Abstract
Histone deacetylase inhibitors (HDACIs) activate the prosurvival nuclear factor-κB (NF-κB) pathway by hyperacetylating RelA/p65, whereas the chemopreventive agent resveratrol inhibits NF-κB by activating the class III histone deacetylase Sirt1. Interactions between resveratrol and pan-HDACIs (vorinostat and panobinostat) were examined in human acute myelogenous leukemia (AML) cells. Pharmacologically achievable resveratrol concentrations (25–50 μM) synergistically potentiated HDACI lethality in AML cell lines and primary AML blasts. Resveratrol antagonized RelA acetylation and NF-κB activation in HDACI-treated cells. However, short hairpin RNA Sirt1 knockdown failed to modify HDACI sensitivity, which suggests that factors other than or in addition to Sirt1 activation contribute to resveratrol/HDACI interactions. These interactions were associated with death receptor 5 (DR5) up-regulation and caspase-8 activation, whereas cells expressing dominant-negative caspase-8 were substantially protected from resveratrol/HDACI treatment, which suggests a significant functional role for the extrinsic apoptotic pathway in lethality. Exposure to resveratrol with HDACI induced sustained reactive oxygen species (ROS) generation, which was accompanied by increased levels of DNA double-strand breaks, as reflected in γH2A.X and comet assays. The free radical scavenger Mn(III)tetrakis(4-benzoic acid)porphyrin chloride blocked ROS generation, DR5 up-regulation, caspase-8 activation, DNA damage, and apoptosis, which indicates a primary role for oxidative injury in lethality. Analyses of cell-cycle progression and 5-ethynyl-2′-deoxyuridine incorporation through flow cytometry revealed that resveratrol induced S-phase accumulation; this effect was abrogated by HDACI coadministration, which suggests that cells undergoing DNA synthesis may be particularly vulnerable to HDACI lethality. Collectively, these findings indicate that resveratrol interacts synergistically with HDACIs in AML cells through multiple ROS-dependent actions, including death receptor up-regulation, extrinsic apoptotic pathway activation, and DNA damage induction. They also raise the possibility that S-phase cells may be particularly susceptible to these actions.
Introduction
Histone deacetylase inhibitors (HDACIs) represent a class of epigenetic agents that regulate gene expression by modifying chromatin structure. HDACIs promote histone acetylation, which leads to a more-relaxed configuration conducive to the transcription of genes implicated in differentiation and cell death (Bolden et al., 2006). However, HDACIs also kill transformed cells through alternative mechanisms, including induction of oxidative injury (Ruefli et al., 2001), interference with DNA repair machinery (Subramanian et al., 2005), and up-regulation of death receptors (Nebbioso et al., 2005), among others. The pan-HDACI vorinostat has been approved for the treatment of cutaneous T-cell lymphomas (Grant et al., 2007), and initial suggestions of HDACI activity in acute myelogenous leukemia (AML) were reported (Garcia-Manero et al., 2008).
HDACs are subdivided into four groups, as follows: class I, HDACs 1 to 3 and 8 (analogous to yeast Rpd); class II, HDACs 4 to 7, 9, and 10 (analogous to yeast HdaI); class III, NAD+-dependent sirtuins 1 to 7; class IV, HDAC11 (Glozak and Seto, 2007). Sirtuins have been implicated in the regulation of tumor initiation, progression, and chemoresistance; consequently, agents that modify sirtuin activity are currently a subject of interest for cancer therapy (Liu et al., 2009). Resveratrol is a naturally occurring polyphenolic compound extracted from grapes, and clinical trials are underway to explore its potential among patients with cardiovascular diseases or diabetes mellitus (Baur and Sinclair, 2006). Resveratrol has been associated with minimal toxicity, and plasma levels of >300 μM are achievable and well tolerated among humans (Howells et al., 2011). In preclinical studies, resveratrol exhibited activity against various malignant cell types, including AML (Tsan et al., 2002), through diverse mechanisms such as inhibition of IKK and NF-κB (Holmes-McNary and Baldwin, 2000), induction of oxidative injury (Low et al., 2010), and autophagy (Puissant et al., 2010). Resveratrol was shown to act as a Sirt1 agonist (Milne et al., 2007), although evidence indicating that this may involve indirect actions has emerged (Pacholec et al., 2010).
In addition to histones, HDACIs promote the acetylation of diverse nonhistone proteins, including transcription factors such as NF-κB (Glozak et al., 2005). In previous studies, we reported that inhibitors of the NF-κB signaling pathway, including IKK and proteasome inhibitors, markedly increased the activity of HDACIs against myeloid leukemia cells (Dai et al., 2005, 2011b). Among other actions, these agents potently block RelA deacetylation which plays an important role in DNA binding and transactivation (Dai et al., 2005). It is known that, like class I HDACs (e.g., HDAC3), the class III HDAC Sirt1 deacetylates RelA and inactivates NF-κB (Chen et al., 2005). However, pan-HDACIs such as panobinostat (LBH-589) and vorinostat fail to target class III HDACs (Xu et al., 2007). Furthermore, sirtuin agonists were shown to inhibit NF-κB function by antagonizing RelA acetylation (Yeung et al., 2004; Dai et al., 2005). These observations raised the possibility that resveratrol might sensitize leukemia cells to HDACIs. To address this question, we examined interactions between resveratrol and two clinically relevant pan-HDACIs (vorinostat and panobinostat) in human myeloid leukemia cells. We report that resveratrol synergistically potentiated HDACI activity against myeloid leukemia cells in association with ROS-dependent activation of the extrinsic apoptotic pathway.
Materials and Methods
Cells and Cell Culture.
U937 and MV-4-11 (bearing internal tandem duplications of FLT3) human leukemia cells were obtained from American Type Culture Collection (Manassas, VA) and were maintained in RPMI 1640 medium containing 10% fetal bovine serum, as described previously (Maggio et al., 2004). U937 cells were stably transfected with dominant-negative caspase-8 or empty vector as described previously (Rosato et al., 2007).
Bone marrow or peripheral blood samples (>65% blasts) were obtained, with informed consent, from patients with histologically documented AML who were undergoing routine diagnostic procedures, in accordance with the Declaration of Helsinki and with institutional review board approval (Virginia Commonwealth University Institutional Review Board no. HM 12517). Mononuclear cells were isolated through centrifugation at 400g for 30 min with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO). Cell viability was regularly >95%, and all samples consisted of >70% blasts. All experiments were performed at a density of 1 × 106 cells/ml, as described previously (Dai et al., 2011b).
Drugs and Chemicals.
Resveratrol (3,4′,5-trihydroxy-trans-stilbene) and sodium azide were purchased from Sigma-Aldrich. Vorinostat (suberoylanilide hydroxamic acid) and panobinostat were provided by Merck (Whitehouse Station, NJ) and Novartis (East Hanover, NJ), respectively. Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP), a cell-permeable superoxide dismutase mimetic and peroxynitrite scavenger, was obtained from Calbiochem (San Diego, CA). The selective NOX1 inhibitor 2-acetylphenothiazine (ML171) was purchased from Millipore Corporation (Billerica, MA). Reagents were formulated in dimethylsulfoxide and stored at −20°C. Sodium azide was dissolved in sterile phosphate-buffered saline before use. Stock solutions were diluted with serum-free RPMI 1640 medium before use, to ensure that the final concentration of dimethylsulfoxide did not exceed 0.02%.
Experimental Format.
Logarithmically growing cells (2.5–4.0 × 105 cells/ml) were exposed to various concentrations of HDACIs in the presence or absence of resveratrol for the indicated periods (generally 24–48 h), after which cells were processed and assayed.
RNA Interference.
SureSilencing shRNA plasmids (neomycin resistance), including shSirt1 (targeting human sirtuin 1; GenBank accession no. 23411) and negative control shRNA (shNC), were purchased from QIAGEN (Valencia, CA). U937 cells were stably transfected with these constructs by using an Amaxa Nucleofector device with cell line-specific Nucleofector kit C (Amaxa GmbH, Cologne, Germany), according to the manufacturer's instructions, and clones with down-regulated expression of Sirt1 were selected with 400 μg/ml G418.
Assessments of Cell Death and Mitochondrial Membrane Potential.
Cells were double-stained for 20 min at 37°C with a solution containing 25 μM 7-aminoactinomycin D and 40 nM 3,3′-dihexyloxacarbocyanine iodide and then were analyzed through flow cytometry with a FACScan flow cytometer (BD Biosciences, San Jose, CA), as described previously (Maggio et al., 2004). In some cases, apoptosis was evaluated with staining for 30 min at room temperature with annexin V/propidium iodide (PI) (BD Biosciences Pharmingen, Franklin Lakes, NJ) and then flow cytometry, as described previously (Rosato et al., 2010).
Measurement of ROS Production.
Cells were treated with 20 μM 2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen, Carlsbad, CA) for 30 min at 37°C and then were analyzed through flow cytometry as described previously (Rosato et al., 2010).
Comet Assays.
Single-cell gel electrophoresis assays were performed to assess both single- and double-strand DNA breaks in cells by using a comet assay kit (Trevigen, Gaithersburg, MD), according to the manufacturer's instructions.
Western Blot Analyses.
Whole-cell pellets were washed in phosphate-buffered saline and were lysed with loading buffer (Invitrogen) as described previously (Maggio et al., 2004). Alternatively, S-100 cytosolic fractions were prepared as described previously (Dai et al., 2005). Thirty micrograms of total protein for each condition were separated with a 4 to 12% Bis-Tris NuPAge precast gel system (Invitrogen) and were electroblotted to nitrocellulose membranes. After incubation with the corresponding primary and secondary antibodies, blots were developed for enhanced chemiluminescence detection (PerkinElmer Life and Analytical Sciences, Waltham, MA). Primary antibodies were as follows: anti-caspase-8 and anti-PARP from Enzo Life Sciences (Plymouth Meeting, PA), anti-caspase-3 from BD Biosciences Transduction Laboratories (Lexington, KY), anti-caspase-9, anti-DR5, anti-cytochrome c, and anti-p65 from BD Biosciences, anti-acetyl-NF-κB p65 (Lys310), anti-cleaved caspase-3, and anti-cleaved caspase-9 from Cell Signaling Technology (Danvers, MA), anti-Sirt1 from Santa Cruz Biotechnology (Santa Cruz, CA), anti-γH2A.X (Ser139) from Millipore Corporation, anti-β-actin from Sigma-Aldrich, and anti-α-tubulin from Calbiochem. Secondary antibodies conjugated to horseradish peroxidase were obtained from KPL (Gaithersburg, MD).
Immunoprecipitation Analyses.
RelA acetylation was evaluated through immunoprecipitation/Western blot analysis as described previously (Dai et al., 2005). Two hundred micrograms of protein for each condition were incubated overnight at 4°C with 1 μg of mouse monoclonal anti-NF-κB p65 antibody (Santa Cruz Biotechnology), with continuous shaking; 20 μl of Dynabeads (goat anti-mouse IgG; Invitrogen) for each condition were then added, and mixtures were incubated for an additional 4 h. After three washes with RIPA buffer, the bead-bound protein was eluted with vortex-mixing and boiling in 20 μl of 1× sample buffer. The samples were separated through SDS-polyacrylamide gel electrophoresis and were subjected to Western blot analysis as described above. Acetylated lysine-specific antibodies (Millipore Corporation) were used as primary antibodies.
ELISA-Based NF-κB p65 Activity Analyses.
Nuclear protein was extracted by using a Nuclear Extract kit (Active Motif Inc., Carlsbad, CA). RelA/p65-specific DNA-binding activity in nuclear extracts was measured by using a TransAM NF-κB p65 kit (Active Motif Inc.), as described previously (Rosato et al., 2010).
Cell-Cycle Analyses.
Cell-cycle analysis of DNA contents on the basis of PI staining was performed through flow cytometry using Modfit LT 2.0 (Verity Software House, Topsham, ME), as described previously (Pei et al., 2011).
DNA Synthesis (S-Phase) Analyses.
Click-iT EdU CellCycle 488-red (7-aminoactinomycin D) assay kit (Invitrogen) was used, according to the manufacturer's instructions, with flow cytometry to determine the S-phase population through incorporation of the thymidine analog EdU into genomic DNA during DNA synthesis. Alternatively, cells were double-stained with EdU-Alexa Fluor 488 and annexin V-allophycocyanin, after which flow cytometry was performed to determine the level of apoptosis (annexin V-positive) in the S-phase (EdU-positive) population.
Statistical Analyses.
For analyses of cell death, ROS production, and NF-κB activity, values represent the mean ± S.D. of at least three separate experiments performed in triplicate. One-way analysis of variance with the Tukey-Kramer multiple-comparison test and Student's two-tailed t test were performed. Analysis of synergism was performed through median dose effect analysis with Calcusyn (Biosoft, Ferguson, MO).
Results
Resveratrol Synergistically Interacts with HDACIs in Human Myeloid Leukemia Cells.
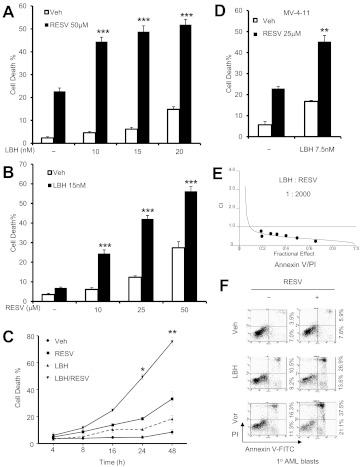
Coadministration (24 h) of a marginally toxic concentration (50 μM) of resveratrol significantly increased the lethality of minimally toxic concentrations (10–20 nM) of LBH-589 in U937 cells (Fig. 1A). This event was observed at resveratrol concentrations as low as 10 μM (Fig. 1B). Time-course analysis revealed increases in lethality for the resveratrol/LBH-589 regimen that were first discernible at 16 h and became more pronounced in the subsequent 24 h (Fig. 1C). Analogous results were obtained when another pan-HDACI (vorinostat, used at 1.5 μM) was used in combination with resveratrol (Supplemental Fig. 1A). Similar interactions were observed in MV-4-11 cells (an AML line bearing internal tandem duplications of FLT3) with relatively lower concentrations (e.g., 7.5 nM) of LBH-589 (Fig. 1D). Median dose effect analysis of data for U937 cells exposed to a range of resveratrol and LBH-589 concentrations at the indicated fixed ratio yielded combination index values of less than 1.0 (combination index, 0.243–0.782; annexin V/PI analysis), which indicated synergistic interactions (Fig. 1E). Synergism between resveratrol and vorinostat also was observed (combination index, 0.614–0.976; annexin V/PI analysis) (Supplemental Fig. 1B). Consistent with these findings, resveratrol interacted synergistically with LBH-589 or vorinostat in triggering a loss of mitochondrial membrane potential (determined through 3,3′-dihexyloxacarbocyanine iodide uptake) (Supplemental Fig. 1C). Primary AML blasts displayed no or minimal toxicity when exposed for 24 h to 50 μM resveratrol, 10 nM LBH-589, or 1.0 μM vorinostat alone, but combined treatments resulted in sharp increases in the levels of cell death (Fig. 1F).
Fig. 1.
Resveratrol interacts synergistically with LBH-589 in human AML cell lines and primary AML blasts. A to C, U937 cells were exposed to 50 μM resveratrol (RESV) with or without 10 to 20 nM LBH-589 (LBH) for 24 h (A), 10 to 50 μM resveratrol with or without 15 nM LBH-589 for 24 h (B), or 50 μM resveratrol with or without 15 nM LBH-589 for 4 to 48 h (C). Veh, vehicle. D, MV-4-11 cells were incubated with 25 μM resveratrol with or without 7.5 nM LBH-589 for 24 h. After treatment, the percentage of cell death was determined through 7-aminoactinomycin D staining and flow cytometry. Values represent the mean ± S.D. for triplicate determinations performed on three separate occasions. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with each agent alone. E, U937 cells were treated with LBH-589 with or without resveratrol at a fixed ratio (1:2000) for 24 h, after which apoptosis was monitored through annexin V/PI analysis. F, blasts from the bone marrow of a patient with AML were exposed to 50 μM resveratrol with or without 10 nM LBH-589 or 1 μM vorinostat for 24 h, after which cell death was analyzed through annexin V-fluorescein isothiocyante (FITC)/PI staining and flow cytometry. top right, annexin-positive/PI-positive (late cell death); bottom right, annexin-positive/PI-negative (early cell death). An additional experiment yielded equivalent results.
Coadministration of HDACIs with Resveratrol Leads to Enhanced DNA Damage, Mitochondrial Injury, and Caspase-3, Caspase-9, and Caspase-8 Activation.
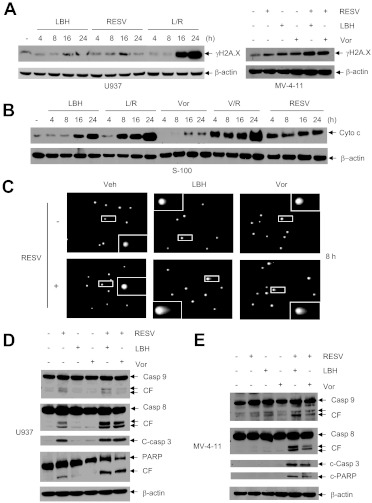
Whereas resveratrol or HDACIs (LBH-589 or vorinostat) administered individually for ≥16 h minimally induced γH2A.X, an indicator of DNA double-strand breaks (Rosato et al., 2010), combined treatments sharply increased the levels of γH2A.X expression in U937 and MV-4-11 cells (Fig. 2A). After drug treatment for 24 h, single- and double-strand DNA breaks in U937 cells were analyzed with comet assays. In this assay, denatured broken DNA fragments migrate out of the cell under the influence of an electric field, producing a comet tail, whereas undamaged DNA migrates more slowly and remains within the confines of the nucleus (Dai et al., 2008). As shown in Fig. 2C, coadministration of resveratrol with either LBH-589 or vorinostat resulted in clear increases in the numbers of comet-positive cells, compared with treatment with the agents individually. The appearance of DNA comet tails in cells exposed to resveratrol with LBH-589 or vorinostat occurred substantially before the induction of massive apoptosis, i.e., 8 h (Fig. 2C) versus 24 h (Fig. 1C). Furthermore, coadministration of resveratrol with LBH-589 or vorinostat resulted in early (4–8 h) and marked increases in the release of mitochondrial cytochrome c into the cytosol, compared with individual treatment (Fig. 2B). Exposure to resveratrol with either LBH-589 or vorinostat led to clearly increased cleavage/activation of caspase-3, caspase-9, and particularly caspase-8, which was accompanied by marked PARP degradation, in U937 (Fig. 2D) and MV-4-11 cells (Fig. 2E).
Fig. 2.
Resveratrol/HDAC inhibitors induce DNA damage, mitochondrial injury, and caspase activation in human leukemia cells. A, U937 cells were incubated with 50 μM resveratrol (RESV) with or without 15 nM LBH-589 (LBH) for the indicated periods (left) and MV-4-11 cells were exposed to 25 μM resveratrol with or without 7.5 nM LBH-589 1 μM vorinostat (Vor) for 24 h (right). After treatment, cells were lysed and Western blot analyses were performed to monitor the expression of γH2A.X. R, resveratrol; L, LBH-589. B, U937 cells were treated with 50 μM resveratrol with or without 15 nM LBH-589 or 1.5 μM vorinostat (Vor) for the indicated periods, after which the S-100 cytosolic fraction was prepared and subjected to Western blot analysis to monitor the release of mitochondrial cytochrome c (Cyto c) into the cytosol. V, vorinostat. C, U937 cells were treated for 8 h as described for B, after which comet assays were performed to assess single- and double-strand DNA breaks. Veh, vehicle. D and E, U937 (D) and MV-4-11 (E) cells were exposed to resveratrol (U937, 50 μM; MV-4-11, 25 μM) with or without LBH-589 (U937, 15 nM; MV-4-11, 7.5 nM) or vorinostat (U937, 1.5 μM; MV-4-11, 1 μM) for 24 h, after which cleavage of caspase-8, caspase-3, caspase-9, and PARP was assessed through Western blot analysis. CF, cleaved fragment; Casp, caspase. Each lane was loaded with 30 μg of protein; blots were stripped and reprobed for β-actin to ensure equivalent loading and transfer.
Resveratrol Blocks HDACI-Mediated RelA Acetylation and NF-κB Activation.
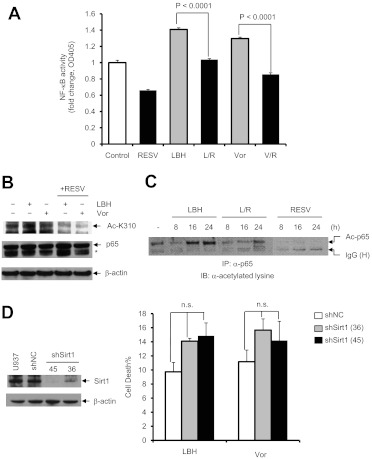
Previous studies showed that interference with RelA/p65 acetylation and NF-κB activation, e.g., by IKK inhibitors, dramatically increased HDACI lethality in AML cells (Dai et al., 2005), which raises the possibility that a Sirt1 agonist such as resveratrol might act similarly by promoting Sirt1-mediated RelA deacetylation. To address this, the effects of resveratrol on RelA/p65 acetylation and NF-κB activation were examined in U937 cells exposed to HDACIs. As shown in Fig. 3A, ELISA-based NF-κB activity analysis of nuclear extracts showed that exposure to both LBH-589 and vorinostat induced p65-specific NF-κB activation, as reported previously (Dai et al., 2005), whereas this event was significantly blocked by resveratrol (P < 0.001 in each case). Western blot analyses of whole-cell lysates demonstrated that coadministration of resveratrol with either LBH-589 or vorinostat clearly decreased K310 acetylation of p65 (Fig. 3B). Immunoprecipitation analyses also revealed that resveratrol coadministration substantially decreased HDACI-induced p65 acetylation (Fig. 3C). Together, these findings indicate that resveratrol attenuates RelA/p65 acetylation and NF-κB activation in HDACI-treated AML cells, similar to effects observed with agents that directly target the NF-κB signaling pathway, such as IKK and proteasome inhibitors (Dai et al., 2005, 2011b).
Fig. 3.
Resveratrol inhibits HDACI-induced RelA/p65 acetylation and NF-κB activation. A, U937 cells were incubated with 50 μM resveratrol (RESV) with or without 15 nM LBH-589 (LBH) or 1.5 μM vorinostat (Vor) for 8 h, after which NF-κB activity was determined with an ELISA-based, p65-specific, NF-κB activity assay. R, resveratrol; L, LBH-589; V, vorinostat. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. B, U937 cells were treated for 24 h as described for A, and Western blot analysis was performed with antibodies specifically recognizing Lys310-acetylated p65. Total p65 was probed for comparison. *, nonspecific bands. C, cells were harvested and lysed at the indicated times, and 200 μg of protein was subjected to immunoprecipitation (IP) with anti-p65 antibody, followed by Western blot analysis with antilysine antibody. IB, immunoblotting; Ac, acetylated; IgG (H), IgG heavy chain. D, U937 cells were stably transfected with constructs encoding shRNA specifically targeting Sirt1 (shSirt1) or a scrambled sequence as negative control (shNC). Western blot analysis was performed to determine the down-regulation of Sirt1 protein in two shSirt1 clones (designed 36 and 45), compared with parental U937 and shNC-transfected cells. Cells were then exposed to 15 nM LBH-589 or 1.5 μM vorinostat for 48 h, after which cell death was monitored through 7-aminoactinomycin D staining and flow cytometry. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. n.s., not significant (P > 0.05). For Western blot analysis, each lane was loaded with 30 μg of protein; blots were stripped and reprobed for β-actin to ensure equivalent loading and transfer.
Knockdown of Sirt1 Fails to Attenuate HDACI Lethality.
To investigate the functional significance of Sirt1 perturbations in the regulation of HDACI lethality, U937 cells were stably transfected with constructs encoding shRNA specifically targeting human Sirt1 (shSirt1) or a scrambled sequence as a negative control (shNC). Two shSirt1 clones (designated 36 and 45) that displayed sharp reductions in Sirt1 expression, compared with control cells, were isolated (Fig. 3D, left). Contrary to expectations that Sirt1 knockdown would exert effects opposite those of the Sirt1 agonist resveratrol, both shSirt1 clones appeared slightly more sensitive (rather than resistant) to LBH-589 or vorinostat, compared with control cells, although differences did not achieve statistical significance (P > 0.05) (Fig. 3D, right). This finding suggests that mechanisms other than or in addition to Sirt1 activation by resveratrol contribute to the potentiation of HDACI antileukemic activity by this agent.
Resveratrol/HDACI Activity Proceeds through a ROS-Dependent Process.
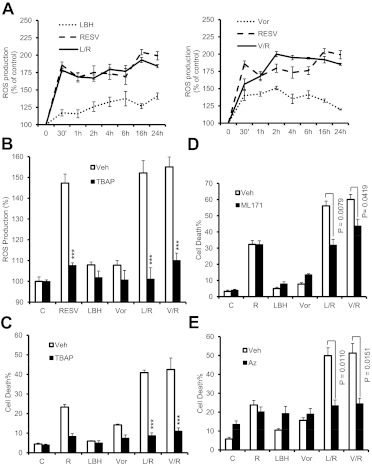
Earlier studies showed that both resveratrol (Schilder et al., 2009; Low et al., 2010) and HDACIs (Ruefli et al., 2001; Rosato et al., 2010) could trigger cell death through an oxidative injury-mediated process. Therefore, the effects of resveratrol with or without HDACIs on the generation of reactive oxygen species (ROS) were examined. Exposure of U937 cells to LBH-589 or vorinostat alone triggered modest increases in ROS levels, which decreased slightly after 24 h of exposure (Fig. 4A). In contrast, resveratrol alone induced sharp increases in ROS levels, which persisted over the 24-h exposure period, and coadministration of HDACIs did not increase ROS accumulation further (Fig. 4A). ROS generation in cells exposed to resveratrol with or without HDACIs was largely abrogated by the ROS scavenger MnTBAP (Fig. 4B), which led to substantial protection from resveratrol/HDACI-induced cell death (Fig. 4C) as well as loss of mitochondrial membrane potential (Supplemental Fig. 2A) and cleavage of caspase-3 and PARP (Supplemental Fig. 2B). It is known that NOXs share the capacity to transport electrons across the plasma membrane and to reduce oxygen to superoxide, thereby generating downstream ROS (Gianni et al., 2010). In this context, the selective NOX1 inhibitor ML171 (Gianni et al., 2010) was used to assess the functional role of NOXs in the lethality of the resveratrol/HDACI regimen. As shown in Fig. 4D, 1-h pretreatment with ML171 significantly prevented apoptosis induced by coadministration of resveratrol and LBH-589 (P = 0.0079, with versus without ML171) or vorinostat (P = 0.0419) in U937 cells. Catalases efficiently decompose H2O2 derived from superoxide (O2−), a reaction that is catalyzed by superoxide dismutase in cells (Mesquita et al., 2010). Therefore, two catalase inhibitors were used to analyze the functional role of catalase in cell death induced by resveratrol with or without HDACIs. It was found that the specific catalase inhibitor 3-amino-1,2,4-triazole (Mesquita et al., 2010) failed to attenuate the lethality of the resveratrol regimen (data not shown). One-hour pretreatment with sodium azide, a known catalase inhibitor and a donor of nitric oxide in the presence of catalase and H2O2 (Ogino et al., 2001), which was reported previously to prevent apoptosis through the extrinsic pathway (Kim et al., 1997) substantially prevented apoptosis induced by exposure to resveratrol and LBH-589 (P = 0.0110, with versus without azide) or vorinostat (P = 0.0151) in U937 cells (Fig. 4E).
Fig. 4.
The resveratrol/HDACI regimen induces ROS production, which plays a functional role in lethality. A, U937 cells were incubated with 50 μM resveratrol (RESV) with or without 15 nM LBH-589 (LBH) (left) or 1.5 μM vorinostat (Vor) (right) for the indicated periods, after which intracellular ROS levels were assessed through staining with 2′,7′-dichlorodihydrofluorescein diacetate (a cell-permeable ROS indicator) and flow cytometry. R, resveratrol; L, LBH-589; V, vorinostat. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. B and C, U937 cells were treated with 50 μM resveratrol with or without 15 nM LBH-589 or 1.5 μM vorinostat in the absence or presence of 400 μM MnTBAP, after which ROS levels were measured as described for A (B, 6 h) and cell death was determined through 7-aminoactinomycin D staining and flow cytometry (C, 24 h). Veh, vehicle; C, control. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions. ***, P < 0.001, compared with the same treatment without MnTBAP. D and E, U937 cells were treated for 24 h with 50 μM resveratrol with or without 15 nM LBH-589 or 1.5 μM vorinostat after 1-h pretreatment with 5 μM ML171 (D) or 5 mM sodium azide (Az) (E), after which cell death was determined through 7-aminoactinomycin D staining and flow cytometry.
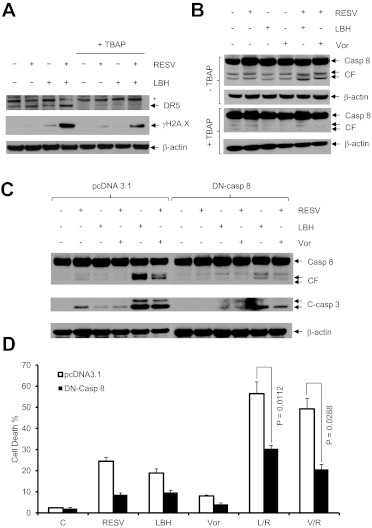
The ROS Scavenger MnTBAP Blocks DR5 Up-regulation, Caspase-8 Cleavage, and DNA Damage in Cells Exposed to Resveratrol and HDACI.
As shown in Fig. 2D, caspase-8 exhibited pronounced cleavage in U937 cells after exposure to resveratrol and HDACI, which indicated activation of the extrinsic apoptotic cascade. In this context, resveratrol by itself induced expression of DR5 (Fig. 5A), as reported for diffuse large B-cell lymphoma cells (Hussain et al., 2011). Coadministration of LBH-589 clearly enhanced resveratrol-mediated DR5 up-regulation (Fig. 5A), which was accompanied by increased caspase-8 cleavage (Fig. 5B) and γH2A.X expression (Fig. 5A). In the presence of MnTBAP, however, DR5 induction and caspase-8 cleavage/activation were entirely abrogated and γH2A.X expression (DNA damage) was largely prevented (Fig. 5, A and B). Together, these findings indicate that induction of ROS acts upstream of other lethal events (e.g., activation of the extrinsic death pathway and DNA damage) and thus plays a primary functional role in the antileukemic activity of the resveratrol/HDACI regimen.
Fig. 5.
MnTBAP blocks DR5 expression, caspase-8 activation, and DNA damage, whereas dominant-negative caspase-8 prevents cell death induced by the resveratrol/HDACI regimen. A and B, U937 cells were treated with 50 μM resveratrol (RESV) with or without 15 nM LBH-589 (LBH) or 1.5 μM vorinostat (Vor) in the absence or presence of 400 μM MnTBAP for 24 h, after which cells were lysed and subjected to Western blot analysis to monitor the expression of DR5, γH2A.X, and caspase-8. Each lane was loaded with 30 μg of protein; blots were stripped and reprobed for β-actin to ensure equivalent loading and transfer. CF, cleaved fragment; Casp, caspase. C, U937 cells stably transfected with dominant-negative (DN) caspaspe-8 or the empty-vector control (pcDNA3.1) were treated with 50 μM resveratrol with or without 15 nM LBH-589 or 1.5 μM vorinostat for 24 h, after which Western blot analysis was performed to monitor the cleavage/activation of caspase-8 and caspase-3. Blots were stripped and reprobed with antibodies to β-actin, to ensure equivalent loading and transfer. D, flow cytometry was performed to assess cell death after 7-aminoactinomycin D staining. C, control; R, resveratrol; L, LBH-589; V, vorinostat. Values represent the mean ± S.D. of triplicate determinations performed on three separate occasions.
There Is Evidence of a Functional Role for the Extrinsic Pathway in Resveratrol/HDACI Lethality.
To assess more definitively the functional significance of activation of the extrinsic apoptotic pathway in this setting, U937 cells expressing dominant-negative caspase-8 were used (Rosato et al., 2007). Cleavage/activation of both caspase-8 and caspase-3 induced by resveratrol with LBH-589 or vorinostat was dramatically decreased in dominant-negative caspase-8–transfected cells, compared with their empty-vector–transfected counterparts (Fig. 5C). Consistent with these results, resveratrol/HDACI lethality was significantly attenuated in dominant-negative caspase-8–transfected cells (P < 0.02 or P < 0.05, compared with empty-vector–transfected control cells) (Fig. 5D). Collectively, these findings indicate that ROS-dependent activation of the extrinsic pathway plays a significant functional role in the antileukemic activity of this regimen.
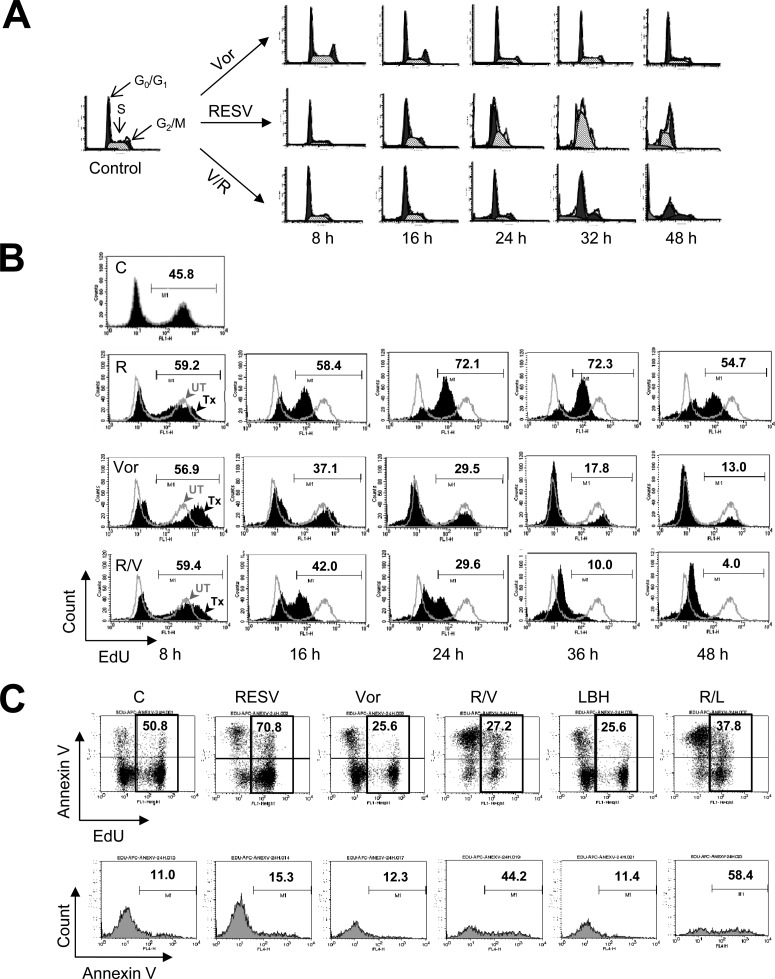
Resveratrol Induces S-Phase Accumulation and Sensitizes Leukemia Cells to HDACIs.
Resveratrol has been shown to induce S-phase arrest in various tumor cell types, including leukemia cells (Bernhard et al., 2000). Therefore, the effects of resveratrol with or without HDACIs on cell-cycle progression were examined. As reported previously (Bernhard et al., 2000), exposure to resveratrol induced a marked increase in the S-phase cell population, in a time-dependent manner (Fig. 6A). In contrast, exposure to HDACIs (e.g., vorinostat) resulted in modest increases in the population of cells in the G0/G1 phase, with slight decreases in the S-phase population, but yielded little increase in the subdiploid population. Coadministration of vorinostat virtually eliminated the S-phase accumulation of cells induced by resveratrol, with a clear increase in the subdiploid (sub-G1) population (Fig. 6A; Supplemental Fig. 2C). In accord with these findings, flow cytometric analysis of EdU incorporation, which reflects DNA synthesis, demonstrated a pronounced increase in the EdU-positive S-phase cell population after exposure to resveratrol (e.g., 72%, compared with 46% in untreated cells), whereas coadministration of vorinostat with resveratrol led to the virtual disappearance of EdU-positive cells (e.g., to 4%) (Fig. 6B). Double-staining with EdU-Alexa Fluor 488 and annexin V-allophycocyanin demonstrated that administration of resveratrol with either LBH-589 or vorinostat induced apoptosis in both the EdU-positive (e.g., 58% or 44% for resveratrol plus LBH-589 or vorinostat, respectively, compared with 15% for resveratrol alone) (Fig. 6C) and EdU-negative populations. Together, these findings raise the possibility that resveratrol may arrest leukemia cells in the S-phase and that such cells may be particularly susceptible to HDACI-mediated lethality.
Fig. 6.
Resveratrol induces S-phase accumulation, an effect abrogated by HDACIs. A, U937 cells were exposed to 50 μM resveratrol (RESV) with or without 1.5 μM vorinostat (Vor) for the indicated periods, after which flow cytometry was performed for analysis of cell-cycle distribution after PI staining. R, resveratrol; V, vorinostat. B and C, flow cytometry was performed to determine the S-phase-specific population with a Click-iT EdU CellCycle 488-red (7-aminoactinomycin D) assay kit (B) or apoptosis in the S-phase population (C). Values indicate the percentage of S-phase (EdU-positive) cells (B) or the percentage of apoptotic (annexin V-allophycocyanin-positive) cells in the S-phase (EdU-Alexa Fluor 488-positive) population (C). C, control; L, LBH-589.
Discussion
Although resveratrol has traditionally been viewed as a chemopreventive agent (Baur and Sinclair, 2006), more-recent studies highlighted its capacity to induce cell death in neoplastic cells, including leukemia cells (Puissant et al., 2010). As for numerous natural products, the mechanisms through which resveratrol triggers transformed cell death are likely to be multifactorial (Athar et al., 2009). Resveratrol acts as an agonist of Sirt1 (Park et al., 2012), a member of the class III HDAC subfamily that pan-HDACIs fail to target (Xu et al., 2007), and exerts inhibitory effects on NF-κB, which contribute to its anticancer activity (Yeung et al., 2004; Dai et al., 2005). Resveratrol also was shown to prevent NF-κB activation and NF-κB-dependent gene expression through its inhibitory effects on IKKs (Holmes-McNary and Baldwin, 2000). In transformed cells, HDACIs activate NF-κB through RelA (Ser536) phosphorylation (Dai et al., 2011a) with an ataxia telangiectasia-mutated/NF-κB essential modifier-dependent mechanism (Rosato et al., 2010). Moreover, HDACIs induce RelA/p65 acetylation, which prevents nuclear export, while promoting DNA binding and transactivation (Chen et al., 2002). These events lead to up-regulation of several antiapoptotic and antioxidant proteins, which decrease HDACI lethality, as well as activation of the stress-related c-Jun N-terminal kinase pathway (Dai et al., 2005). In this context, agents that prevent NF-κB activation, such as IKK (Dai et al., 2010) and proteasome inhibitors (Dai et al., 2011b), were shown to increase HDACI lethality markedly in malignant human hematopoietic cells, including leukemia cells. We hypothesized that resveratrol, like NF-κB–inhibitory agents, might enhance HDACI-mediated antileukemia activity. Consistent with this hypothesis, resveratrol clearly decreased p65 acetylation (e.g., K310) and NF-κB activation in HDACI-treated leukemia cells, with a sharp increase in cell death.
The observation that most HDACIs do not target class III HDACs, including Sirt1, provides a rationale for combining Sirt1 agonists such as resveratrol with HDACIs for cancer treatment. Although the Sirt1 agonist activity of resveratrol may involve an indirect mechanism (Pacholec et al., 2010), it was demonstrated that this agent exerts its effects by activating Sirt1, which negatively regulates p65 acetylation (an event essential for sustained NF-κB activation) (Chen et al., 2001). It was anticipated that, if Sirt1 activation by resveratrol (Milne et al., 2007) was responsible for potentiating HDACI lethality, then knockdown of Sirt1 would attenuate HDACI-mediated cell death, contrary to the effects of a Sirt1 agonist. Unexpectedly, Sirt1 knockdown failed to protect leukemia cells from HDACI-mediated lethality, which raises several possibilities. Other resveratrol actions (e.g., pro-oxidant activity or disruption of the cell cycle) (Athar et al., 2009) may be primarily responsible for the potentiation of HDACI lethality. Alternatively, aside from deacetylation of p65, Sirt1 actions may play predominantly cytoprotective roles (Chen et al., 2009). For example, Sirt1 is known to mediate deacetylation of numerous nonhistone substrates (e.g., Forkhead box proteins, p53, NF-κB, peroxisome proliferator-activated receptor γ, and peroxisome proliferator-activated receptor γ coactivator 1α). In support of this notion, pharmacological Sirt1 antagonists were shown recently to promote chronic myelogenous leukemia stem cell death by increasing the acetylation and transcriptional activity of p53 (Li et al., 2012).
Although the chemopreventive actions of resveratrol may result from its antioxidant properties (de la Lastra and Villegas, 2007), resveratrol was shown to be a potent inducer of tumor cell oxidative injury (Chandra, 2009). In leukemia cells, oxidative injury also was shown to represent an important mechanism of HDACI lethality (Ruefli et al., 2001; Rosato et al., 2003; Petruccelli et al., 2011). The observation that ROS generation played a critical functional role in the actions of this combination regimen is not surprising. Resveratrol administered alone induced a pronounced sustained increase in ROS levels, but this was not accompanied by marked DNA damage (expression of γH2A.X or formation of comet tails) or cell death. Although HDACIs did not further increase ROS production in resveratrol-treated cells, coadministration sharply increased DNA damage and cell death. Given evidence that HDACIs interfere with DNA repair processes by hyperacetylating DNA repair proteins such as Ku70 (Subramanian et al., 2005) and down-regulating others (e.g., Rad50 or MRE11) (Lee et al., 2010), it is tempting to speculate that HDACIs amplify the lethal consequences of resveratrol-mediated oxidative injury and the resulting DNA damage (Subramanian et al., 2005).
Coadministration of resveratrol and HDACIs triggered the activation of caspases, particularly caspase-8, which indicates activation of the extrinsic apoptotic cascade (Scaffidi et al., 1998). In this context, HDACIs are known to up-regulate death receptors in human leukemia cells (Insinga et al., 2005), and resveratrol was shown to up-regulate death receptors, including DR5, in lymphoma cells (Hussain et al., 2011). Consistent with these observations, exposure of leukemia cells to resveratrol and HDACIs increased DR5 expression, compared with the effects of each agent administrated individually. This finding provides a possible explanation for the marked activation of the extrinsic pathway produced by the resveratrol/HDACI regimen. Blockade of the extrinsic pathway with dominant-negative caspase-8 substantially decreased resveratrol/HDACI lethality, which demonstrates a significant functional role for the activation of this pathway in the antileukemia activity of this strategy. Both up-regulation of DR5 and activation of the extrinsic pathway were essentially abrogated by the ROS scavenger MnTBAP. It is interesting to note that similar phenomena were observed in human lymphoma (diffuse large B-cell lymphoma) cells exposed to resveratrol alone (Hussain et al., 2011). Together, these findings suggest that ROS-dependent induction of death receptors (e.g., DR5) and the resulting activation of the extrinsic apoptotic pathway represent an important mechanism underlying antileukemic synergism between these agents. Finally, although the ability of ML171 to protect cells from resveratrol/HDACI lethality implicates NOXs in this phenomenon, additional studies will be required to identify more definitively the sources of the ROS generated.
Resveratrol was reported to synchronize cells in the S-phase in association with the inhibition of cdc2 (Tyagi et al., 2005) or NF-κB (Estrov et al., 2003). The results of the present study demonstrated that resveratrol exposure sharply increased the S-phase population and the proportion of cells positive for EdU (a specific S-phase marker that reflects DNA synthesis) (Kramer and Wesierska-Gadek, 2009), in a time-dependent manner. Coadministration of HDACIs prevented resveratrol-induced S-phase accumulation and DNA synthesis, with a marked increase in the subdiploid fraction, which reflects apoptosis. One possible explanation for these findings is that cells exposed to resveratrol may accumulate in the S-phase, which is characterized by active DNA synthesis, and such cells may be particularly sensitive to HDACIs, as observed for the classic S-phase synchronizer hydroxyurea (Krämer et al., 2008). Previous findings that abrogation of G0/G1 arrest through blockade of induction of the endogenous cyclin-dependent kinase inhibitor p21CIP1 markedly sensitized leukemia cells to HDACIs (Rosato et al., 2002) are consistent with this notion. Alternatively, the cytoprotective effects of HDACI-mediated NF-κB activation, particularly its antioxidant actions attributable to induction of proteins such as superoxide dismutase 2 (Dai et al., 2005), may be critical for the survival of S-phase cells, which are known to be particularly vulnerable to oxidative injury (Ge et al., 2006). Finally, HDACIs kill transformed cells through diverse mechanisms, including up-regulation of proapoptotic proteins such as Bim (Zhao et al., 2005), induction of death receptors (Nebbioso et al., 2005), and promotion of DNA damage (Lee et al., 2010), among numerous others. Additional studies will be required to define the relative contributions of these actions to HDACI lethality toward S-phase cells.
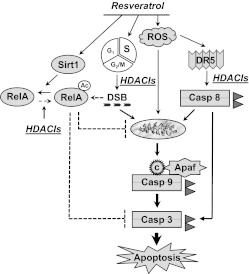
In summary, the present studies demonstrate that resveratrol, administered at pharmacologically achievable concentrations (Howells et al., 2011), significantly increases HDACI lethality in human AML cells through oxidative injury-mediated activation of the extrinsic apoptotic pathway. The results also indicate that, although resveratrol does block HDACI-mediated NF-κB acetylation and activation, which were previously implicated in the potentiation of HDACI-mediated leukemic cell death (Dai et al., 2005; Rosato et al., 2010), actions other than or in addition to Sirt1 activation are likely to be responsible for the observed synergism. A hypothetical model summarizing mechanisms through which these agents may interact is shown in Fig. 7. According to this model, exposure of leukemia cells to resveratrol triggers multiple interrelated actions, including activation of Sirt1 (Milne et al., 2007), induction of ROS (Chandra, 2009), and synchronization of cells in the S-phase (Estrov et al., 2003). Activation of Sirt1 decreases HDACI-mediated p65 acetylation and NF-κB activation, which are events known to promote HDACI lethality (Dai et al., 2005). Resveratrol-mediated ROS generation, in cooperation with HDACIs (Insinga et al., 2005), triggers up-regulation of death receptors (e.g., DR5) (Hussain et al., 2011), which leads to activation of the extrinsic cascade, release of mitochondrial death proteins (e.g., cytochrome c), and full engagement of the apoptotic cascade. ROS also cause DNA damage, the lethal consequences of which may be exacerbated by HDACI-mediated interference with or down-regulation of DNA repair proteins (Subramanian et al., 2005; Lee et al., 2010). S-phase–synchronized leukemia cells may be particularly susceptible to the oxidative injury (Ge et al., 2006) and DNA damage triggered by the preceding events. The net effect of these cooperative actions is a pronounced induction of cell death. Given the relative lack of toxicity of resveratrol concentrations considerably higher than those used in the present study (Howells et al., 2011), a strategy combining HDACIs with resveratrol warrants further attention for AML and possibly other hematological malignancies.
Fig. 7.
A theoretical model of synergistic interactions between resveratrol and HDAC inhibitors is presented. Resveratrol triggers multiple actions in human leukemia cells, including Sirt1 activation, ROS induction, and S-phase synchronization. Activation of Sirt1 blocks cytoprotective NF-κB activation by preventing HDACI-mediated p65 acetylation. In addition, resveratrol induces ROS generation, which, in conjunction with HDACIs, promotes up-regulation of DR5 and activation of the extrinsic apoptotic cascade, followed by release of mitochondrial death proteins (e.g., cytochrome c), thus fully engaging apoptotic signaling cascades. Alternatively, ROS may directly cause mitochondrial damage. Resveratrol synchronizes cells in S-phase and they may be particularly susceptible to HDACI- and/or ROS-mediated DNA damage, which contributes to the activation of cell death pathways. Casp, caspase; DSB, double-strand breaks; c, cytochrome c; Apaf, apoptotic protease-activating factor. Solid lines, prodeath signals; dashed lines, prosurvival signals.
Supplementary Material
Acknowledgments
We thank Dr. Kapil Bhalla for providing the dominant-negative caspase-8 cDNA.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grants CA93738, CA100866, 1P50-CA142509-01, 1P50-CA130805-01]; the Leukemia and Lymphoma Society of America [Grant 6181-10]; the V Foundation; and the Multiple Myeloma Research Foundation.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- HDACI
- histone deacetylase inhibitor
- HDAC
- histone deacetylase
- PI
- propidium iodide
- AML
- acute myelogenous leukemia
- shRNA
- short hairpin RNA
- ELISA
- enzyme-linked immunosorbent assay
- ML171
- 2-acetylphenothiazine
- MnTBAP
- Mn(III)tetrakis(4-benzoic acid)porphyrin chloride
- ROS
- reactive oxygen species
- DR5
- death receptor 5
- NF-κB
- nuclear factor-κB
- IKK
- IκB kinase
- NOX
- NADPH oxidase
- PARP
- poly(ADP-ribose) polymerase
- EdU
- 5-ethynyl-2′-deoxyuridine.
Authorship Contributions
Participated in research design: Yaseen, Chen, Rosato, Dent, Dai, and Grant.
Conducted experiments: Yaseen, Chen, Hock, and Dai.
Contributed new reagents or analytic tools: Chen, Dai, and Grant.
Performed data analysis: Yaseen, Chen, and Dai.
Wrote or contributed to the writing of the manuscript: Yaseen, Dai, and Grant.
References
- Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. (2009) Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch Biochem Biophys 486:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506 [DOI] [PubMed] [Google Scholar]
- Bernhard D, Tinhofer I, Tonko M, Hübl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. (2000) Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ 7:834–842 [DOI] [PubMed] [Google Scholar]
- Bolden JE, Peart MJ, Johnstone RW. (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784 [DOI] [PubMed] [Google Scholar]
- Chandra J. (2009) Oxidative stress by targeted agents promotes cytotoxicity in hematologic malignancies. Antioxid Redox Signal 11:1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. (2009) Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun 378:389–393 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. (2005) SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J Biol Chem 280:40364–40374 [DOI] [PubMed] [Google Scholar]
- Chen LF, Fischle W, Verdin E, Greene WC. (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653–1657 [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J 21:6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen S, Pei XY, Almenara JA, Kramer LB, Venditti CA, Dent P, Grant S. (2008) Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood 112:2439–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen S, Wang L, Pei XY, Funk VL, Kramer LB, Dent P, Grant S. (2011a) Disruption of IκB kinase (IKK)-mediated RelA serine 536 phosphorylation sensitizes human multiple myeloma cells to histone deacetylase (HDAC) inhibitors. J Biol Chem 286:34036–34050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Chen S, Wang L, Pei XY, Kramer LB, Dent P, Grant S. (2011b) Bortezomib interacts synergistically with belinostat in human acute myeloid leukaemia and acute lymphoblastic leukaemia cells in association with perturbations in NF-κB and Bim. Br J Haematol 153:222–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Guzman ML, Chen S, Wang L, Yeung SK, Pei XY, Dent P, Jordan CT, Grant S. (2010) The NF (nuclear factor)-κB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol 151:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Dent P, Grant S. (2005) Blockade of histone deacetylase inhibitor-induced RelA/p65 acetylation and NF-κB activation potentiates apoptosis in leukemia cells through a process mediated by oxidative damage, XIAP downregulation, and c-Jun N-terminal kinase 1 activation. Mol Cell Biol 25:5429–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I. (2007) Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans 35:1156–1160 [DOI] [PubMed] [Google Scholar]
- Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Aggarwal BB. (2003) Resveratrol blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102:987–995 [DOI] [PubMed] [Google Scholar]
- Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, et al. (2008) Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 111:1060–1066 [DOI] [PubMed] [Google Scholar]
- Ge Y, Montano I, Rustici G, Freebern WJ, Haggerty CM, Cui W, Ponciano-Jackson D, Chandramouli GV, Gardner ER, Figg WD, et al. (2006) Selective leukemic-cell killing by a novel functional class of thalidomide analogs. Blood 108:4126–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H. (2010) A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5:981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Sengupta N, Zhang X, Seto E. (2005) Acetylation and deacetylation of non-histone proteins. Gene 363:15–23 [DOI] [PubMed] [Google Scholar]
- Glozak MA, Seto E. (2007) Histone deacetylases and cancer. Oncogene 26:5420–5432 [DOI] [PubMed] [Google Scholar]
- Grant S, Easley C, Kirkpatrick P. (2007) Vorinostat. Nat Rev Drug Discov 6:21–22 [DOI] [PubMed] [Google Scholar]
- Holmes-McNary M, Baldwin AS., Jr (2000) Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IκB kinase. Cancer Res 60:3477–3483 [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, Gescher AJ. (2011) Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases: safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 4:1419–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain AR, Uddin S, Bu R, Khan OS, Ahmed SO, Ahmed M, Al-Kuraya KS. (2011) Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS One 6:e24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG. (2005) Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med 11:71–76 [DOI] [PubMed] [Google Scholar]
- Kim YM, Talanian RV, Billiar TR. (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272:31138–31148 [DOI] [PubMed] [Google Scholar]
- Kramer MP, Wesierska-Gadek J. (2009) Monitoring of long-term effects of resveratrol on cell cycle progression of human HeLa cells after administration of a single dose. Ann NY Acad Sci 1171:257–263 [DOI] [PubMed] [Google Scholar]
- Krämer OH, Knauer SK, Zimmermann D, Stauber RH, Heinzel T. (2008) Histone deacetylase inhibitors and hydroxyurea modulate the cell cycle and cooperatively induce apoptosis. Oncogene 27:732–740 [DOI] [PubMed] [Google Scholar]
- Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. (2010) Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci USA 107:14639–14644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R. (2012) Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell 21:266–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Liu PY, Marshall GM. (2009) The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res 69:1702–1705 [DOI] [PubMed] [Google Scholar]
- Low IC, Chen ZX, Pervaiz S. (2010) Bcl-2 modulates resveratrol-induced ROS production by regulating mitochondrial respiration in tumor cells. Antioxid Redox Signal 13:807–819 [DOI] [PubMed] [Google Scholar]
- Maggio SC, Rosato RR, Kramer LB, Dai Y, Rahmani M, Paik DS, Czarnik AC, Payne SG, Spiegel S, Grant S. (2004) The histone deacetylase inhibitor MS-275 interacts synergistically with fludarabine to induce apoptosis in human leukemia cells. Cancer Res 64:2590–2600 [DOI] [PubMed] [Google Scholar]
- Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leão C, Costa V, Rodrigues F, Burhans WC, Ludovico P. (2010) Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci USA 107:15123–15128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, Alvarez R, Schiavone EM, Ferrara F, Bresciani F, et al. (2005) Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med 11:77–84 [DOI] [PubMed] [Google Scholar]
- Ogino K, Kodama N, Nakajima M, Yamada A, Nakamura H, Nagase H, Sadamitsu D, Maekawa T. (2001) Catalase catalyzes nitrotyrosine formation from sodium azide and hydrogen peroxide. Free Radic Res 35:735–747 [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. (2010) SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285:8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al. (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XY, Dai Y, Youssefian LE, Chen S, Bodie WW, Takabatake Y, Felthousen J, Almenara JA, Kramer LB, Dent P, et al. (2011) Cytokinetically quiescent (G0/G1) human multiple myeloma cells are susceptible to simultaneous inhibition of Chk1 and MEK1/2. Blood 118:5189–5200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli LA, Dupéré-Richer D, Pettersson F, Retrouvey H, Skoulikas S, Miller WH., Jr (2011) Vorinostat induces reactive oxygen species and DNA damage in acute myeloid leukemia cells. PLoS One 6:e20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S, Auberger P. (2010) Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res 70:1042–1052 [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Cartee L, Betts V, Chellappan SP, Grant S. (2002) The cyclin-dependent kinase inhibitor flavopiridol disrupts sodium butyrate-induced p21WAF1/CIP1 expression and maturation while reciprocally potentiating apoptosis in human leukemia cells. Mol Cancer Ther 1:253–266 [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Coe S, Grant S. (2007) The multikinase inhibitor sorafenib potentiates TRAIL lethality in human leukemia cells in association with Mcl-1 and cFLIPL down-regulation. Cancer Res 67:9490–9500 [DOI] [PubMed] [Google Scholar]
- Rosato RR, Almenara JA, Grant S. (2003) The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res 63:3637–3645 [PubMed] [Google Scholar]
- Rosato RR, Kolla SS, Hock SK, Almenara JA, Patel A, Amin S, Atadja P, Fisher PB, Dent P, Grant S. (2010) Histone deacetylase inhibitors activate NF-κB in human leukemia cells through an ATM/NEMO-related pathway. J Biol Chem 285:10064–10077 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ruefli AA, Ausserlechner MJ, Bernhard D, Sutton VR, Tainton KM, Kofler R, Smyth MJ, Johnstone RW. (2001) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA 98:10833–10838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder YD, Heiss EH, Schachner D, Ziegler J, Reznicek G, Sorescu D, Dirsch VM. (2009) NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic Biol Med 46:1598–1606 [DOI] [PubMed] [Google Scholar]
- Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. (2005) Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 102:4842–4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan MF, White JE, Maheshwari JG, Chikkappa G. (2002) Anti-leukemia effect of resveratrol. Leuk Lymphoma 43:983–987 [DOI] [PubMed] [Google Scholar]
- Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, Agarwal R. (2005) Resveratrol causes Cdc2-Tyr15 phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis 26:1978–1987 [DOI] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. (2007) Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26:5541–5552 [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. (2005) Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA 102:16090–16095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.