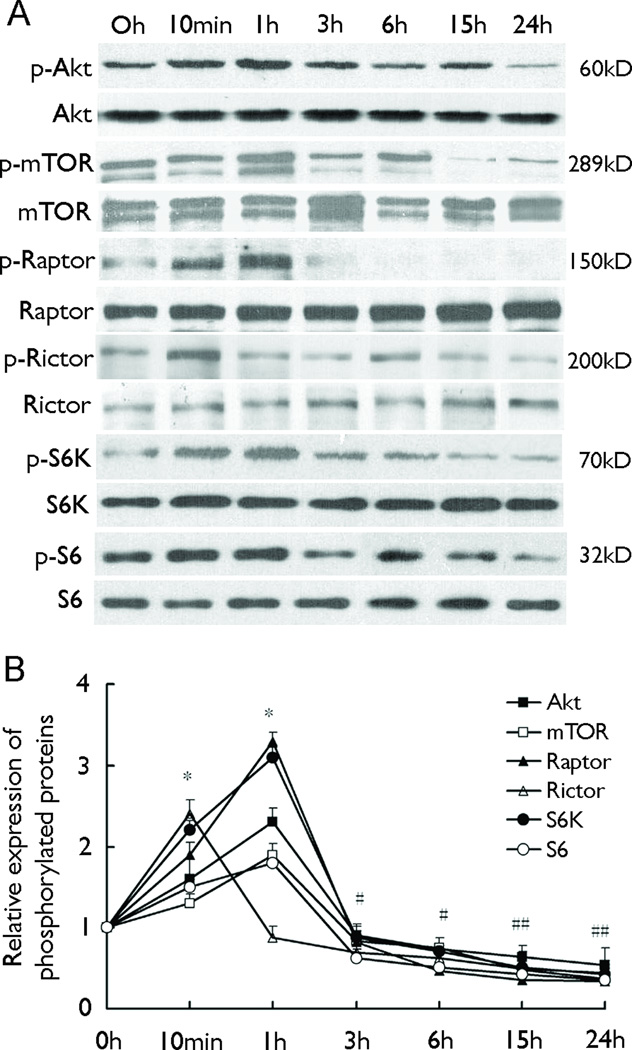
Figure 5.
The paradoxical effect of rapamycin on S6 phosphorylation is correlated with upstream mTOR signaling. Rats were injected with rapamycin once at the dose of 3 mg/kg and sacrificed at different time intervals. A: Representative blots of expression of proteins in hippocampus. B: Quantification of relative expression of phosphorylated proteins. p-Akt, p-mTOR, p-Raptor, p-S6K showed the same pattern of change as that of p-S6, which increased at 10 min and 1h as compared to 0 h, while p-Rictor peaked at 10 min (n=6 rats per group). *p<0.05 increase compared to the 0 h group in all groups except 1h Rictor.. # P<0.05 decrease compared to the 0 h group in all groups (F=41.5). The 0 h group was injected with rapamycin and sacrificed immediately.
Rapamycin has paradoxical effects on KA seizure-induced increase of S6 phosphorylation
We have previously reported that S6 phosphorylation increases with KA-induced acute seizures and that rapamycin treatment for 3 consecutive days prior to KA injection abolishes the KA-induced increase in p-S6 (Zeng et al., 2009). Here, we first treated rats with a single injection of rapamycin 3 mg/kg at various times prior to KA injection and assessed mTOR activation 1 h after status epilepticus (SE). As shown in Fig.2A, rats injected with KA exhibited a significant increase of p-S6 as compared to control rats, indicating activation of the mTOR signaling pathway by KA-induced seizure. Pretreatment with rapamycin at 1, 3 and 6 hours before KA injection, however, promoted an additional increase in mTOR activation evident by S6 phosphorylation. If administered 10 hours or longer before KA injection, rapamycin could almost completely inhibit the KA-induced increase of p-S6,which was identical to our previous report (Zeng et al., 2009).
Figure 2.
Rapamycin had paradoxical effects on KA-induced p-S6 elevation in hippocampus. Adult rats were injected with vehicle (Cont), KA (12 mg/kg, i.p.), or rapamycin (3 mg/kg) at different intervals before KA. A: Rats were sacrificed 1 h after rapamycin injection. KA alone (KA) caused increased p-S6 compared to vehicle (Cont). Pretreatment with rapamycin over 10 h prior to KA inhibited the KA-induced p-S6 elevation. In contrast, rapamycin administered 1 to 6 hours before KA caused a paradoxical increase in the KA-induced activation of S6. *p < 0.05, ***p < 0.001 compared to the KA group; #p<0.001 compared to the control group (F(4,15)=15.6). B: Rapamycin was administrated 1 h before KA and rats were sacrificed at different time intervals after SE (Rats were sacrificed immediately after receiving KA at 0 h group). Elevated p-S6 was observed from 1 to 6 h after KA-induced SE, and an additional increase in p-S6 expression occurred in rapamycin treated groups from 1 to 3 h. *p < 0.05 compared to the time-matched vehicle group; #p<0.001 compared to the 0h vehicle group (F(1,20)=10.5). C: Rapamycin was administrated 10 h before KA and no elevation of P-S6 was observed at any time point after SE. # P<0.001 compared to the control group (F(4,15)=2.43). n=6 rats for all groups. Control groups were injected with the same volume of saline 1 h (panel A) or 10 h (panel C) before vehicle injection, and sacrificed 1 h later.
Our previous data with acute KA-induced seizures showed that the p-S6 increase started at around 1 h after seizure onset, peaked at 3–6 h, and returned to baseline by 24 h in both hippocampus and neocortex (Zeng et al., 2009). In this study, we also tested the effect of pretreatment of rapamycin on p-S6 expression at different time intervals after KA administration. When rapamycin was pretreated 1 h prior to KA injection, the paradoxical increase in p-S6 expression to a level higher than KA alone was observed at 1–3 h after SE (Fig. 2B). p-S6 expression then returned to baseline by 15 h as compared to vehicle. In contrast, if rapamycin was injected 10 h prior to KA administration, it completely abolished the KA seizure-associated increase in S6 phosphorylation (Fig. 2C). Parallel treatments as Fig.2A and C were also performed in control rats, and no obvious changes were observed in different time point (Supplemental Fig. 1B and C). The paradoxical exacerbation of the KA seizure-induced p-S6 expression with 1 h rapamycin pretreatment is similar to the short-term paradoxical effects of rapamycin observed in normal rats.
Immediate rapamycin pretreatment aggravates KA-induced seizures
We examined whether paradoxical activation of mTOR by rapamycin was associated with changes in the seizures. Compared with vehicle-pretreated rats, rats pretreated with rapamycin 1 h prior to KA injection demonstrated exacerbated SE, including a higher seizure stage (Fig. 3B) and longer seizure duration (Fig. 3C) (P<0.05), while rats pretreated with rapamycin 10 h before KA injection exhibited no change. No change in seizure latency was observed among the three groups (Fig. 3A).
Figure 3.
Rapamycin administered immediately before KA aggravates KA-induced SE. Rapamycin at 3 mg/kg was pretreated 1 h or 10 h before injection. Rats pretreated with rapamycin 1 h prior to KA showed more severe seizures (B, P<0.05, F=10.8) and longer duration (C, P<0.05, F=4.06). No change was seen in seizure latency (P>0.05, F=0.161). In contrast, rapamycin pretreated 10 h prior to KA had no effect on the seizures. *p < 0.05 by ANOVA compared to the Veh+KA group (n=10 rats per group).
Immediate rapamycin pretreatment exacerbates KA-induced neuronal cell death
Next, we tested whether the paradoxical effect of rapamycin subsequently affects KA-induced neuronal death. As we reported previously (Zeng et al., 2009), KA injected rats exhibited robust neuronal cell death by Fluoro-Jade B (FJB) staining. All vehicle pretreated rats (n=6 of 6) demonstrated evidence of neuronal cell death by FJB staining after KA-induced SE in hippocampal CA1, CA3 and dentate hilus (Fig. 4B, CA1 is shown). Pretreatment with rapamycin 10 h before KA injection can significantly decrease neuronal death (Fig. 4D). In contrast, rats treated with rapamycin 1 h before KA-induced SE had more neuronal death compared with vehicle-pretreated KA rats (Fig. 4C).
Figure 4.
Rapamycin causes paradoxical effects on KA-induced cell death. KA induced SE caused cell death in the CA1 region of hippocampus, as detected by FJB staining 7 days after SE (B). Pretreatment with rapamycin 10 h prior to KA inhibited the KA-induced neuronal death (D). In contrast, rapamycin administered within 1 h before KA caused a paradoxical increase in cell death (C). *p<0.05 compared to the KA group. # P<0.001 compared to the control group (A) (F(2,20)=47.4). The control group was injected with the same volume of saline 1 h before vehicle injection. (n=10 rats per group). E. Quantification of FJB-positive cells per field in each group. Scale bar = 200 µm.
The paradoxical effect of rapamycin is induced by upstream Akt-mTOR signaling
To explore the possible mechanism which mediated the paradoxical effect of rapamycin within short periods after administration, we assessed the phosphorylation of several upstream signals of S6, including Akt, mTOR, Raptor, Rictor and S6K, in normal rats with one dose of rapamycin injection. All these proteins, except Rictor, exhibited increased phosphorylation starting at 10 min after rapamycin administration, peaking at 1 h, and then decreasing after 3 h (Fig. 5). In contrast, the increase of Rictor phosphorylation appeared earlier, which peaked around 10 min and began to decrease at 1 h.
Pretreatment with an Akt inhibitor abolished the rapamycin-induced transient increase of S6 phosphorylation
To further determine whether the paradoxical effect of rapamycin on S6 phosphorylation was related to upstream signals of Akt-mTOR, we treated rats with perifosine (20 mg/kg, ip, once), an Akt inhibitor, 30 min before rapamycin administration. Rats were sacrificed 1 h or 6 h after rapamycin injection. While rats treated with rapamycin exhibited increased S6 and Akt phosphorylation at 1 h, pretreatment with perifosine markedly decreased rapamycin induced activation of S6 and Akt (Fig. 6A, B). No significant difference was observed in rats treated with vehicle, rapamycin or both perifosine and rapamycin if the rats were sacrificed 6 h after rapamycin administration. Effects of perifosine per se on p-Akt and p-S6 in normal rats were also detected. As shown in Sup Fig. 2, perifosine markedly decreased p-Akt from 10 min to 24 hours and subsequently, moderately decreased p-S6 from 1h to 24 h after injection.
Figure 6.
The Akt inhibitor perifosine abolishes the rapamycin-induced transient paradoxical increase of p-S6. Perifosine (Peri) was administrated 30 min prior to rapamycin injection. Hippocampal homogenates were obtained 1 h or 6 h after vehicle or rapamycin injection. A: Representative blots of the expression of p-Akt, Akt, p-S6 and S6. B: Quantitative summary of p-Akt/Akt and p-S6/S6 at 1 h. Compared to the group of NS + Veh, phosphorylation of S6 and Akt were increased in the group of NS + Rap, whereas no significant increase was observed in the group of Peri + Rap (*p<0.01, F(2,12)=33.2, n=6 rats per group). The same volume of saline or vehicle were injected in NS + Veh group and sacrificed as the other groups.
Discussion
This study reports the surprising finding that the mTOR inhibitor, rapamycin, may cause a paradoxical, albeit transient, increase in mTOR pathway activation. We previously observed this phenomenon in the KA model, which involved conditions of intense neuronal activity due to SE (Zeng et al., 2010). In the present study, we confirm this previous observation with KA-induced seizures and provide further details of the critical timing during which paradoxical activation of mTOR occurs in relation to rapamycin and KA injection. Rapamycin administered within 1–6 hours of KA injection causes a higher level of S6 phosphorylation for 1–3 hours than KA alone. If rapamycin is injected at greater intervals from KA, the expected inhibition of S6 phosphorylation is observed. Beyond the KA model, the even more unexpected finding of the present study is that a similar phenomenon was also observed in control rats, indicating that this paradoxical effect of rapamycin is not dependent on KA or increased neuronal activity. In normal rats, rapamycin treatment paradoxically activates mTOR pathway evident by increased phosphorylation of S6 within 1–2 hours. Three hours after administration, rapamycin then converts to the expected long-term inhibition of S6 phosphorylation lasting at least 24 hours. The mechanistic basis and functional implications of these findings are currently unclear, but deserve further investigation.
The mechanisms by which rapamycin exerts paradoxical effects on the mTOR pathway could involve other upstream regulators of the mTOR pathway. In particular, S6 phosphorylation is regulated by other upstream signaling pathways, besides mTOR itself, such as PI3K-Akt, and is subjected to multiple positive and negative feedback loops (Sarbassov et al., 2006; Guertin et al., 2006; Huang et al., 2009; Toschi et al., 2009; Dibble et al., 2009; Julien et al., 2010). In the KA model, during the acute phase of KA-induced seizure activity, PI3K-Akt may be activated by seizures and by-pass and overcompensate for the direct mTOR inhibition by rapamycin. Similarly, in normal rats, direct mTOR inhibition by rapamycin may initially cause a release of feedback inhibition on PI3K-Akt, which then directly stimulates S6 phosphorylation. We have found the upstream mTOR signaling proteins of S6, including Akt, mTOR, Raptor and S6K, show transient elevations in phosphorylation, similar to that of S6. These possibilities were also tested with dual inhibitor studies, as perifosine pretreatment abolishes the rapamycin-induced S6 phosphorylation. Other reports have also demonstrated that rapamycin induces Akt phosphorylation in cancer cell lines, although the mechanism remains controversial (Breuleux et al., 2009; Wang et al., 2008; Chen et al., 2010; Sun et al., 2005; O’Reilly et al., 2006). Our results demonstrate that phosphorylation of S6 is correlated with that of upstream mTOR signaling proteins.
The functional implications of the paradoxical mTOR activation are also uncertain. In previous studies of animal models of epilepsy, rapamycin inhibited mTOR activation and correspondingly had anticonvulsant or antiepileptogenic actions (Zeng et al., 2008; Zeng et al. 2009; Huang et al., 2010; Raffo et al., 2011; Sunnen et al., 2011; Talos et al., 2012; van Vliet et al., 2012). In the present study in the KA model, the paradoxical mTOR activation by rapamycin was associated with more severe SE, characterized by higher seizure score and longer duration. One limitation of this study was the exclusive use of video monitoring, which may not be as accurate or sensitive as EEG. Nevertheless, we were still able to detect a significant effect of rapamycin on seizures using video monitoring alone, indicating that this is likely a robust effect. Furthermore, the period of paradoxical increased mTOR activity correlated with the timing of the increased seizure severity, both occurring within the first several hours after rapamycin. Thus, it is uncertain whether the paradoxical mTOR activation causes the more severe seizures or is simply a result of the increased seizure activity. However, the similar finding of paradoxical mTOR activation with rapamycin in normal rats suggests that this is a primary phenomenon, not secondary to seizures. Subsequently, the paradoxical mTOR activation effect of rapamycin is also associated with greater neuronal death. Again, this could reflect a direct effect of increased mTOR signaling on cell death mechanisms or may be related to the increased seizures, which secondarily cause cell death (Yellen et al., 2011; Yang et al., 2011). In either case, these paradoxical effects need to be considered in clinical applications, as mTOR inhibitors are being considered as potential treatments for epilepsy and other neurological disorders (Zeng et al., 2009; Huang et al., 2010; Franz et al., 2006; Krueger at al, 2010).
Taken together, our present data stimulate the question of just how much increase of S6 phosphorylation following rapamycin actually contribute to seizure. One could speculate that rapamycin induced S6 activation within short time may abolish the anti-epileptic activity of rapamycin, which is under investigation in our group now. However, our results, together with others, demonstrate that there is a high level of complexity associated with the regulation of S6 phosphorylation induced by rapamycin.
Supplementary Material
Supplemental figure 1: Effects of vehicle on S6 phosphorylation in normal rats. Representative blots of protein expression in hippocampus and statistical results were shown. A: SD rats were injected i.p. with 500 – 800 µl vehicle once and sacrificed at various time points. B: SD rats were injected with vehicle at various time points prior to saline and sacrificed 1 h after saline administration. C. Rats were injected with vehicle 10 h prior to saline and sacrificed at various time points after saline administration. Control rats did not receive any injection. No significant change was observed in each group.
Supplemental figure 2: Effects of perifosine on S6 and Akt phosphorylation in normal rats. SD rats were injected i.p. with perifosine at the dose of 20 mg/kg once and sacrificed at various time points for Western blotting analysis. A: Representative blots of protein expression in hippocampus. B. Quantitative summary of p-Akt/Akt and p-S6/S6. Perifosine inhibition of Akt phosphorylation was observed 10min after administration and lasted untill 24 h, while the inhibition in S6 phosphorylation occurred from 1 h to 24 h *p < 0.001 (F=77.3) compared to the control group. Control group received saline and sacrificed in 10 min (n=6 rats per group).
Acknowledgments
This project was supported by National Natural Science Foundation of China (81072621), Zhejiang Provincial Natural Science Foundation of China (Y2100417), Foundation of Qianjiang Talents (QJD1002012), Scientific Research Foundation for Returned Scholars, Ministry of Education of China(2011)and National Institutes of Health (NIH R01 NS056872 to MW). We thank Dr. Xiaoyi Sun for her kind help in statistics.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Basso MS, Subramaniam P, Tredger M, Verma A, Heaton N, Rela M, Mieli-Vergani G, Dhawan A. Sirolimus as renal and immunological rescue agent in pediatric liver transplant recipients. Pediatr Transplant. 2011;15:722–727. doi: 10.1111/j.1399-3046.2011.01560.x. [DOI] [PubMed] [Google Scholar]
- Breuleux M, Klopfenstein M, Stephan C, Cheryl A, Doughty CA, Barys L, Kwiatkowski D, Lane HA. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8:742–753. doi: 10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XG, Liu F, Song XF, Wang ZH, Dong ZQ, Hu ZQ, Lan RZ, Guan W, Zhou TG, Xu XM, Lei H, Ye ZQ, Peng EJ, Du LH, Zhuang QY. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinog. 2010;49:603–610. doi: 10.1002/mc.20628. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC1 components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKC alpha but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Huang JX, Wu SL, Wu CL, Manning BD. Signaling events downstream of mammalian target of rapamycin complex2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res. 2009;69:6107–6114. doi: 10.1158/0008-5472.CAN-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raffo E, Coppola A, Ono T, Briggs Sw, Galanopoulo AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–328. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turclos E, Mukhi S, Parghi D, D'Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen F. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTORC1) pathway. PLoS ONE. 2012;7:e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, Den Burger JCG, Sinjewel A, De Vries HE, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin(mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-I nitiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Xiao X, Meng X, Leslie KK. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLoS One. 2011;6:e26343. doi: 10.1371/journal.pone.0026343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatscoff RW, LeGatt DF, Kneteman NM. Therapeutic monitoring of rapamycin: a new immunosuppressive drug. Ther Drug Monit. 1993;15:478–482. doi: 10.1097/00007691-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Yellen P, Saqcena M, Salloum D, Feng J, Preda A, Xu L, Feng J, Preda A, Xu L, Rodrik-Outmezguine V, Foster DA. High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1. Cell Cycle. 2011;10:3948–3956. doi: 10.4161/cc.10.22.18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, McDaniel S, Rensing NR, Wong M. Regulation of cell death and epileptogenesis by the mammalian target of rapamycin (mTOR): a double-edged sword? Cell Cycle. 2010;9:2281–2285. doi: 10.4161/cc.9.12.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wong M. Pentylenetetrazole-induced seizures cause acute, but not chronic, mTOR pathway activation in rat. Epilepsia. 2012;53:506–511. doi: 10.1111/j.1528-1167.2011.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Effects of vehicle on S6 phosphorylation in normal rats. Representative blots of protein expression in hippocampus and statistical results were shown. A: SD rats were injected i.p. with 500 – 800 µl vehicle once and sacrificed at various time points. B: SD rats were injected with vehicle at various time points prior to saline and sacrificed 1 h after saline administration. C. Rats were injected with vehicle 10 h prior to saline and sacrificed at various time points after saline administration. Control rats did not receive any injection. No significant change was observed in each group.
Supplemental figure 2: Effects of perifosine on S6 and Akt phosphorylation in normal rats. SD rats were injected i.p. with perifosine at the dose of 20 mg/kg once and sacrificed at various time points for Western blotting analysis. A: Representative blots of protein expression in hippocampus. B. Quantitative summary of p-Akt/Akt and p-S6/S6. Perifosine inhibition of Akt phosphorylation was observed 10min after administration and lasted untill 24 h, while the inhibition in S6 phosphorylation occurred from 1 h to 24 h *p < 0.001 (F=77.3) compared to the control group. Control group received saline and sacrificed in 10 min (n=6 rats per group).