Abstract
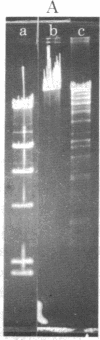
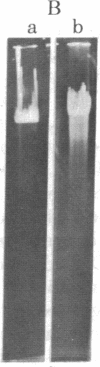
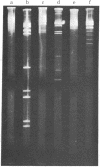
An endonuclease has been isolated from human B lymphoblastoid cells that copurifies with an exonucleolytic activity and has been shown to produce double-strand breaks and a high proportion of single-strandedness in phage lambda DNA in vitro. The data are consistent with a model in which single-strand cuts are made by the endonucleolytic activity, possibly in A+T-rich regions of the DNA, followed by creation of single-stranded regions (gaps) precessing from the site of a cut. Generation of overlapping gaps on opposite strands or of a gap opposite a nick would lead to the creation of the banding patterns that we have seen on electrophoretic gels. This endonucleolytic activity copurifies with other enzymes induced by Epstein-Barr virus that relate to the process of viral DNA replication in productively infected cells. However, a more general role is proposed for this class of eukaryotic endonuclease activities. A marked degree of single-strandedness has been found in the replicating DNAs of many eukaryotes, ad these gaps could be generated by endonucleases with associated exonucleolytic activity such as that reported here. This Epstein-Barr virus-induced nuclease activity has been shown to resemble the recBC nuclease isolated from the prokaryote Escherichia coli and also the endonuclease isolated from the eukaryote Chlamydomonas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Electron microscopic analysis of replicating DNA of sea urchin embryos. Cell. 1978 Nov;15(3):1095–1107. doi: 10.1016/0092-8674(78)90293-3. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Gussander E., Lindahl T. Long regions of single-stranded DNA in human cells. Nature. 1979 Aug 2;280(5721):420–423. doi: 10.1038/280420a0. [DOI] [PubMed] [Google Scholar]
- Burton W. G., Roberts R. J., Myers P. A., Sager R. A site-specific single-strand endonuclease from the eukaryote Chlamydomonas. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2687–2691. doi: 10.1073/pnas.74.7.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case S. T., Baker R. F. Investigation into the use of Aspergillus oryzae S1 nuclease in the presence of solvents which destabilize or prevent DNA secondary structure: formaldehyde, formamide, and glyoxal. Anal Biochem. 1975 Apr;64(2):477–484. doi: 10.1016/0003-2697(75)90457-1. [DOI] [PubMed] [Google Scholar]
- Chetsanga C. J., Boyd V., Peterson L., Rushlow K. Single-stranded regions in DNA of old mice. Nature. 1975 Jan 10;253(5487):130–131. doi: 10.1038/253130a0. [DOI] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Berry D. E., Cobbs C. S. Structure of parental deoxyribonucleic acid of synchronized HeLa cells. Biochemistry. 1977 Dec 13;16(25):5438–5444. doi: 10.1021/bi00644a006. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Schreier P. H., Buchel D. E. Nucleotide sequence of the attachment site of coliphage lambda. Nature. 1977 Dec 22;270(5639):757–760. doi: 10.1038/270757a0. [DOI] [PubMed] [Google Scholar]
- Dickinson D. G., Baker R. F. Evidence for translocation of DNA sequences during sea urchin embryogenesis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5627–5630. doi: 10.1073/pnas.75.11.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint S. J., Berget S. M., Sharp P. A. Characterization of single-stranded viral DNA sequences present during replication of adenovirus types 2 and 5. Cell. 1976 Dec;9(4 Pt 1):559–571. doi: 10.1016/0092-8674(76)90038-6. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Goodman S. R., Prezyna C., Benz W. C. Two Epstein-Barr virus-associated DNA polymerase activities. J Biol Chem. 1978 Dec 10;253(23):8617–8628. [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulatory function of simian virus 40 DNA replication for late viral gene expression. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4831–4834. doi: 10.1073/pnas.74.11.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Pereira M., Scharff R. DNA synthesis involving a complexes form of DNA polymerase I in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2099–2103. doi: 10.1073/pnas.72.6.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. The presence of single-stranded regions in mammalian DNA. J Mol Biol. 1978 Mar 15;119(4):487–506. doi: 10.1016/0022-2836(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Landy A. Structure of the lambda att sites generated by int-dependent deletions. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5437–5441. doi: 10.1073/pnas.75.11.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. DNase induced after infection of KB cells by herpes simplex virus type 1 or type 2. II. Characterization of an associated endonuclease activity. J Virol. 1979 Nov;32(2):449–457. doi: 10.1128/jvi.32.2.449-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA VIII. Properties of the replicating DNA. J Virol. 1977 Aug;23(2):394–411. doi: 10.1128/jvi.23.2.394-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean J. H., Ben-Porat T. Appearance in vivo of single-stranded complementary ends on parental herpesvirus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2674–2678. doi: 10.1073/pnas.73.8.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Malone R. E., Chattoraj D. K., Faulds D. H., Stahl M. M., Stahl F. W. Hotspots for generalized recombination in the Escherichia coli chromosome. J Mol Biol. 1978 Jun 5;121(4):473–491. doi: 10.1016/0022-2836(78)90395-9. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Old R., Murray K., Boizes G. Recognition sequence of restriction endonuclease III from Hemophilus influenzae. J Mol Biol. 1975 Feb 25;92(2):331–339. doi: 10.1016/0022-2836(75)90232-6. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Rosamond J., Endlich B., Telander K. M., Linn S. Mechanisms of action of the type-I restriction endonuclease, ecoB, and the recBC DNase from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1049–1057. doi: 10.1101/sqb.1979.043.01.114. [DOI] [PubMed] [Google Scholar]
- SCHACHMAN H. K., ADLER J., RADDING C. M., LEHMAN I. R., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VII. Synthesis of a polymer of deoxyadenylate and deoxythymidylate. J Biol Chem. 1960 Nov;235:3242–3249. [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]