Abstract
The amygdala is a key structure of the brain’s reward system. Existing theories view its role in decision-making as restricted to an early valuation stage that provides input to decision mechanisms in downstream brain structures. However, the extent to which the amygdala itself codes information about economic choices is unclear. Here, we report that individual neurons in the primate amygdala predict behavioral choices in an economic decision task. We recorded the activity of amygdala neurons while monkeys chose between saving liquid reward with interest and spending the accumulated reward. In addition to known value-related responses, we found that activity in a group of amygdala neurons predicted the monkeys’ upcoming save–spend choices with an average accuracy of 78%. This choice-predictive activity occurred early in trials, even before information about specific actions associated with save–spend choices was available. For a substantial number of neurons, choice-differential activity was specific for free, internally generated economic choices and not observed in a control task involving forced imperative choices. A subgroup of choice-predictive neurons did not show relationships to value, movement direction, or visual stimulus features. Choice-predictive activity in some amygdala neurons was preceded by transient periods of value coding, suggesting value-to-choice transitions and resembling decision processes in other brain systems. These findings suggest that the amygdala might play an active role in economic decisions. Current views of amygdala function should be extended to incorporate a role in decision-making beyond valuation.
Keywords: neurophysiology, abstract representation, subjective value, emotion
The amygdala is a key structure of the brain’s reward system, and it is involved in value-guided behavior. Damage to the amygdala in humans is related to changes in decision-making under conditions of ambiguity (1) and risk (2). In monkeys and rats, amygdala lesions impair reward-related and affective behavior (3, 4). Individual amygdala neurons respond to basic rewarding and aversive stimuli (5, 6), code expectations about rewarding and aversive outcomes (7–10), and update the positive and negative values of conditioned stimuli during learning (9–11). In human imaging studies, amygdala activation is associated with basic rewards (12), decision variables (13), and decision-related emotions (14). Together, these findings suggest an important contribution of the amygdala to economic decision-making in addition to its well-known roles in emotion and fear conditioning (5, 14–19). However, the specific nature of this contribution is currently unknown.
Existing theories of the amygdala view its role in decision-making as restricted mainly to the evaluation of choice options (1, 14, 20), which may serve as input for decision mechanisms in downstream brain structures. Although this view ties in well with known amygdala functions in reward (3, 5, 21), Pavlovian learning (4, 11), and emotion (14–20), it may be premature to conclude that the role of the amygdala in decision-making is confined to the valuation stage. Crucially, the information coded by individual amygdala neurons during economic decision-making has not been systematically explored. Therefore, it is currently unclear whether information processing in the amygdala ends with the coding of values or whether its neurons also carry information about upcoming economic choices.
Here, we report that the activity of single neurons in the primate amygdala predicted behavioral choices in an economic reward–saving task. The decision task temporally dissociated the economic choice from the process of action selection, which allowed us to assess neuronal choice coding independently from action coding.
Results
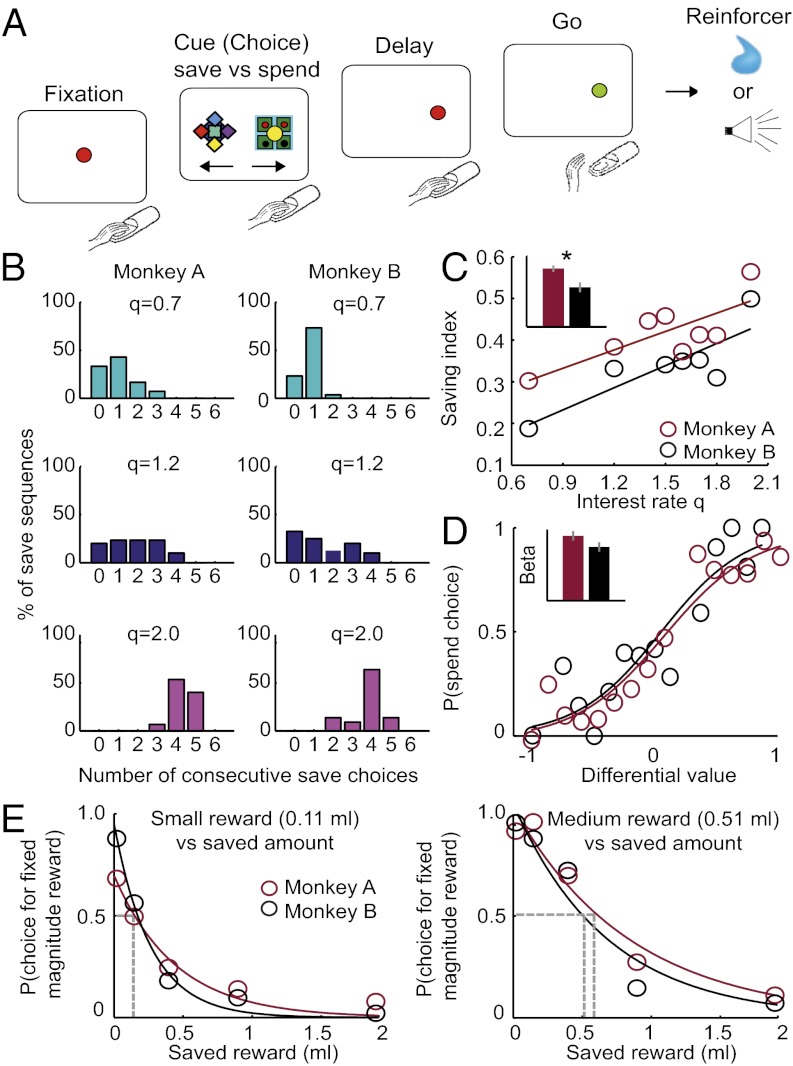
Two monkeys performed in a free choice economic task (Fig. 1A). The animals chose between saving a liquid reward with interest for future trials and spending the already accumulated reward immediately. The increase of reward magnitude over successive save choices was determined by a geometric series (Eq. 1),
Fig. 1.
Behavioral task and economic choice behavior. (A) Sequence of events in the free choice save–spend task. Monkeys indicated their choices with a saccade to one of two visual cues. Specific cues predicted save and spend options; different save cues indicated different interest rates. (B) Monkeys made more consecutive save choices with higher interest rates. Percentage of observed save sequences in six representative sessions with different interest rates (q). (C) Saving index increased as a function of interest rate (monkey A: R2 = 0.61; monkey B: R2 = 0.71; both P < 0.03, linear regression), and mean index differed between monkeys (Inset; P = 0.005, paired t test; error bars denote ± SEM). (D) Spend probability as a function of differential subjective value between save and spend options (color code is the same as in C, and curves represent logistic fits to choice data). Inset shows standardized logistic regression coefficients (both P < 1 × 10−8, t test for logistic regression coefficient; error bars denote ± SEM). (E) Control test with fixed reward. On random trials (in sessions with the same interest rate), monkeys chose between a fixed reward (0.11 or 0.51 mL, indicated by different cues) and the accumulated saved amount. Intersections between horizontal gray lines and choice curves (exponential fits) indicate points of subjective indifference between fixed and saved rewards.
 |
with xn as the reward magnitude on trial n, b as the base rate of reward magnitude, and q as the interest rate, resulting in exponential increases for higher interest rates (Fig. S1). Monkeys indicated their choices by a saccade to the visual save or spend cue. Notably, the task temporally dissociated the internal process of save vs. spend choice, which could occur before the saccade targets were presented, from the process of left vs. right action selection.
The monkeys also performed in an imperative task with the same visual cues but small dots indicating the required target choice. This imperative task was a useful control to examine the extent to which choice-related neuronal activity would also occur when choices were externally instructed. We matched the ratio of save to spend trials in the imperative task to the ratio observed in the free choice task for a given monkey and a given interest rate.
Behavioral Data.
The monkeys took advantage of the nature of the reward–saving task by making more consecutive save choices with higher interest rates (Fig. 1B). The spend probability at any point in a save choice sequence depended on the number of preceding save choices since the last spend choice (P < 0.003, repeated measures ANOVA). There were also slight differences in saving between monkeys (interaction between interest rate and animal identity; P < 0.003, repeated measures ANOVA). To further examine these effects, we constructed an index that reflected preferences for longer save sequences (SI Methods). For both monkeys, this index increased with higher interest rates (P < 0.03, linear regression) (Fig. 1C). The average index across interest rates was also higher for monkey A compared with monkey B (P = 0.005, paired t test) (Fig. 1C Inset), indicating a stronger preference for saving over multiple trials. Thus, in both animals, save sequences became longer with higher interest rates, although saving behavior differed slightly between animals.
We modeled the monkeys’ trial-by-trial choices with a logistic function of the differential subjective value of spending or saving on a given trial (Methods). Logistic regressions suggested that the differential value of spending on the present trial vs. spending on any potential subsequent trial of the same save sequence provided a good fit to the monkeys’ behavior (Fig. 1D, Fig. S2, and Table S1). Thus, the monkeys’ choices were guided by the reward value of potential future trials in a save sequence. Moreover, saving behavior was better explained by this differential value model compared with a simpler model based only on the monkeys’ average choice probabilities (SI Results). This finding suggested that monkeys incorporated trial-by-trial variations in subjective value rather than using a simple counting strategy.
To confirm that monkeys tracked accumulated rewards over consecutive save choices, we offered them, on randomly selected control trials, a choice between the accumulated reward and fixed reward amounts, which were indicated by pretrained visual cues. Both animals consistently chose the fixed reward when it exceeded the saved magnitude (Fig. 1E) (P < 0.001, Mann–Whitney test). This result suggested that monkeys kept track of accumulated rewards over successive save trials and based their choices on this information.
Analysis of licking durations confirmed that monkeys distinguished save–spend trials in both free choice and imperative tasks even before cue appearance (Fig. S3). Furthermore, performance levels were similar for both tasks (80% and 76% correct trials in the free choice and imperative tasks, respectively). These observations suggested that monkeys anticipated save and spend choices in the imperative task.
Neuronal Activity.
We recorded the activity of 329 task-related amygdala neurons and used multiple regression analysis to test for coding of the monkeys’ upcoming save–spend choices before the behavioral responses (Methods). We included several reward value measures as regression covariates and estimated their coefficients simultaneously with choice coefficients. This method ensured that significant choice coefficients were not confounded by value coding.
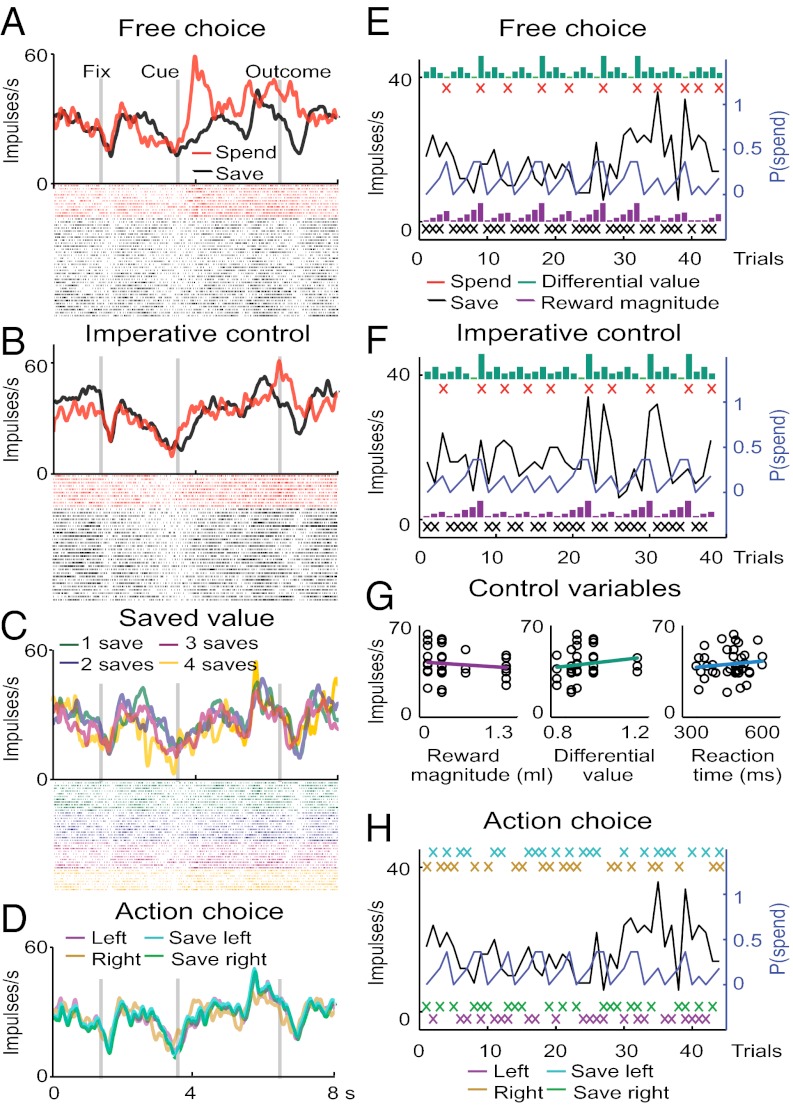
The choice-related activity of the neuron shown in Fig. 2 was higher before spend choices compared with save choices (P = 0.026, t test for the save–spend coefficient) (Fig. 2 A and E). Importantly, save–spend differences occurred only in free choice and not imperative trials (Fig. 2 B and F). Multiple regression revealed that the neurons’ activity was unrelated to different measures of value (Fig. 2 C, E, and G). The activity did not predict upcoming left or right eye movements, and it was independent of visual cue position or reaction time (Fig. 2 D, G, and H). Taken together, the neuron’s response predicted the behavioral choice to save or spend irrespective of value, action, and other measured choice parameters.
Fig. 2.
Choice coding in an example amygdala neuron. (A) Choice-predictive activity in the free choice task. Activity time courses and raster plots for save (black) and spend (red) choices. Each line in the raster plots represents one trial; each dot represents one impulse. The regression coefficient for save–spend choice was significant (P = 0.026, t test) in the cue period before the behavioral response. The differential response did not reflect cue differences, as both cues appeared in all trials. (B) Activity in the imperative control task did not distinguish between save–spend trials. (C) Activity in save trials did not track accumulated reward over successive save trials (one to four saves). (D) Activity failed to distinguish between left/right eye movements or visual cue positions (save cue left/right). (E) Trial-by-trial record of activity across save–spend choices (black trace, neuronal activity in the cue period; blue trace, spend probability; black/red crosses, save–spend trials; vertical bars, value; magenta/green, reward magnitude/differential value). (F) Trial-by-trial record during the imperative task. (G) Activity did not track reward magnitude, differential value, or reaction times (all P > 0.3, t test on regression coefficients). (H) Trial-by-trial record across left–right actions and cue positions.
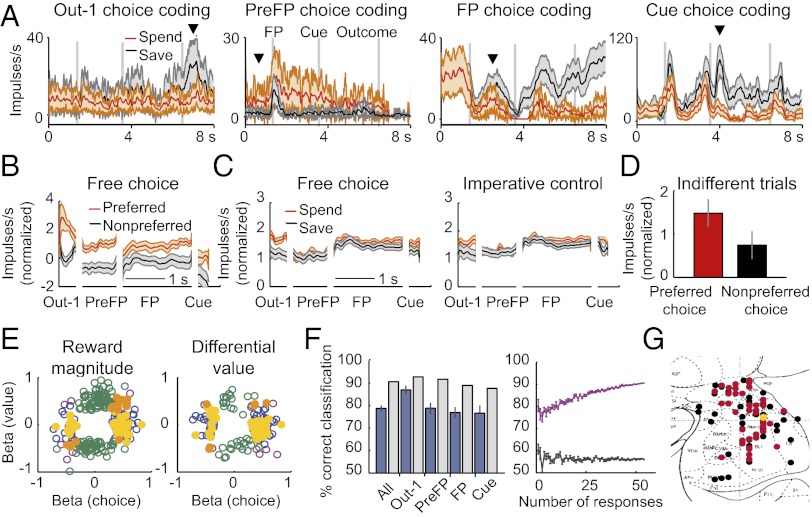
Of 846 task-related responses in 329 neurons, 127 responses in 94 neurons (29% of the neuronal population; 50 and 44 neurons from monkeys A and B, respectively) showed choice-predictive activity (i.e., differential activity for upcoming save–spend choices as defined by a significant choice regressor) (Fig. 3A and Table 1). Task-related responses were defined by comparing a neuron’s activity in a given task period with the neuron’s activity in a control period (P < 0.05, Wilcoxon test) (Methods). In addition to choice coding, we confirmed the known value coding (8, 11, 22) in 140 amygdala neurons (43% of the neuronal population). Among the 127 choice-predictive responses, 85 responses (67%) showed no value coding, and 42 responses showed conjoint choice and value coding (Table 1). Only a few neurons showed relationships with left vs. right actions or reaction times (SI Results).
Fig. 3.
Choice-predictive activity in amygdala neurons. (A) Four example neurons with choice-predictive activity in different task periods. Out-1 data were sorted according to choice on the next trial. Arrows indicate first period with significant choice regressor. Shaded regions indicate SEM. (B) Population time courses of z-normalized activity for 57 responses that predicted choice in the free task but not in the imperative task [Out-1 (outcome period of previous trial): n = 8; PreFP (before fixation spot): n = 16; FP (during fixation): n = 20; Cue (cue period): n = 13). (C) Population time courses of z-normalized activity for all 421 responses that were tested in both tasks (not selected for choice prediction) sorted into save–spend trials. (D) Mean normalized activity of 55 choice-predictive responses on trials in which monkeys were indifferent between spending and saving sorted according to trial-by-trial choice. (E) Relationships between choice and value coding. Standardized choice regression coefficients plotted against coefficients for reward magnitude (Left) and differential value (Right). Blue, significant choice but not value coefficients (Left, n = 87 responses; Right, n = 106 responses); green, significant value but not choice coefficients (Left, n = 137 responses; Right, n = 37 responses); magenta, significant choice and value coefficients (Left, n = 36 responses; Right, n = 14 responses); yellow, 37 responses (31 neurons) coding choice only during free choices without coding value; orange, 20 responses (14 neurons) coding choice only during free choices and coding value. (F) Decoding choices from neuronal activity. Accuracy (percent correct classification) of a biologically plausible classifier (23) using 57 responses with choice coding only during free choice. Blue bars, mean accuracy (±SEM) for classification based on individual responses; gray bars, accuracy for combining data across responses. Chance performance was 50%. (Right) Increases in accuracy (mean ± SEM) as responses were combined. Black trace, accuracy for randomly permuted data. (G) Histological reconstruction of recording sites. Locations of all 94 choice-predictive neurons (black symbols) and 45 neurons with choice coding only in the free choice task (red symbols) overlaid on a section from a stereotaxic atlas showing approximate amygdala subdivisions [45; the rhesus monkey brain in stereotaxic coordinates, Paxinos G, Huang XF, Toga AW, p 1, Copyright Elsevier (2000)]. Yellow symbol, example neuron from Fig. 2. Collapsing in the anterior–posterior dimension resulted in symbol overlap.
Table 1.
Numbers of neurons with significant value and choice coefficients in different task periods and numbers of total significant responses summed over task periods
| Total neurons* | Total responses† | Out-1 | Pre FP | FP | Cue | |
| All | ||||||
| Task-related | 329 | 846 | 144 | 327 | 210 | 165 |
| Value‡ | 140 (43%) | 225 (27%) | 65 (45%) | 49 (15%) | 67 (32%) | 44 (27%) |
| Choice§ | 94 (29%) | 127 (15%) | 19 (13%) | 27 (8%) | 38 (18%) | 43 (26%) |
| No value/value/complex¶ | 59/25/10 | 85/42/— | 9/10/— | 22/5/— | 26/12/— | 28/15/— |
| Imperative‖ | ||||||
| Task-related | 156 | 421 | 76 | 155 | 104 | 86 |
| Value | 76 (49%) | 115 (27%) | 28 (37%) | 25 (16%) | 34 (33%) | 28 (33%) |
| Choice | 56 (36%) | 73 (17%) | 10 (13%) | 18 (12%) | 23 (22%) | 22 (26%) |
| Free choice only** | 45 (29%) | 57 (14%) | 8 (11%) | 16 (10%) | 20 (19%) | 13 (15%) |
| No value/value/complex | 31/12/2 | 37/20/— | 3/5/— | 11/5/— | 13/7/— | 10/3/— |
*Numbers of individual neurons with significant responses in at least one task period; some neurons showed effects in multiple periods.
†Numbers of significant responses summed over task periods.
‡Significant reward magnitude or differential value coefficient.
§Significant save–spend choice coefficient.
¶Complex indicates neurons that coded choice both with and without value in different periods; it only applies for the total neurons column.
‖Neurons tested in both free choice and imperative tasks.
**Significant choice coefficients in the free choice task but not the imperative task.
These data suggest that a substantial fraction of amygdala neurons carried choice-predictive information. The question arises whether these neurons genuinely coded economic choices or whether choice-predictive activity reflected differential reward expectation on save–spend trials. To address this question, we tested 156 neurons in both the free choice and imperative control tasks. If a neuron coded economic choices rather than reward expectations, its choice-predictive activity should be specific to the free choice task. Of 156 neurons tested in both tasks, 56 neurons showed choice-predictive activity during free choices. Among them, 45 neurons (80%) failed to predict choices on imperative trials (P < 0.001, binomial test for choice coding only in free choice vs. both tasks). In these 45 neurons, 41 responses showed higher activity on spend than save trials, and 16 responses showed the opposite pattern (Fig. 3B) (P < 0.001, two-tailed binomial test). Across all neurons tested in both tasks (not selected for choice coding), simple save–spend differences were small and mainly restricted to cue and outcome periods (Fig. 3C). As behavioral data showed, reward expectation was similar in free choice and imperative tasks (Fig. S3). Thus, specificity of save–spend differences for free choices in these 45 neurons implied that their activity did not reflect differential reward expectation.
To examine relationships between choice-predictive activity and other decision parameters, we performed additional tests. Across choice-predictive responses, we searched for trials in which monkeys were, on average, indifferent between saving and spending. We identified 55 responses in 39 neurons in which this criterion was fulfilled [median P (spend) = 0.5; median number of successive save choices after which indifference was observed was three, depending on the interest rate]. We then tested whether neuronal activity on indifference trials tracked trial-by-trial choices. Across these 55 responses, activity was significantly higher for each neuron’s preferred compared with nonpreferred choices (Fig. 3D) (P < 0.001, Wilcoxon test), despite identical choice probability and value-related decision variables. In another test, we evaluated whether choice coding remained constant over changes in interest rate. Among the 45 neurons in which choice-predictive activity was specific to the free choice but not the imperative task, 20 neurons were also tested with different interest rates. The majority of these neurons (16/20, 80%) showed a significant choice regressor, despite changed interest rate and visual save cues (Fig. S4).
A subgroup of 37 choice-predictive responses (31 neurons, 20% of neurons tested in both tasks; 16 and 15 neurons from monkeys A and B, respectively) showed the same characteristics as the neuron in Fig. 2; this subgroup predicted upcoming save–spend choices, but it failed to predict choice in imperative trials and failed to code value, action, visual stimulus position, and reaction time (Fig. 3E). As a useful control, less than 5% (our statistical threshold) of neurons exhibited such characteristics only in the imperative task and not in the free choice task, suggesting that this response pattern was not caused by random variability (P < 0.001, χ2 test comparing proportions of such response types in both tasks). Thus, a group of amygdala neurons coded the monkeys’ economic choices largely independent of value, action, and other choice parameters.
To quantify the degree to which economic choices could be predicted from neuronal activity, we used a biologically plausible classifier (23) as well as, independently, linear discriminant analysis to decode choices from trial-by-trial impulse rates. Notably, classifications used data from individual trials, which reflect the information propagated to the next downstream neuron during decision-making. We focused on those 57 responses that predicted choices in the free but not imperative task (although similar results were obtained with all 127 choice-predictive responses) (Fig. S5). On average, neuronal responses predicted save–spend choices with an accuracy of 78% (80% for the biologically plausible classifier; 76% for linear discriminant analysis) (Fig. 3F and Fig. S6). Combining responses, the classifier predicted choices with an accuracy of 91% (P < 0.001, permutation test with 1,000 iterations) (Fig. 3F). Increases in accuracy as responses were combined (Fig. 3F) indicated that neurons contributed partly independently to the prediction.
Of 45 neurons with choice coding only in the free choice task, 23 neurons were from the dorsal amygdala, 1 neuron was from the lateral amygdala, 10 neurons were from the basomedial amygdala, 9 neurons were from the basolateral amygdala, and 2 neurons were from the basoventral amygdala (Fig. 3G and Fig. S7). We were unable to identify systematic differences between recording sites (nonsignificant χ2 tests); therefore, in line with previous studies (6, 24), we present the neuronal data as one set.
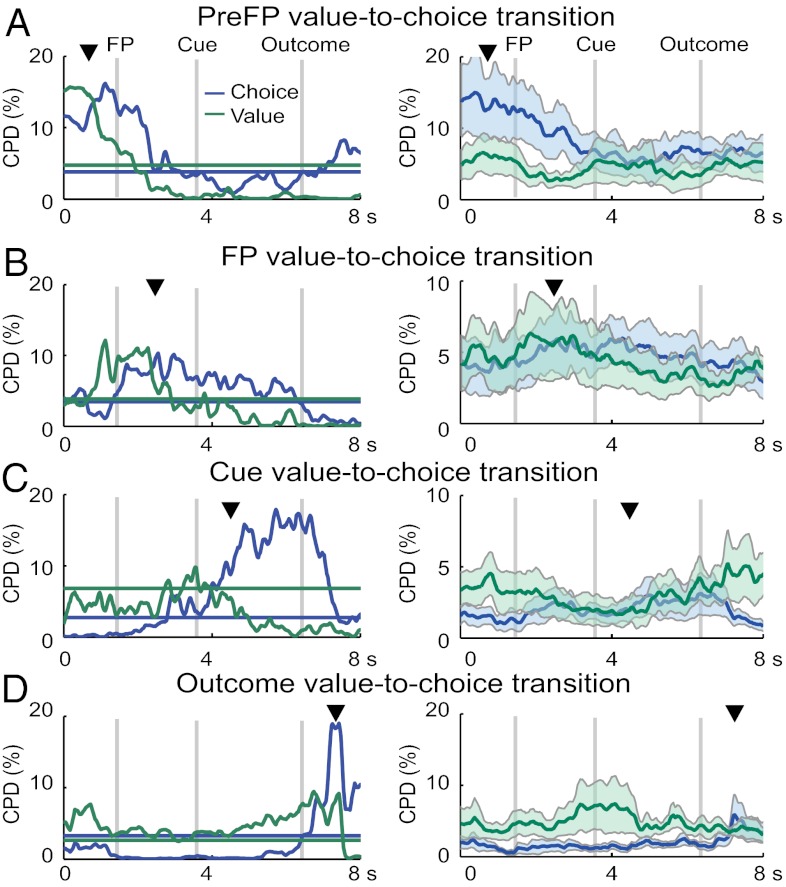
In perceptual decision-making, individual neurons in parietal cortex and related systems exhibit transitions from coding of decision variables to coding of perceptual choices (25, 26). Such signals are often interpreted as correlates of decision processes. To test for analogous value-to-choice transitions, we identified neurons with significant choice coding and in the same or preceding task period, significant value coding, and we examined the related temporal dynamics using sliding window regressions (Methods). We found 39 responses (33 neurons) in which value coding in different task periods preceded choice coding (Fig. 4). Among them, 22 responses (19 neurons) were also tested in the imperative task. None of them coded choices in imperative trials. The mean latency difference between the onset of value and choice coding was 1,741 ± 302 ms (SEM). Such responses might reflect translations from value to choice coding, resemble perceptual decision processes in other brain systems (25, 26), and match predictions from computational models of decision mechanisms (27–29).
Fig. 4.
Value-to-choice transitions. Four example neurons (Left) and related subpopulation means (Right; shaded regions indicate SEM) in which choice coding in a given task period was preceded by value coding. Coefficients of partial determination (CPDs) for value (green trace) and choice (blue trace) regressors from a sliding regression quantify the percent of variance in neuronal activity explained by one regressor in a multiple regression model. Arrows indicate task periods with significant choice coding. Horizontal lines indicate 3 SDs above the mean CPD obtained from randomly permuted data. Value-to-choice transitions were found in 39 responses [33 neurons; (A) PreFP: n = 6; (B) FP: n = 12; (C) Cue: n = 12; (D) Out-1: n = 9]. Value-to-choice transitions occurred within each individual response, and exact timing varied across responses; the population CPDs reflect the relative strength of value and choice coding across responses without necessarily also showing these transitions.
Discussion
The present data show that the activity of individual amygdala neurons predicts behavioral choices during economic decision-making. In many neurons, choice-predictive activity occurred before information about specific behavioral responses was available to the monkeys. This finding suggested neuronal coding of the abstract economic save–spend choice rather than specific actions. For a large proportion of choice-predictive neurons that were tested in both free choice and imperative tasks, choice-predictive activity was specific to free, internally generated choices. The inclusion of value covariates ensured that choice coding could not be explained in terms of reward value or related decision variables. Choice-predictive activity in a subgroup of neurons was irrespective of value, action, visual cue position, or other measured parameters. Taken together, neurons with such responses seem to code the monkeys’ economic choices in a predictive manner.
A potential alternative explanation is that the choice-predictive activity might reflect differences in reward expectation, because immediate rewards were only available on spend but not save trials. However, several observations seem incompatible with this interpretation. First, in the majority of neurons tested in both tasks, choice-differential activity was not observed during the externally instructed imperative task, despite similar reward timing (although in some neurons, choice-predictive activity persisted during imperative trials). Second, reaction time and licking differences between save–spend trials, potentially reflecting differential reward expectation, were similar in both tasks (Fig. S3), and many choice-predictive neurons did not track them (SI Results). Third, a previous study showed that reward expectation-related activity in amygdala neurons covaried with the temporal distance to reward receipt (24). By contrast, activity in many choice-predictive neurons in the present study did not covary with distance to reward (SI Results). Taken together, these observations argue against an explanation of choice-predictive activity solely in terms of reward expectation.
What role might neurons with choice-predictive responses serve in the context of general amygdala function? One potential role of the amygdala in decision-making might be to provide valuation signals that inform decision processes in downstream brain structures. This view fits well with current theories of amygdala function (1, 14, 20), known value coding in the amygdala in nonchoice situations (6, 11), and findings that human amygdala neurons track values during decision-making (22). Furthermore, decision impairments in humans with amygdala lesions are usually interpreted as valuation deficits based on concomitant changes in autonomic responses to decision outcomes (1, 2). A second possibility is that choice-predictive responses in the amygdala reflect the output of decision processes in other brain structures. The convergence of information about already computed decisions with value signals in the amygdala could be useful in learning processes (for example, by comparing values between expected and obtained outcomes). Conceptually similar chosen value neurons are found in the orbitofrontal cortex and striatum along with other neurons that code the value of individual choice options (30–35). However, many amygdala neurons in the present study coded information about upcoming choices without also coding their values. Indeed, supplementary analyses showed that the majority of choice-predictive responses could not be explained by these different forms of value coding (SI Results, Table S2).
A third and perhaps, more tantalizing possibility is that choice-predictive responses could reflect decision computations within the amygdala, which might directly instruct the selection of actions. The presently observed value-to-choice transitions could be interpreted as initial evidence for such a local decision mechanism. Current views of the role of the amygdala in fear conditioning also emphasize its potential to directly guide behavior through outputs to the striatum (17). Although this possibility could explain some of the behavioral deficits associated with amygdala lesions (1–3), it might seem inconsistent with evidence from reinforcer devaluation paradigms. In these studies, amygdala inactivation does not cause deficits in object choice after reward values have been updated, which is in contrast to inactivation of the orbitofrontal cortex (36, 37). However, the absence of behavioral deficits after inactivation does not, per se, preclude amygdala involvement in choices. For example, the amygdala might code choices in parallel with other brain systems, including the orbitofrontal cortex. Indeed, a recent study showed largely parallel value coding in amygdala and orbitofrontal cortex (10). By analogy, studies of perceptual decision-making showed parallel choice coding throughout multiple neural systems (38). Furthermore, reward structures, such as the amygdalae, consist of functionally heterogeneous neuronal populations (5, 6), and the behavioral consequences of lesions may reveal only a small part of the information processing in such brain structures. Nevertheless, a conclusive understanding of choice-predictive activity within the amygdala will require additional experimental investigation.
This discussion raises the question of whether similar coding of economic choices exists in other reward structures. The work by Padoa-Schioppa and Assad (34) described neurons in the orbitofrontal cortex that coded the chosen taste in an economic decision task. Recent observations suggest that such responses can occur early in trials before action information (39). Accordingly, chosen taste responses in the orbitofrontal cortex could potentially reflect the output of a decision mechanism for translating values into choices. However, it will be important to determine whether these responses reflect genuine economic choice coding or the expectation of specific taste rewards.
Our findings have implications for an ongoing debate about the nature of economic choice coding in the primate brain. Evidence for action-based coding of decision variables in the parietal cortex and related systems (25, 40) has led to views that economic decisions take place primarily among actions (40). By contrast, evidence for action-independent value coding in reward structures has led to the proposal that economic decisions take place in an abstract space of economic goods (34, 39). Thus, a fundamental, unresolved question is whether economic choices can exist as action-independent neuronal representations. Recent studies indicated that perceptual choice coding in parietal (41) and frontal cortices (42) and even the superior colliculus (43) can occur in an action-independent manner. Here, we extended these observations to value-based economic choices. We found choice-predictive responses before action information in the amygdala, a reward structure that is conceptually even farther upstream of action selection. The present behavioral testing with eye movements should not imply that the observed choice-related activity is specific for eye movements. As the absence of relationships to saccadic reaction times suggests, the observed activity may well occur with economic decisions involving other effector systems. Thus, taken together with previous evidence, our findings imply that abstract, action-independent neuronal representations may provide the basis for both perceptual and economic decisions.
In conclusion, our findings show that, in addition to providing value inputs to decision-making, the amygdala also codes economic choices in a predictive manner. Conceptually, choice coding in the amygdala seems to occupy an intermediate stage in neuronal information processing that is situated between valuation and action selection. Existing views of the amygdala as a pure valuation structure may, therefore, need to be extended to incorporate a more direct role in economic decisions.
Methods
Animals.
Two adult male rhesus monkeys (Macaca mulatta) weighing 9.2 and 12.0 kg participated in the experiment. All animal procedures conformed to US National Institutes of Health Guidelines and were approved by the Home Office of the United Kingdom.
Free Choice Task.
In different blocks of typically 50–100 consecutive trials, different stimuli were used as save cues to indicate different interest rates. We tested interest rates ranging from q = 0.7 to q = 2.0. Each neuron was typically tested with one or two different interest rates.
Imperative Control Task.
A small visual cue was presented next to either the save or the spend cue to indicate the correct choice on each trial that was otherwise identical to a free choice trial.
Electrophysiological Recordings.
We recorded the activity of single amygdala neurons from extracellular positions during task performance using standard electrophysiological techniques. We sampled activity from about 700 amygdala neurons in exploratory tests with the save–spend task, resulting in a database of 329 neurons with task-related responses that we analyzed statistically.
Data Analysis.
We counted impulses in each neuron relative to different task events with fixed time windows: 1,000 ms before fixation spot (PreFP), 1,775 ms after fixation spot but before cues (FP; starting 25 ms after fixation spot onset), 300 ms after cues (Cue; starting 20 ms after cue onset), and 500 ms during the reward/outcome period of the preceding trial (Out-1; starting 50 ms after reward onset). We used the following multiple regression model to assess relationships to different variables (P < 0.05) (Eq. 2):
with SS as save vs. spend choice, RM as the sum of objective reward magnitudes available for save and spend choices, DV as the subjective differential value used for behavioral modeling, LR as left vs. right action, SL as spatial cue position (save cue left vs. right), and RT as saccadic reaction time; β1–6 are corresponding regression coefficients, β0 is the intercept, and ε is error.
Standardized regression coefficients (β values) in Figs. 1D and 3E were defined as xi (si/sy); xi is the raw slope regression coefficient for regressor i, and si and sy are the SDs of independent variable i and the dependent variable, respectively (44).
Decoding Choices from Neuronal Data.
We used a leave-one-out cross-validation procedure, in which every trial was decoded based on the distribution of impulse rates from all other trials For combining data across responses, we used z-normalized neuronal data.
Supplementary Material
Acknowledgments
We thank Dr. Ken-ichiro Tsutsui for help with task design and preliminary data analysis; Prof. Anthony Dickinson, Dr. Shunsuke Kobayashi, Mr. Raymundo Baez Mendoza, Dr. William Stauffer, and Mr. Armin Lak for discussions; Dr. Mercedes Arroyo for expert histology; and Dr. Corinna Zygourakis for preliminary analysis of behavioral data. We also thank the Wellcome Trust, the European Research Council (ERC), and the Cambridge Behavioural and Clinical Neuroscience Institute (BCNI) for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212706109/-/DCSupplemental.
References
- 1.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand M, Grabenhorst F, Starcke K, Vandekerckhove MM, Markowitsch HJ. Role of the amygdala in decisions under ambiguity and decisions under risk: Evidence from patients with Urbach-Wiethe disease. Neuropsychologia. 2007;45(6):1305–1317. doi: 10.1016/j.neuropsychologia.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 4.Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- 5.Rolls ET. Neurophysiology and functions of the primate amygdala, and the neural basis of emotion. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. 2nd Ed. Oxford: Oxford Univ Press; 2000. pp. 447–478. [Google Scholar]
- 6.Bermudez MA, Schultz W. Reward magnitude coding in primate amygdala neurons. J Neurophysiol. 2010;104(6):3424–3432. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19(5):1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermudez MA, Schultz W. Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity) J Neurophysiol. 2010;103(3):1158–1170. doi: 10.1152/jn.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453(7199):1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SE, Saez A, Lau B, Salzman CD. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71(6):1127–1140. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grabenhorst F, Rolls ET, Parris BA, d’Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2010;20(5):1082–1091. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- 13.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 14.Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):787–799. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Markowitsch HJ, Staniloiu A. Amygdala in action: Relaying biological and social significance to autobiographical memory. Neuropsychologia. 2011;49(4):718–733. doi: 10.1016/j.neuropsychologia.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: Evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- 20.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11(11):489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 22.Jenison RL, Rangel A, Oya H, Kawasaki H, Howard MA. Value encoding in single neurons in the human amygdala during decision making. J Neurosci. 2011;31(1):331–338. doi: 10.1523/JNEUROSCI.4461-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quian Quiroga R, Snyder LH, Batista AP, Cui H, Andersen RA. Movement intention is better predicted than attention in the posterior parietal cortex. J Neurosci. 2006;26(13):3615–3620. doi: 10.1523/JNEUROSCI.3468-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugase-Miyamoto Y, Richmond BJ. Neuronal signals in the monkey basolateral amygdala during reward schedules. J Neurosci. 2005;25(48):11071–11083. doi: 10.1523/JNEUROSCI.1796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 26.Romo R, Hernández A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41(1):165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 27.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Hunt LT, et al. Mechanisms underlying cortical activity during value-guided choice. Nat Neurosci. 2012;15(3):470–476. doi: 10.1038/nn.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XJ. Decision making in recurrent neuronal circuits. Neuron. 2008;60(2):215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59(1):161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58(3):451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roesch MR, Singh T, Brown PL, Mullins SE, Schoenbaum G. Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. J Neurosci. 2009;29(42):13365–13376. doi: 10.1523/JNEUROSCI.2572-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 34.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14(12):1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West EA, DesJardin JT, Gale K, Malkova L. Transient inactivation of orbitofrontal cortex blocks reinforcer devaluation in macaques. J Neurosci. 2011;31(42):15128–15135. doi: 10.1523/JNEUROSCI.3295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25(18):4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66(2):300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Padoa-Schioppa C. Neurobiology of economic choice: A good-based model. Annu Rev Neurosci. 2011;34:333–359. doi: 10.1146/annurev-neuro-061010-113648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glimcher PW, Dorris MC, Bayer HM. Physiological utility theory and the neuroeconomics of choice. Games Econ Behav. 2005;52(2):213–256. doi: 10.1016/j.geb.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennur S, Gold JI. Distinct representations of a perceptual decision and the associated oculomotor plan in the monkey lateral intraparietal area. J Neurosci. 2011;31(3):913–921. doi: 10.1523/JNEUROSCI.4417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merten K, Nieder A. Active encoding of decisions about stimulus absence in primate prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109(16):6289–6294. doi: 10.1073/pnas.1121084109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol. 2004;91(5):2281–2296. doi: 10.1152/jn.00872.2003. [DOI] [PubMed] [Google Scholar]
- 44.Cai X, Kim S, Lee D. Heterogeneous coding of temporally discounted values in the dorsal and ventral striatum during intertemporal choice. Neuron. 2011;69(1):170–182. doi: 10.1016/j.neuron.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.