Abstract
The biosynthesis of thymine nucleotides in Saccharomyces cerevisiae can be inhibited either by genetic lesions in the structural gene for thymidylate synthetase (TMP1) or by drugs that prevent the methylation of dUMP to dTMP. This methylation can be blocked by folate antagonists. We find that 5-fluoro-dUMP (FdUMP) is also an effective inhibitor in vivo. Inhibition of dTMP biosynthesis by these three different routes causes thymineless death. In addition to being cytotoxic, we find that FdUMP is highly recombinagenic in yeast but does not induce nuclear gene mutations. Provision of exogenous dTMP eliminates this induced mitotic recombination and cell killing. Similar results were obtained when a thymineless condition was provoked in cells by antifolate drugs or by dTMP deprivation in strains auxotrophic for this nucleotide. These findings show that, in contrast to the situation in prokaryotes, starvation for thymine nucleotides in yeast induces genetic recombination but is not mutagenic.
Full text
PDF
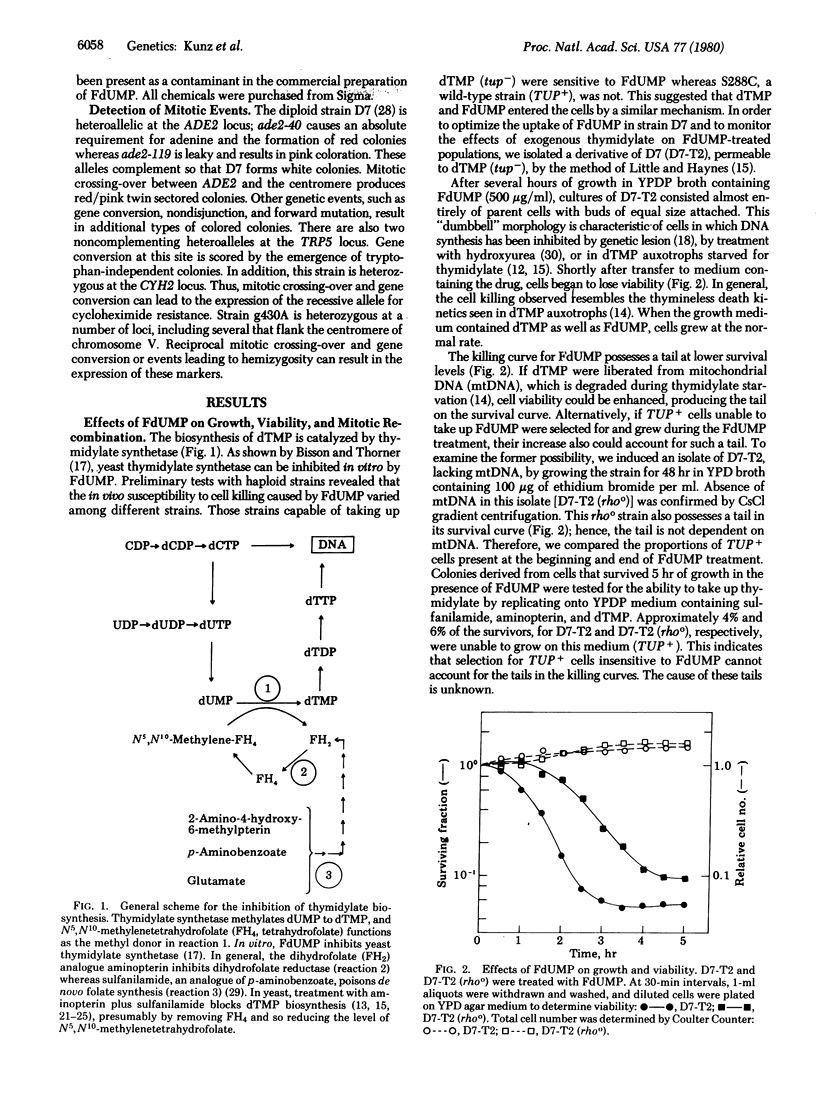

Images in this article
Selected References
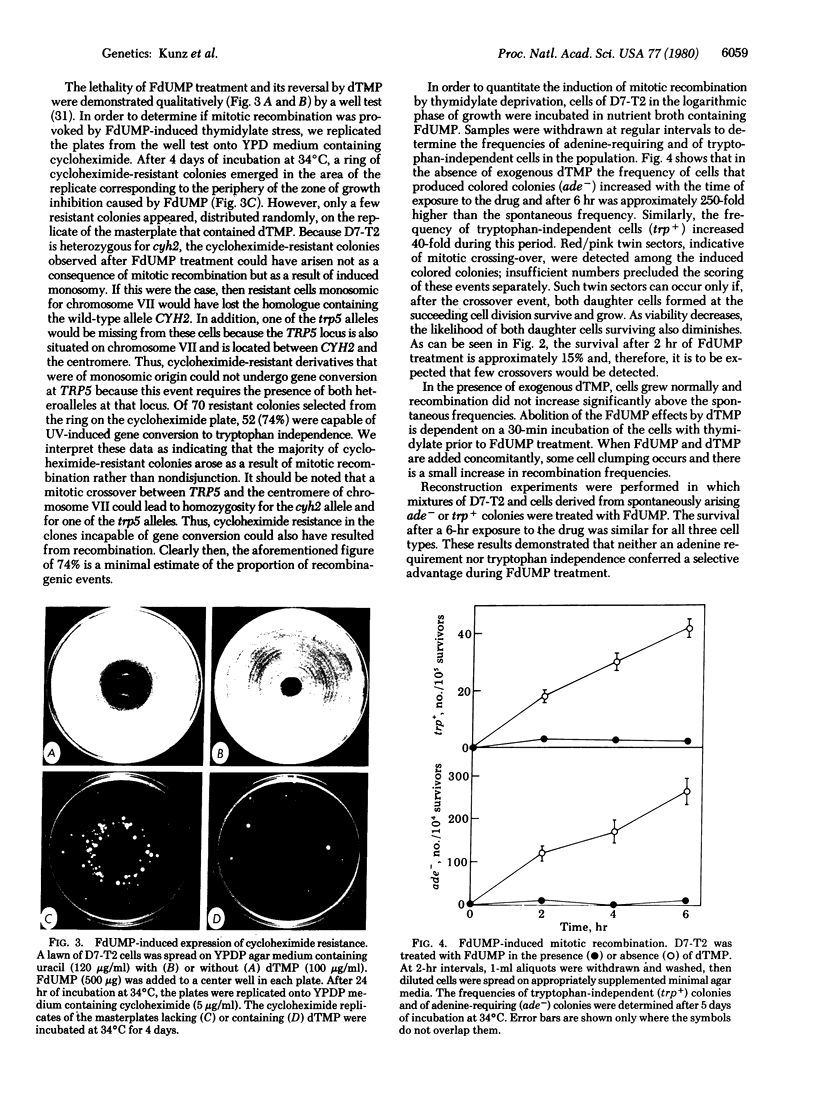
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., COUGHLIN C. A. Bacterial mutation induced by thymine starvation. Nature. 1956 Sep 8;178(4532):531–532. doi: 10.1038/178531a0. [DOI] [PubMed] [Google Scholar]
- Barclay B. J., Little J. G. Genetic damage during thymidylate starvation in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Mar 20;160(1):33–40. doi: 10.1007/BF00275116. [DOI] [PubMed] [Google Scholar]
- Barclay B. J., Little J. G. Selection of yeast auxotrophs by thymidylate starvation. J Bacteriol. 1977 Dec;132(3):1036–1037. doi: 10.1128/jb.132.3.1036-1037.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousque J. L., Sicard N. Size and transforming activity of deoxyribonucleic acid in Diplococcus pneumoniae during thymidine starvation. J Bacteriol. 1976 Nov;128(2):540–548. doi: 10.1128/jb.128.2.540-548.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel M., Langjahr U. G. "Thymineless death" in a strain of Saccharomyces cerevisiae auxotrophic for deoxythymidine-5'-monophosphate. Mol Gen Genet. 1974;131(4):351–358. doi: 10.1007/BF00264865. [DOI] [PubMed] [Google Scholar]
- Bresler S. E., Mosevitsky M. I., Vyacheslavov L. G. Mutations as possible replication errors in bacteria growing under conditions of thymine deficiency. Mutat Res. 1973 Sep;19(3):281–293. doi: 10.1016/0027-5107(73)90228-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
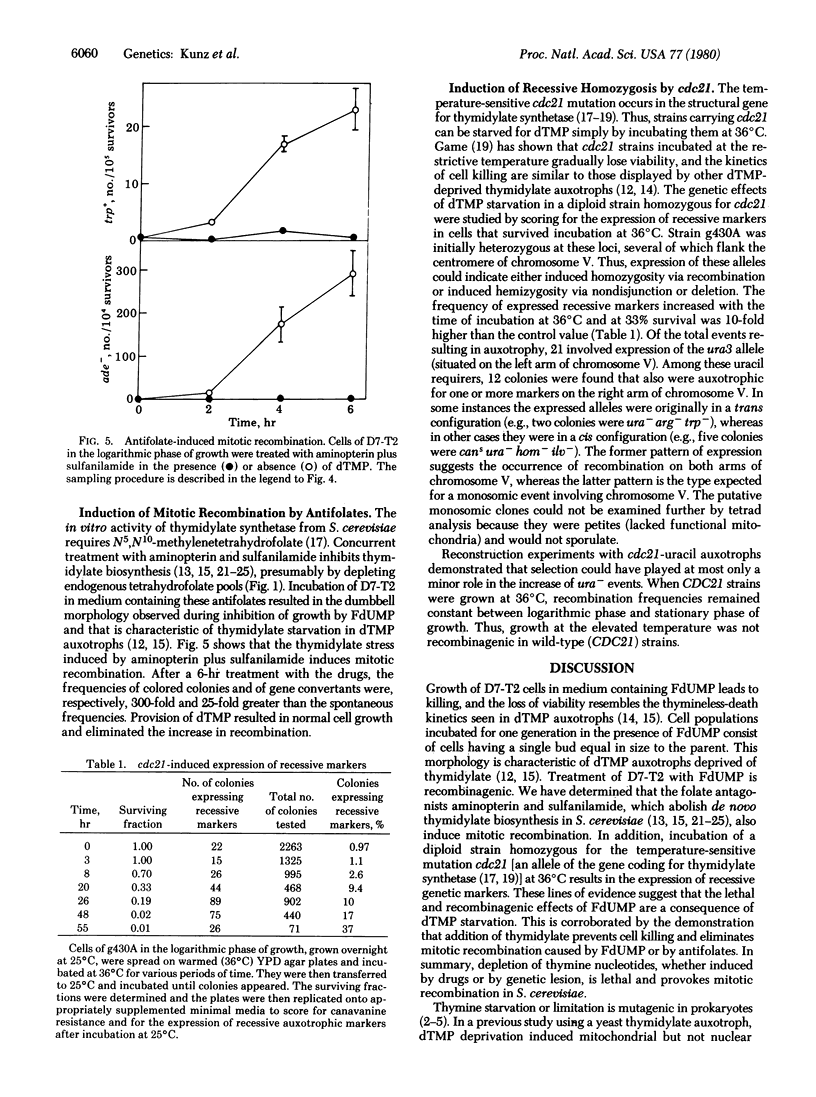
- Eckardt F., Haynes R. H. Induction of pure and sectored mutant clones in excision-proficient and deficient strains of yeast. Mutat Res. 1977 Jun;43(3):327–338. doi: 10.1016/0027-5107(77)90056-2. [DOI] [PubMed] [Google Scholar]
- Fäth W. W., Brendel M., Laskowski W., Lehmann-Brauns E. Economizing DNA-specific labelling by deoxythymidine-5'-monophosphate in Saccharomyces cerevisiae. Mol Gen Genet. 1974;132(4):335–345. doi: 10.1007/BF00268573. [DOI] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. doi: 10.1128/jb.82.2.187-194.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Spottswood T. The recombinogenic effect of thymidylate starvation in Escherichia coli merodiploids. Genetics. 1965 Jul;52(1):107–118. doi: 10.1093/genetics/52.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C., Johnston L. H., von Borstel R. C. Enhanced mitotic recombination in a ligase-defective mutant of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4589–4592. doi: 10.1073/pnas.76.9.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Game J. C. Yeast cell-cycle mutant cdc21 is a temperature-sensitive thymidylate auxotroph. Mol Gen Genet. 1976 Aug 2;146(3):313–315. doi: 10.1007/BF00701257. [DOI] [PubMed] [Google Scholar]
- Goulian M., Bleile B., Tseng B. Y. Methotrexate-induced misincorporation of uracil into DNA. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1956–1960. doi: 10.1073/pnas.77.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell A. R., Jackson J. F. Thymidine kinase: evidence for its absence from Neurospora crassa and some other micro-organisms, and the relevance of this to the specific labelling of deoxyribonucleic acid. J Gen Microbiol. 1968 Dec;54(2):307–317. doi: 10.1099/00221287-54-2-307. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Three additional genes required for deoxyribonucleic acid synthesis in Saccharomyces cerevisiae. J Bacteriol. 1973 Sep;115(3):966–974. doi: 10.1128/jb.115.3.966-974.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. J., Eisenstark A. The mutagenic effect of thymine-starvation on Salmonella typhimurium. Mutat Res. 1968 Jan-Feb;5(1):15–21. doi: 10.1016/0027-5107(68)90076-6. [DOI] [PubMed] [Google Scholar]
- Hryniuk W. M., Bertino J. R. Growth rate and cell kill. Ann N Y Acad Sci. 1971 Nov 30;186:330–342. [PubMed] [Google Scholar]
- Jannsen S., Witte I., Megnet R. Mutants for the specific labelling of DNA in Saccharomyces cerevisiae. Biochim Biophys Acta. 1973 Apr 11;299(4):681–685. doi: 10.1016/0005-2787(73)90243-8. [DOI] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Laskowski W., Lehmann-Brauns E. Mutants of Saccharomyces able to grow after inhibition of thymidine phosphate synthesis. Mol Gen Genet. 1973 Sep 12;125(3):275–277. doi: 10.1007/BF00270749. [DOI] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. G., Hanawalt P. C. Thymineless death and ultraviolet sensitivity in Micrococcus radiodurans. J Bacteriol. 1973 Jan;113(1):233–240. doi: 10.1128/jb.113.1.233-240.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
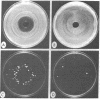
- Little J. G., Haynes R. H. Isolation and characterization of yeast mutants auxotrophic for 2'-deoxythymidine 5'-monophosphate. Mol Gen Genet. 1979 Jan 10;168(2):141–151. doi: 10.1007/BF00431440. [DOI] [PubMed] [Google Scholar]
- Little J. W. The effect of 5-bromouracil on recombination of phage lambda. Virology. 1976 Jul 15;72(2):530–535. doi: 10.1016/0042-6822(76)90184-7. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Hanawalt P. Sedimentation analysis of deoxyribonucleic acid from thymine-starved Escherichia coli. J Bacteriol. 1975 Feb;121(2):537–547. doi: 10.1128/jb.121.2.537-547.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney R. J., Jacob A. E., Hedges R. W., Smith J. T. Dislodgement and thymineless elimination of N-group plasmids. Genet Res. 1977 Aug;30(1):21–30. doi: 10.1017/s0016672300017432. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- Reichenbach D. L., Schaiberger G. E., Sallman B. The effect of thymine starvation on chromosomal structure of Escherichia coli JG-151. Biochem Biophys Res Commun. 1971 Jan 8;42(1):23–30. doi: 10.1016/0006-291x(71)90356-1. [DOI] [PubMed] [Google Scholar]
- Slater M. L. Effect of reversible inhibition of deoxyribonucleic acid synthesis on the yeast cell cycle. J Bacteriol. 1973 Jan;113(1):263–270. doi: 10.1128/jb.113.1.263-270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Green R. R., Ripley L. S., Drake J. W. Thymineless mutagenesis in bacteriophage T4. Genetics. 1973 Jul;74(3):393–403. doi: 10.1093/genetics/74.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Lehman I. R. Excision repair of uracil incorporated in DNA as a result of a defect in dUTPase. J Mol Biol. 1977 Dec 5;117(2):293–306. doi: 10.1016/0022-2836(77)90128-0. [DOI] [PubMed] [Google Scholar]
- Walker J. R. Thymine Starvation and Single-Strand Breaks in Chromosomal Deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1391–1392. doi: 10.1128/jb.104.3.1391-1392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz E. A., Sears B. B., Rabert D. K., Shepherd H. S., Gillham N. W., Boynton J. E. A specific increase in chloroplast gene mutations following growth of Chlamydomonas in 5-fluorodeoxyuridine. Mol Gen Genet. 1979 Mar 5;170(3):235–242. doi: 10.1007/BF00267056. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K. Double-strand scission of DNA involved in thymineless death of Escherichia coli 15 TAU. Biochim Biophys Acta. 1973 Jan 19;294(2):204–213. [PubMed] [Google Scholar]
- Zimmermann F. K. A yeast strain for visual screening for the two reciprocal products of mitotic crossing over. Mutat Res. 1973 Oct;21(5):263–269. [PubMed] [Google Scholar]