Abstract
Flower and fruit development in tomato (Lycopersicon esculentum Mill.) were severely affected when plants were grown at low temperatures, displaying homeotic and meristic transformations and alterations in the fusion pattern of the organs. Most of these homeotic transformations modified the identity of stamens and carpels, giving rise to intermediate organs. Complete homeotic transformations were rarely found and always affected organs of the reproductive whorls. Meristic transformations were also commonly observed in the reproductive whorls, which developed with an excessive number of organs. Scanning electron microscopy revealed that meristic transformations take place very early in the development of the flower and are related to a significant increase in the floral meristem size. However, homeotic transformations should occur later during the development of the organ primordia. Steady-state levels of transcripts corresponding to tomato MADS-box genes TM4, TM5, TM6, and TAG1 were greatly increased by low temperatures and could be related to these flower abnormalities. Moreover, in situ hybridization analyses showed that low temperatures also altered the stage-specific expression of TM4.
In several plant species flower and fruit development are highly sensitive to low, nonfreezing temperatures (Polowick and Sawhney, 1985; Lynch, 1990; Shuff and Thomas, 1993). In fact, when tomato (Lycopersicon esculentum) and pepper plants are exposed to low temperatures, they produce flowers showing alterations in the number, morphology, and pattern of fusion of floral organs. As a consequence, abnormal fruits of low economic value are produced from these flowers (Sawhney, 1983; Polowick and Sawhney, 1985; Barten et al., 1992). Many of these flower and fruit abnormalities have been reproduced by exogenous treatments of young flower buds with GA3 or cytokinins, suggesting a role for these plant hormones as mediators in the LT effects (Sawhney, 1983; Sawhney and Shukla, 1994; Venglat and Sawhney, 1996). Work with snapdragon and Arabidopsis thaliana has allowed the genetic and molecular characterization of several families of regulatory genes that control flower initiation and development. However, the effect of environmental factors or hormone treatments on these regulatory genes has so far only been analyzed in a few instances (Estruch et al., 1993).
In Arabidopsis the specification of floral meristem identity requires at least two genes, LEAFY (LFY) and APETALA1 (AP1), the functions of which have been shown to be sufficient to promote flower initiation (Mandel and Yanofsky, 1995; Weigel and Nilsson, 1995). In this species and in snapdragon, floral organ identity depends on three homeotic functions, A, B, and C, which define three overlapping regions, each comprising two adjacent whorls (for reviews, see Coen, 1991; Coen and Meyerowitz, 1991). Mutations in genes responsible for any of these functions promote homeotic transformations and, consequently, abnormal floral organogenesis. Cloning and sequence analyses have revealed that many of the genes determining floral organ and floral meristem identities encode proteins belonging to the same family of transcriptional activators sharing a conserved DNA-binding domain known as the MADS box (Schwarz-Sommer et al., 1990). However, meristic mutations altering the number of floral organs have also been characterized in Arabidopsis. Genes such as CLAVATA1 (CLV1) and CLAVATA3 (CLV3) seem to be required to regulate the size of the shoot and floral meristems, which in turn affects the number of floral organs that develop in each whorl. clv1 and clv3 plants develop enlarged shoot and floral meristems, which give rise to flowers with additional organs in each whorl, with the third (stamen) and fourth (carpel) whorls being the most affected by these mutations (Clark et al., 1993, 1995, 1997).
In tomato several members of the MADS-box gene family have recently been cloned and characterized (Pnueli et al., 1991, 1994a, 1994b). One of these genes, TM4, has been considered an “early” gene, since its transcripts are not detected in mature flower organs and it shows sequence similarities with AP1 and SQUAMOSA (SQUA). Two other genes, TM5 and TM6, have been considered “late” genes because their transcripts are more abundant in mature flower organs than in floral meristems (Pnueli et al., 1991, 1994a). TM6 shows the highest sequence similarity to DEFICIENS (DEF), a class B gene of snapdragon. However, its expression pattern, like that of TM5, does not correspond to the patterns of any of the homeotic functions mentioned above, since they are expressed and required for organ differentiation in the three inner whorls (Pnueli et al., 1994a). Finally, TAG1, a C-function homeotic gene homologous to the AGAMOUS (AG) gene of Arabidopsis, controls stamen and carpel development in tomato flowers and prevents the indeterminate growth of floral meristems (Pnueli et al., 1994b).
To understand the molecular mechanisms that are responsible for the abnormal development of tomato flowers and fruits at low temperatures, we have characterized tomato flower development at standard and at low temperatures using SEM, and analyzed the expression patterns of different tomato MADS-box genes under the two temperature conditions. We show here that low temperatures cause the production of both homeotic and meristic transformations and that these transformations are associated with a drastic increase in the steady-state expression levels of the tomato MADS-box genes considered. The possibility that these alterations in gene expression could be responsible for the morphogenetic changes affecting the identity, the number, and the fusion patterns of floral organs at low temperature is discussed.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of tomato (Lycopersicon esculentum Mill. cv Rambo) (Novartis Seeds, Enkhuizen, The Netherlands) were sown in plastic pots filled with a sphagnum peat/vermiculite substrate mixture (3:1, v/v) and germinated under dark conditions at 25°C for 8 to 10 d. After germination plants were grown at standard temperatures of 26°C day/18°C night, and when they had developed three to four true leaves, were transferred to growth chambers at two different temperature conditions: standard and low temperatures, 17°C day/7°C night. In both cases, plants were grown under a 16-h light photoperiod provided by wide-spectrum tubes (450 μmol s−1 m−2; Gro-lux, Sylvania, Germany), and watered twice a week with a mineral nutrient solution commonly used in greenhouse culture conditions for this species. Young flowers collected for RNA isolation belonged to two size classes, one smaller than 0.5 cm (class 0, samples ST0 and LT0), and the other larger than 1 cm in length (class 2, samples ST2 and LT2). Although both developmental stages correspond to fully differentiated flower buds, only the latter includes young flowers with a completely developed gynoecium.
Light Microscopy and SEM
To determine the size of the floral meristems in ST and LT plants, floral buds were fixed in FAE (2% formaldehyde, 5% acetic acid, and 60% absolute ethanol) and stored in 70% ethanol. The size of a floral meristem in a given developmental stage was determined as the largest diameter at the time of initiation of organ primordia in the preceding whorl. Thus, measurements at the prepetal stage mean the largest width of the floral meristem at the time of sepal primordia initiation, and so on. Sizes were measured through a Nikon stereomicroscope equipped with a calibrated ocular micrometer. For SEM analysis, floral buds were fixed, prepared, visualized, and photographed as previously described (Sommer et al., 1990).
RNA-Blot Hybridization
Total RNA was isolated from young flowers of the two size classes mentioned above, as described by Nagy et al. (1988). Poly(A+) RNA was selected from total RNA by using the Quick-Prep mRNA purification kit (Pharmacia). Samples were size fractionated on 1% formaldehyde agarose gels and transferred to nitrocellulose membranes (Schleicher & Schuell), following the protocol provided by the manufacturer. Integrity and equal loading of the poly(A+) samples were confirmed by hybridization of the filters to an oligo-dT probe. Quantification of the hybridization signal was obtained with a Bioimage plate reader (Millipore). The probes were labeled with [α-32P]dCTP (3000 Ci mmol−1) by random primer extension (Feinberg and Vogelstein, 1983). Prehybridization and hybridization were performed at 65°C following standard protocols (Sambrook et al., 1989). After washing, filters were exposed to Kodak X-Omat film at −80°C.
Probes were gene specific and were obtained from standard PCR amplifications using the corresponding clones as the templates. Appropriate enzyme digestions were subsequently performed to eliminate the conserved and 5′-located MADS box. The 3′ probe for TM4 was synthesized from a 780-bp DdeI fragment of the amplification product from pLE8, which is the TM4 cDNA inserted as a 911-bp EcoRI fragment into the pBluescript SK+ vector (Stratagene). The 3′ TM5 probe was synthesized from a 708-bp ScaI fragment obtained from the amplification of pLE9, a clone containing the TM5 cDNA as a 910-bp EcoRI fragment in the pBluescript SK+ vector. The 3′ probe for TM6 probe was synthesized from a 448-bp EcoRV fragment of pLE14, which is the TM6 cDNA inserted as a 902-bp EcoRI fragment into the pBluescript SK+ vector. The 3′ TAG1 probe was synthesized from a 875-bp HindIII fragment obtained from the PCR amplification of pAS5, a clone containing the TAG1 cDNA as a 1084-bp EcoRI fragment in the pGEM7Z vector. A β-ATPase probe (Boutry and Chua, 1985) from tobacco was used as a control for mRNA not inducible by low temperatures.
In Situ-Hybridization Analysis
For in situ hybridization, inflorescences containing floral buds of different sizes and selected immature flowers were fixed in FAE, embedded in Paraplast Plus, and sliced into 8-μm sections following standard procedures. Subsequently, the material was prepared and in situ hybridization was performed using the protocol described by Huijser et al. (1992) with minor modifications. Plasmids containing specific probes were digested to avoid transcription and labeling of the MADS-box sequence. The TM4 35S-labeled antisense mRNA was synthesized with the T7 RNA polymerase from a DdeI-digested pLE8 template. The TM5 35S-labeled antisense mRNA was synthesized with T7 RNA polymerase from an ScaI-digested pLE9 template. The TM6 35S-labeled antisense mRNA was synthesized with the T7 RNA polymerase from a BglII-digested pLE14 template. The TAG1 35S-labeled antisense mRNA was synthesized with Sp6 RNA polymerase from an SphI-digested pAS5 template. The corresponding 35S-labeled sense transcripts were synthesized and used as the controls. Probes were used at a final concentration of 1.2 to 1.5 × 106 cpm mL−1.
RESULTS
Effects of Low Temperature on Tomato Flower Development
Tomato plants grown at low temperatures showed similar vegetative development as plants grown at standard temperatures. They follow a sympodial developmental pattern in which, after the production of seven to eight true leaves, the apical meristem gives rise to a lateral monochasial inflorescence (Fig. 1a) (Sawhney and Greyson, 1972; Hareven et al., 1994). In these inflorescences the apical meristem (Fig. 1b) acquires the identity of a floral meristem (Fig. 1c), whereas lateral meristems take over to maintain the inflorescence meristem. ST tomato flowers are composed of four whorls. The two outer whorls contain five to seven organs, sepals and petals, respectively, whereas the reproductive whorls normally contain six stamens fused in a staminal cone and four fused carpels (Fig. 2a).
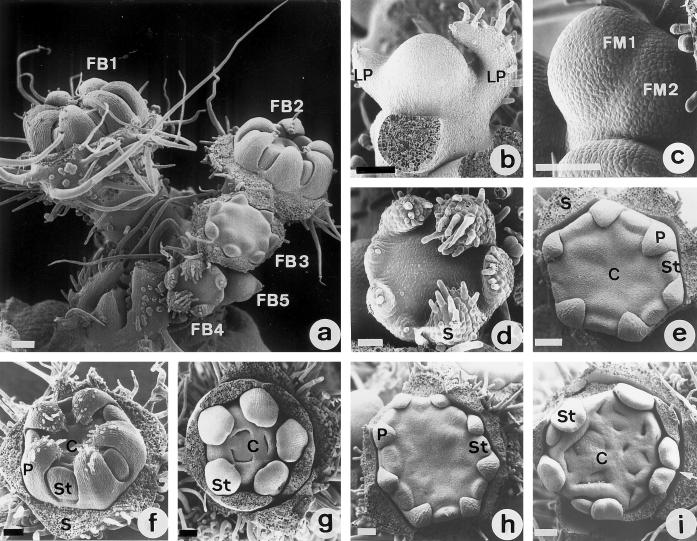
Figure 1.
SEM analysis of tomato floral organogenesis at standard and low temperature. a, ST inflorescence. FB1 through FB5 represent floral buds at different developmental stages. Sepals of FB1 through FB3 have been removed to show the internal whorls. b, Apical meristem from an ST plant flanked by two leaf primordia (LP). c, Floral meristem (FM1) showing the characteristic flattened morphology in contrast to the round shape of the vegetative apex (see b). Note that a new floral meristem (FM2) is laterally initiated. d, Prepetal stage of an ST floral bud in which six sepal primordia (S) are initiated following an helical pattern. Epidermal hairs are differentiating on the top of the sepal primordia. e, Floral bud from an ST inflorescence showing the simultaneous development of six petal primordia (P) and an equal number of stamen primordia. Sepals (S) were removed. f, ST floral bud showing organ primordia of petals (P), stamens (St), and carpels (C). Stamen primordia are in alternate positions to petals and opposite to sepals (removed). Note the initiation of carpel primordia (C) in the innermost whorl. g, Initiation of five stamens (St) and three carpel (C) primordia in an ST floral bud. The former are initiated independently, although later they fuse longitudinally. Carpel primordia are initiated at the center of the meristematic region following a regular pattern (sepal and petal primordia have been removed). h, LT floral bud at a similar stage of development as that of (e). Note the larger size and the higher number of petal (P) and stamen (St) primordia that have been initiated. i, LT floral bud at the same developmental stage as that of Figure 5g. Note the excessive number of stamen (St) and carpel (C) primordia, as well as the abnormal pattern of organ distribution and development. Splitting of the stamen primordia (arrow) and the larger size of the meristem are also remarkable features of LT floral buds. Bars = 100 μm, except for a, in which the bar = 200 μm.
Figure 2.
Homeotic and meristic transformations affecting tomato flowers and floral organs grown at low temperatures. a, Wild-type tomato flower showing seven yellow petals and the staminal cone. b, LT flower showing a higher number of stamens. These remain unfused and the flower lacks the staminal cone. c, Abnormal LT flower showing an excessive number of floral organs in all of the whorls. d, Gynoecium with additional carpels forming a multilocular ovary. Note the splitting of the style and the presence of petaloid sepals (arrow) (petals and stamens removed). e, Abnormal fusion between a stamen and the pistil in an LT flower. f, Petal (left) and petaloid sepals (right) at the first whorl of an LT flower. g, Staminoid petal (arrowhead) formed by anther and petal tissues at the second whorl. h, Petaloid stamen at the third whorl (arrow). Note that this intermediate organ is not fused to the forming staminal cone. i, Abaxial view of carpelloid stamens (left and center) developed in LT flowers compared with normal stamens (right). j, Adaxial view of the same organs as shown in i. k, Stamen-like tissue (strong yellow) forming part of a carpel.
Flower developmental abnormalities promoted by low temperatures in tomato plants could be grouped into three categories, i.e. changes in the organ number (meristic changes), in the pattern of organ fusion, and in the organ identity (homeotic changes). Moreover, many LT flowers show more than one type of such abnormalities. Meristic changes affecting the reproductive whorls of the flowers were the most evident effects of low temperatures (Table I). LT flowers showed additional stamens and almost twice the number of carpels (Fig. 2, b–d). Up to 12 stamens and more than 15 carpels could be observed in the most severely affected flowers, which split into two flowers in some cases. The increase in organ number could be due to an increased size of the floral meristem or to the splitting of floral organ primordia after their initiation. To test these possibilities, we measured the floral meristem size in ST and LT plants and performed a SEM analysis under both temperature conditions. Floral meristems initiated under low-temperature conditions were significantly larger than those initiated under standard-temperature conditions, irrespective of their developmental stage (Table II). The increase in floral meristem size produced by low temperature was related to a higher number of cells rather than to an increase in cell size (data not shown). This increase could be the cause of the initiation and development of a higher number of floral organs and could also promote, in extreme cases, the splitting of the floral meristem and the development of twin flowers.
Table I.
Organ number in whorls of ST and LT flowers
| Floral Organ | ST | LT | P |
|---|---|---|---|
| Sepal | 6.22 ± 0.69 | 6.35 ± 1.13 | n.s. |
| Petal | 6.26 ± 0.66 | 6.31 ± 0.92 | n.s. |
| Stamens | 6.38 ± 0.73 | 8.33 ± 2.02 | < 0.001 |
| Carpels | 4.12 ± 0.97 | 7.00 ± 2.49 | < 0.001 |
The sizes of ST and LT samples were 50 and 45, respectively. Mean values were compared by means of a Student's t test and are shown ± sd. n.s., Not significantly different.
Table II.
Meristem sizes at different stages of floral development in tomato plants grown at ST and LT conditions
| Stage of Development | ST | LT | P |
|---|---|---|---|
| μm | |||
| Presepal | 197.50 ± 11.18 (20) | 206.67 ± 14.84 (15) | < 0.05 |
| Prepetal | 227.17 ± 16.71 (23) | 239.58 ± 14.59 (24) | < 0.01 |
| Prestamen | 250.00 ± 22.36 (16) | 269.64 ± 22.31 (14) | < 0.05 |
| Prepistil | 281.82 ± 31.80 (11) | 312.50 ± 29.46 (10) | < 0.05 |
Mean values were compared by means of a Student's t test and are shown ± sd. Numbers in parentheses indicate sample size.
The initiation of a higher number of organ primordia under low temperature was confirmed by the results of the SEM analysis. In control ST plants floral organ differentiation started with the development of five to seven sepal primordia from the flanks of the floral meristem in a helical pattern (Fig. 1d). In each of the remaining whorls, organ primordia developed simultaneously from the inner meristematic cells, producing five to seven petals (Fig. 1e), five to six stamens, and three to four carpels (Fig. 1, f and g). Organs of a given whorl alternate in position with respect to those of neighboring whorls in such a way that stamens alternate with petals, which alternate with sepals (Fig. 1, e and f). SEM analysis showed that in LT plants, flower meristems initiated a higher number of stamen and carpel primordia (Fig. 1, h and i). Stamen primordia initiated independently, as in control flowers, although they did not always maintain an alternate position with respect to petal primordia and were usually heterogeneous in size (Fig. 1i). Frequently, larger stamen primordia showed splitting phenomena, giving rise to twin stamens (Fig. 1i). Carpel primordia in the innermost whorl of LT flowers were initiated in a higher number and did not follow the regular pattern of spatial distribution observed under standard temperature, resulting in a disordered appearance (Fig. 1i). Therefore, these results indicate that the low-temperature promoted increase in the number of reproductive organs is the consequence of both the initiation of a higher number of organ primordia and the splitting of larger primordia giving rise to twin organs.
Low temperatures also generated alterations in the fusion pattern of reproductive organs (Table III). Unlike normal flowers, in which stamens appear ontogenetically fused through trichome interlocking (Fig. 2a), more than 60% of the LT flowers showed a lack of anther fusion, preventing the formation of the staminal cone (Fig. 2, b and c). Carpel fusion at the level of the style was also partially prevented in more than 30% of the LT flowers (Fig. 2d). Furthermore, low temperatures caused unusual fusion events between stamens and carpels, generally involving late longitudinal fusions between poorly developed stamens and abnormal ovaries (Fig. 2e). Fusion patterns of perianth organs were not affected by the experimental low- temperature conditions.
Table III.
Frequency of different types of abnormalities in LT tomato flowers
| Floral Abnormalitiesa | Percent |
|---|---|
| Organ-fusion changesb | |
| Lack of fusion of the stamens | 63.1 |
| Splitting of the style | 31.8 |
| Stamens fused to carpels | 15.4 |
| Unfused carpels | 2.0 |
| Identity changesb | |
| Petaloid sepals | 4.1 |
| Staminoid petals | 2.0 |
| Petaloid stamens | 2.0 |
| Carpelloid stamens | 17.9c |
| Staminoid carpels | 8.7 |
| Petals replacing stamens | 3.6 |
Of the sample of 195 LT flowers, 40 showed some kind of abnormality.
Abnormal flowers usually showed more than one type of abnormality.
Twenty-three percent of these were bearing external ovules.
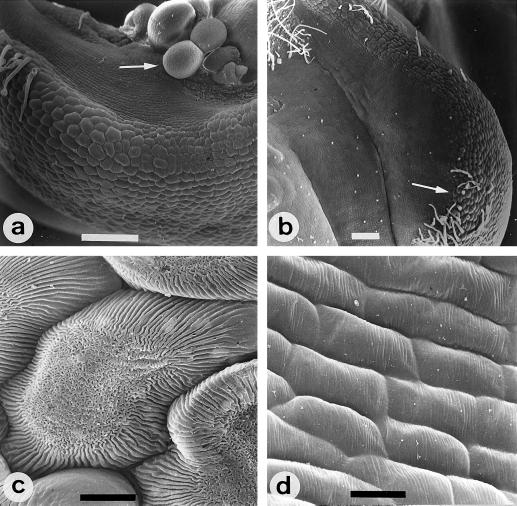
Parallel to the meristic alterations already described, nearly 20% of the flowers grown under low-temperature conditions showed alterations in the identity of their organs (Fig. 2). The frequency of organ-fusion and organ-identity abnormalities is shown in Table III. Identity changes gave rise to chimeric organs, showing tissue sectors with the identity of adjacent whorls. Complete homeotic changes were also observed, although at a much lower frequency. In the perianth the chimeric organs that we found were petaloid sepals (Fig. 2f) and staminoid petals (Fig. 2g), which were observed in 4 and 2%, respectively, of the analyzed flowers. Sepal and petal sectors in these abnormal organs displayed their typical glandular and filliformed hairs, respectively. Reproductive whorls showed a higher frequency of organ identity changes. Floral organs in the third whorl could develop either as petaloid or carpelloid structures. Petaloid stamens made up of a petal-colored tissue spreading along the anther or even fully developed petals were observed in 2% of the LT flowers (Fig. 2h). Carpelloid stamens showing carpel tissue either on the incipient filament or in the anther were observed in 18% of the LT flowers (Fig. 2, i and j). In both cases, the morphology of cells and hairs that develop on each organ section confirmed the identity of the organ (Fig. 3). One-fourth of the carpelloid stamens showed naked ovules on the internal side of the carpel sectors (Fig. 3a) and, occasionally, anthers failed to develop. Identity abnormalities in the gynoecium, consisting of stamen-like tissue associated to carpels (Figs. 2k and 3b), appeared in 9% of the LT flowers (Table III). Although pollen grains were not observed, the yellow color of this tissue and particularly the morphology of the cells confirmed its stamen nature (Fig. 3, b and c). SEM analyses performed on these intermediate organs proved their origin as the combination of patches of cells with different fates. So, typical epidermal cells of stamens (Fig. 3c) and carpels (Fig. 3d), identical to those existing in stamens and carpels of wild-type flowers (data not shown), were clearly present both in carpelloid stamens and in staminoid carpels (Fig. 3, a and b).
Figure 3.
Cell morphology of chimeric reproductive organs developed in LT flowers. Both carpelloid stamens (a) and staminoid carpels (b) develop tissue regions in which epidermal cells have the same features of wild-type stamens and carpels. At the mid-region of these chimeric organs, stamen cells were irregular in shape, were of a large size, and were carriers of cuticular and reticulate thickenings on their surface (c). The shape of the carpel cells was elongated and regular; they were smaller and showed cuticular ridges parallel to the shorter cell axis (d). Carpelloid stamens (a) are able to develop naked ovules from the carpel tissue (arrow). Note that the stamen cells (arrow) in the central region of the staminoid carpel (b) are larger than the flanking carpel cells. Bar = 200 μm in a and b; bar = 10 μm in c and d.
Expression of Tomato MADS-Box Genes in ST and LT Flowers
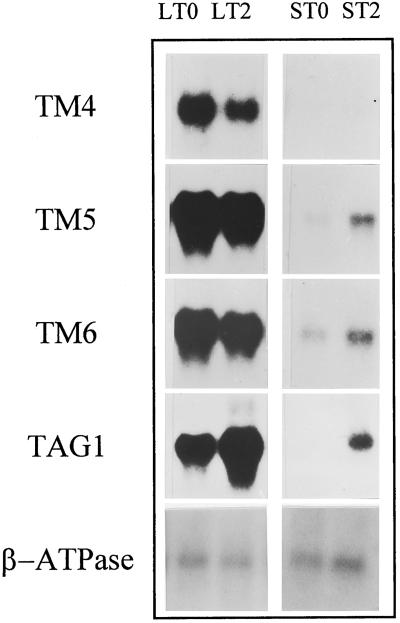
The observation of homeotic changes in tomato flowers grown at low temperatures led us to characterize the expression levels and patterns of the tomato MADS-box homeotic genes TM4, TM5, TM6, and TAG1 identified previously (Pnueli et al., 1991, 1994b). RNA blot-hybridization experiments using poly(A+) RNA isolated from young flower buds corresponding to two different developmental stages (see Methods) revealed that low temperatures caused a drastic accumulation of transcripts hybridizing with probes of the above-mentioned genes in both developmental stages (Fig. 4). In tomato flowers grown at standard temperatures, TM4 was only scarcely detected in young flower buds, whereas the expression of either TM5, TM6 or TAG1 increased with the size of flowers when the gynoecium had fully developed (Fig. 4), in agreement with what has been described for the expression of these floral organ-identity genes (Pnueli et al., 1991, 1994a, 1994b). Transcripts corresponding to the mitochondrial β-ATP synthase, used as a control, did not show any significant change in its steady-state level, supporting the specificity of the effect of low temperatures on the expression of MADS-box genes (Fig. 4).
Figure 4.
Northern-blot analysis of MADS-box gene expression in LT and ST tomato flowers. For each temperature, poly(A+) RNA was isolated from floral buds of two classes, 0 and 2, and hybridized with the probes prepared according to the descriptions in Methods. A β-ATP synthase from tobacco was used as the expression control of an mRNA not inducible by low temperatures.
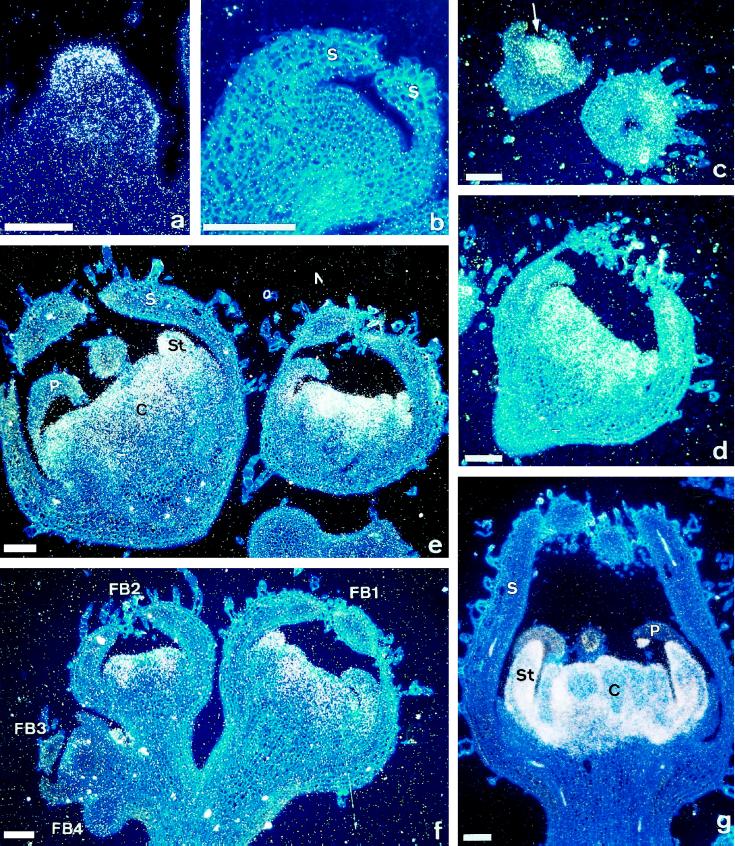
Spatial expression patterns of TM4, TM5, TM6, and TAG1 were analyzed by in situ-hybridization experiments using flower buds in different developmental stages (Fig. 5). Under standard temperatures expression of TM4 was found early in floral meristems (Fig. 5a), disappearing when floral organ primordia started to emerge (Fig. 5b). Under low temperatures, TM4 transcripts were still detected after the initiation of sepal primordia in the central region of floral meristems, with the signals being confined to cells fated to develop the most internal organs (Fig. 5c). Expression of TM4 decreased later in the development of LT flowers, although a weak signal could still be observed in all of the organs of fully developed flowers (Fig. 5d). These results were consistent with the results of the RNA blot-hybridization experiments previously shown. In spite of the strong low-temperature-induced accumulation observed for TM5, TM6, and TAG1 transcripts (Fig. 4), we could not observe any sign of ectopic expression of these messengers in the in situ hybridization experiments. In our experimental conditions, TM5 and TM6 messengers accumulated in the floral meristem region that gives rise to petal, stamen, and carpel primordia in both ST and LT flowers, and were particularly abundant in developed stamens and carpels (Fig. 5, e and f). However, we could not find any hybridization signal of these genes in the earliest stages of flower development either in ST (data not shown) or in LT flowers (see FB3 and FB4 in Fig. 5f). As previously observed in ST flowers (Pnueli et al., 1994b), TAG1 was detected in the primordia and mature organs of the two inner whorls of LT flowers, although the highest hybridization signal was found in developed stamens and carpels (Fig. 5g). Although in situ-hybridization experiments have failed to detect changes in the expression patterns of TM5, TM6, and TAG1 in LT flowers, we cannot rule out the possibility that these changes do take place. Given the fact that genes TM5 and TM6 are expressed in whorls 2, 3, and 4, their ectopic expression would only be expected in specific sectors of petaloid sepals, which only occur as late events in 4% of the studied flowers (Table III). In the case of TAG1, a gene regularly expressed in reproductive whorls (stamens and carpels), ectopic expression would only be expected in staminoid petals. Again, the relatively low frequency with which these intermediate organs appear (2%) hampers the identification of ectopic TAG1 expression by means of in situ hybridization experiments.
Figure 5.
In situ-hybridization patterns of MADS-box gene mRNA transcripts in ST and LT tomato flower buds. a, Longitudinal section of an ST floral meristem displaying TM4 expression. TM4 transcripts are not present, however, when sepal primordia (S) emerge (b). In LT flowers, TM4 transcripts are visible in the central region of the floral meristem (c, arrow). d, Longitudinal section of a young LT flower hybridized with a TM4 antisense probe. e, Expression of TM5 in two longitudinal sections of young LT flowers. Signals are restricted to cells from which the three inner whorls will develop. f, Longitudinal section through a young LT inflorescence hybridized with a TM6 probe. Expression of this gene is visible at the first (FB1) and second (FB2) floral buds but not at earlier stages (FB3 and FB4). g, Distribution of TAG1 transcripts in a young LT flower. Hybridization signals are restricted to stamens (St) and carpels (C). P, Petals. Probes were prepared according to descriptions made in Methods. All pictures are dark-field-fluorescence double exposures, which makes the silver grains (representing RNA expression) appear white on blue-fluorescent tissue. Bars = 100 μm.
DISCUSSION
Low Temperatures Promote Meristic Changes in the Reproductive Organ Whorls of Tomato Flowers
Tomato plants grown under low-temperature conditions during their reproductive development show a dramatic increase in the number of reproductive floral organs, in agreement with previous studies performed in tomato and pepper (Shawney, 1983; Chandra Sekhar and Shawney, 1984). Our SEM analyses show that this increase in organ number is mainly due to the initiation of a higher number of organ primordia in the floral meristems. Nevertheless, splitting events taking place in the largest organ primordia can also contribute to this phenomenon, particularly in the staminal whorl. These meristic changes are related to a significant increase in the size of floral meristems that results from a higher number of cells. The size increase of the floral meristem and the higher sensitivity of whorls 3 and 4 to the increase in organ number resemble the phenotype caused by weak alleles at loci CLV1 to CLV3 of Arabidopsis. These phenotypic similarities could suggest an effect of low temperature on the expression or activity of the corresponding tomato genes. Unfortunately, tomato orthologs of the Arabidopsis CLV1 and CLV3 genes have not been isolated, which precludes the analysis of the effects of low temperature on their expression. Additionally, the size increase of the floral meristem could be related to the up-regulation of the TM5 gene expression, since Pnueli et al. (1994a) have suggested a possible role for the TM5 protein in the establishment of the correct size of the floral meristem. Furthermore, Zachgo et al. (1995) showed that early expression of DEF (most similar to TM6) controls the initiation of fourth whorl-organ (carpel) development. Therefore, elevated TM6 transcription could also affect carpel initiation on the fourth whorl.
Alterations in the pattern of organ fusion in whorls 3 and 4 could be the consequence of the initiation and development of a high number of organ primordia. This is particularly applicable to the formation of the staminal cone, where the excessive number of carpels can hinder the fusion of anthers around the pistil. However, the existence of flowers with regular carpel number but unfused stamens, or vice versa, flowers with increased carpel number and a well-developed staminal cone indicates that low temperatures directly affect anther fusion events in the absence of organ number alterations. Carpel fusion does not seem to be related to the increased number of carpels that lead to the formation of a multilocular ovary. In only 2% of the analyzed flowers is it possible to find unfused isolated carpels that develop separately from the main multilocular ovary. Similarly, carpel fusion does not seem to be affected in clv mutants of Arabidopsis also displaying a high carpel number (Clark et al., 1995).
Low Temperatures Induce Late Homeotic Changes Mainly Affecting Organs in the Reproductive Whorls
Nearly 19% of the flowers initiated and developed at low temperature show some of the homeotic transformations described above, as identified by morphological markers of cell identity. As expected for homeotic transformations, these changes always reproduce the organ identity of adjacent whorls and the intermediate organs formed keep the growth pattern characteristic of the whorl they occupy. The observed homeotic transformations always follow normal patterns in their time of appearance, the kind of transformation, and the whorls affected. Regarding the time of appearance, homeotic transformations take place late in flower organ development, giving rise to chimeric organs. As shown by SEM analyses, low temperatures do not affect the temporal and spatial patterns of organ initiation and, apart from occasional primordia splitting, they do not seem to alter the first stages of organ primordia development. Thus, the observed identity changes would be the consequence of late homeotic transformations taking place in specific cells and cell lineages of the developing organs. However, since only a small number of altered sectors are found in most flowers, the effects of low temperatures seem to be restricted to a few cells in specific stages of their cell cycle or in specific physiological conditions. Once a cell has suffered an epigenetic change of fate, this would be maintained by autoregulatory mechanisms which, in essence, could be similar to the positive autoregulation proposed for homeotic genes such as DEF and GLO (Schwarz-Sommer et al., 1992). With respect to the whorls affected and the kind of transformations, low-temperature-induced homeotic transformations are more frequently produced in the reproductive whorls (85%), promoting the formation of petaloid and carpelloid stamens, staminoid carpels, and petals replacing the stamens. Considering the flower as a whole, transformations most commonly reproduce identity features of the adjacent inner whorl. The only exceptions to this rule are the production of petaloid stamens and staminoid carpels. These results suggest that low temperature would more frequently displace the fate of cells in a given primordium toward the primordia identity of the next whorl. Since morphogenetic alterations conferred by TM5 antisense RNA display a clear tendency to vegetative features of the floral organs (Pnueli et al., 1994a), it is conceivable that the observed TM5 overexpression induced by low temperatures could cause the opposite effect.
RNA blot-hybridization analyses indicate that abnormal flowers bearing homeotic and meristic alterations show elevated steady-state mRNA levels of all of the MADS genes analyzed in this work, TM4, TM5, TM6, and TAG1. This increase is specific for these mRNAs and is related to the magnitude of the temperature decrease at night. In fact, transcripts of these genes, and particularly of TM4, accumulated at higher levels in tomato flowers developed under winter conditions in the greenhouse (4.5°C average night temperature) than in plants grown under the experimental low-temperature treatments provided in growth chambers (data not shown). The effect of low temperature on the expression levels of homeotic genes could be mediated through changes on hormone biosynthesis and/or sensitivity. In fact, treatment of tomato flower buds with GA3 produces similar transformations to the ones observed under low-temperature-growing conditions (Sawhney, 1983), and hormonal regulation of homeotic gene expression has been suggested in several cases (Estruch et al., 1993; Okamuro et al., 1996, 1997; Venglat and Sawhney, 1996). Additional evidence supporting this possibility comes from the quantification of higher levels of GAs in LT than in ST flowers and from the observed increase in the mRNA levels of some of these homeotic genes, which is caused by GA3 treatments of tomato flower buds at standard-temperature conditions (T. Angosto, C. Payan, P. Gómez, and R. Lozano, unpublished results).
The increase in TM4 expression has been related to an altered temporal and spatial expression pattern along the development of the LT flowers by in situ-hybridization experiments. Similar effects on spatial distribution have not been detected for transcripts TM5, TM6, and TAG1 in the same flowers. However, it is reasonable to think that these changes could take place in specific groups of cells, although with a frequency below the level of detection for the number of experiments performed, since only a small percentage of flowers showed homeotic transformations (see Table III). Overexpression of TM4 could be responsible in part for the overexpression found for the other homeotic genes, given its early expression in the floral meristem. Because not all of the flower buds that have been exposed to low temperatures and that show elevated levels of homeotic gene expression on RNA-blot hybrid develop homeotic transformations, it is not possible to establish a full correlation between these two effects of low temperatures. Other variables, as yet unknown, should participate in determining the origin of a homeotic transformation in a group of cells. As mentioned before, these unknown variables could be related to the physiological state of the cell or its cell-cycle phase. Additional studies will be required to identify these variables and to understand the network of interactions that can affect or determine the final size and shape of fruits.
ACKNOWLEDGMENTS
We are grateful to Dr. E. Lifschitz (Technion-Israel Institute of Technology, Israel) and Dr. M.F. Yanofsky (University of California, San Diego) for providing us with the probes of tomato MADS-box genes.
Abbreviations:
- LT
low-temperature-grown
- SEM
scanning electron microscopy
- ST
standard-temperature-grown
Footnotes
This work was supported in part by a grant from the Fundación para la Investigación Agraria de la Provincia de Almeria (FIAPA) and by a grant from the Ministerio de Educación y Ciencia (CICYT; project no. AGF95-0432). P.G. and C.P. received research fellowships from FIAPA and R.L. was supported by a fellowship from CICYT for a long stay at the Centro de Investigación y Tecnología, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria and Max Planck Institut laboratories.
LITERATURE CITED
- Barten JHM, Scott JW, Kedar N. Low temperatures induce rough blossom-end scarring of tomato fruit during early flower development. Characterization of blossom-end morphology genes in tomato and their usefulness in breeding for smooth blossom-end scars. J Am Soc Hortic Sci. 1992;117:298–303. [Google Scholar]
- Boutry M, Chua N-H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985;4:2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra Sekhar KN, Sawhney VK. A scanning electron microscope study of the development and surface features of floral organs of tomato (Lycopersicon esculentum) Can J Bot. 1984;62:2403–2413. [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. Development. 1993;119:397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development. 1995;121:2057–2067. [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Coen ES. The role of homeotic genes in flower development and evolution. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:241–279. [Google Scholar]
- Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Estruch JJ, Granell A, Hansen G, Prinsen E, Redig P, Van Onckelen H, Schwarz-Sommer Z, Sommer H, Spena A. Floral development and expression of floral homeotic genes are influenced by cytokinins. Plant J. 1993;4:379–384. doi: 10.1046/j.1365-313x.1993.04020379.x. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hareven D, Gutfinger T, Pnueli L, Bauch L, Cohen O, Lifschitz E. The floral system of tomato. Euphytica. 1994;79:235–243. [Google Scholar]
- Huijser P, Klein J, Lönnig W-E, Meijer H, Saedler H, Sommer H. Bracteomania, an inflorescence anomaly, is caused by the loss of function of the MADS box gene squamosa in Antirrhinum majus. EMBO J. 1992;11:1239–1249. doi: 10.1002/j.1460-2075.1992.tb05168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DV. Chilling injury in plants: the relevance of membrane lipids. In: Katterman E, editor. Environmental Injury in Plants. San Diego, CA: Academic Press; 1990. pp. 17–34. [Google Scholar]
- Mandel MA, Yanofsky MF. A gene triggering flower formation in Arabidopsis. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua N-H. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–29. [Google Scholar]
- Okamuro JK, Bart den Boer GW, Lotys-Prass C, Jofuku KD. Flowers into shoots: photo and hormonal control of a meristem identity switch in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:13831–13836. doi: 10.1073/pnas.93.24.13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro JK, Szeto W, Lotys-Prass C, Jofuku KD. Photo and hormonal control of meristem identity in the Arabidopsis flower mutants apetala2 and apetala1. Plant Cell. 1997;9:37–47. doi: 10.1105/tpc.9.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Abu-Abeid M, Zamir D, Nacken W, Schwarz-Sommer Z, Lifschitz E. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. Plant J. 1991;1:255–266. [PubMed] [Google Scholar]
- Pnueli L, Hareven D, Broday L, Hurwitz C, Lifschitz E. The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell. 1994a;6:175–186. doi: 10.1105/tpc.6.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Hareven D, Rounsley SD, Yanofsky MF, Lifschitz E. Isolation of the tomato AGAMOUS gene TAG1 and analysis of its homeotic role in transgenic plants. Plant Cell. 1994b;6:163–173. doi: 10.1105/tpc.6.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polowick PL, Sawhney VK. Temperature effects on male fertility and flower and fruit development in Capsicum annuum L. Sci Hortic. 1985;25:117–127. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis TJ. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sawhney VK. The role of temperature and its relationship with gibberellic acid in the development of floral organs of tomato (Lycopersicon esculentum) Can J Bot. 1983;61:1258–1265. [Google Scholar]
- Sawhney VK, Greyson RI. On the initiation of the inflorescence and floral organs in tomato (Lycopersicon esculentum) Can J Bot. 1972;50:1493–1495. [Google Scholar]
- Sawhney VK, Shukla A. Male sterility in flowering plants: are plant growth substances involved? Am J Bot. 1994;81:1640–1647. [Google Scholar]
- Schwarz-Sommer Z, Hue Y, Huijser P, Flor PJ, Hansen R, Tetens F, Lönnig W-E, Saedler H, Sommer H. Characterization of the Antirrhinum floral homeotic MADS-box gene DEFICIENS: evidence for DNA binding and autoregulation of its persistent expression throughout flower development. EMBO J. 1992;11:251–263. doi: 10.1002/j.1460-2075.1992.tb05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, Sommer H. Genetic control of flower development by homeotic genes in Antirrhinum majus. Science. 1990;250:931–936. doi: 10.1126/science.250.4983.931. [DOI] [PubMed] [Google Scholar]
- Shuff T, Thomas JF. Normal floral ontogeny and cool temperature-induced aberrant floral development in Glycine max (Fabaceae) Am J Bot. 1993;80:429–448. [Google Scholar]
- Sommer H, Beltrán JP, Huijser P, Pape H, Lönnig W-E, Saedler H, Schwarz-Sommer Z. Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J. 1990;9:605–613. doi: 10.1002/j.1460-2075.1990.tb08152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat SP, Sawhney VK. Benzylaminopurine induces phenocopies of floral meristem and organ identity mutants in wild-type Arabidopsis plants. Planta. 1996;198:480–487. doi: 10.1007/BF00620066. [DOI] [PubMed] [Google Scholar]
- Weigel D, Nilsson O. A developmental switch sufficient for flower initiation in diverse plants. Nature. 1995;377:495–500. doi: 10.1038/377495a0. [DOI] [PubMed] [Google Scholar]
- Zachgo S, De Andrade Silva E, Motte P, Tröbner W, Saedler H, Schwarz-Sommer Z. Functional analysis of the Antirrhinum floral homeotic DEFICIENS gene in vivo and in vitro using a temperature sensitive mutant. Development. 1995;121:2861–2875. doi: 10.1242/dev.121.9.2861. [DOI] [PubMed] [Google Scholar]