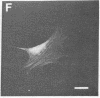
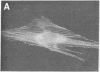
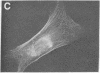
Abstract
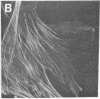
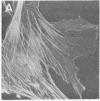
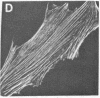
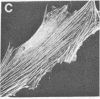
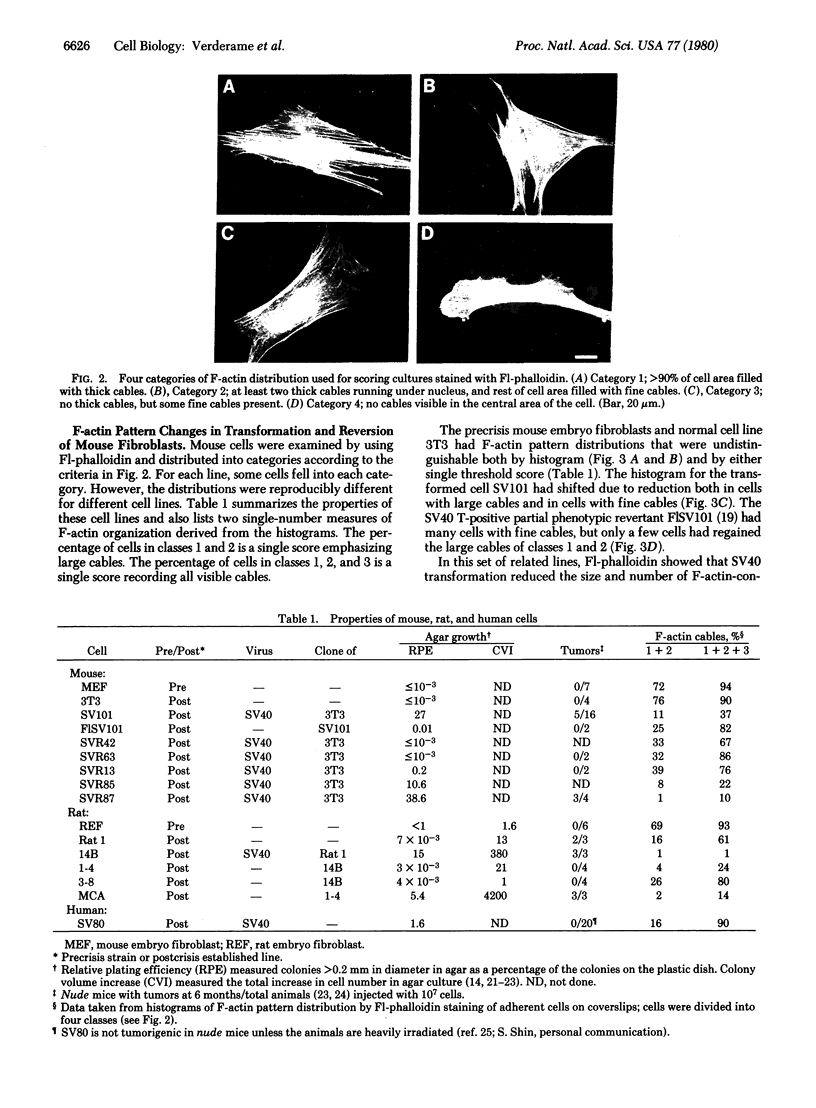
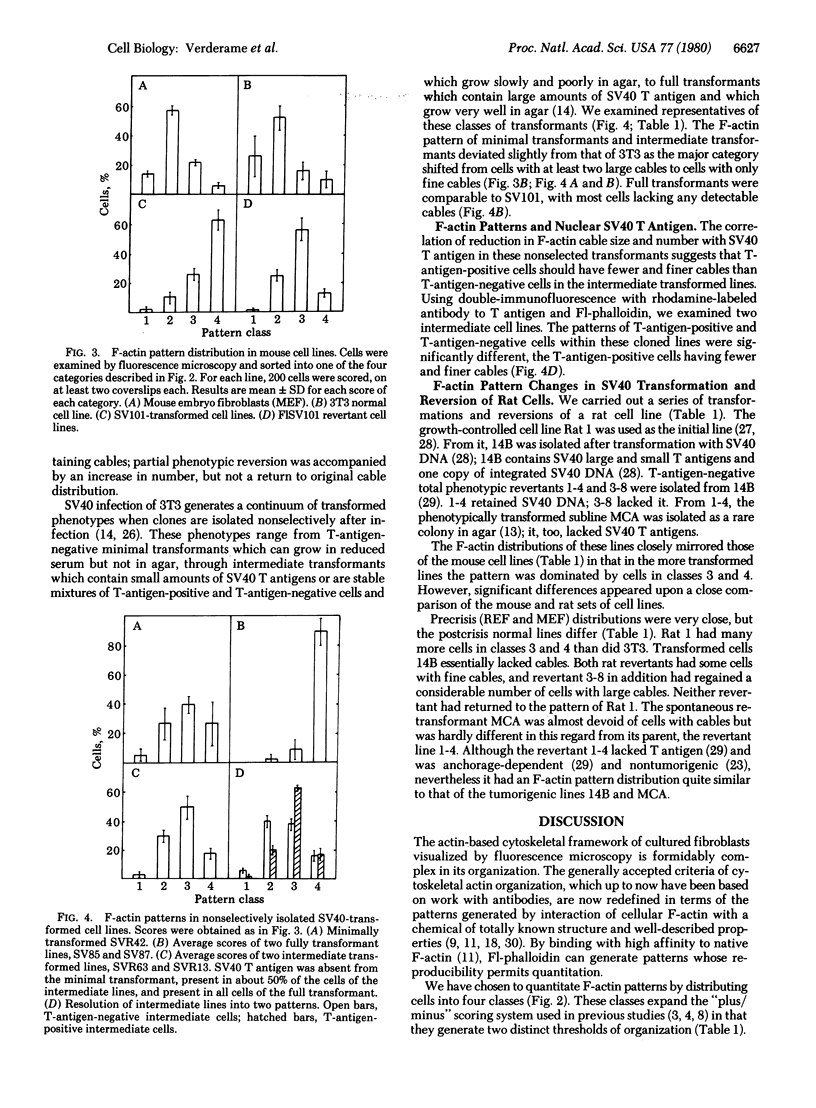
Actin in cultured fibroblasts is organized into a complex set of fibers. Patterns of organization visualized with antibody to actin are similar but not identical to those visualized with fluorescein isothiocyanate-phalloidin (Fl-phalloidin), a chemical that binds to F-actin polymer with a dissociation constant of 2.7 X 10(-7) M [Wulf, E., Deboben, A., Bautz, F. A., Faulstich, H. & Wieland, T. (1979) Proc. Natl. Acad. Sci. USA 76, 4498-4502]. Fl-phalloidin reveals that transformed cells have fewer, finer, and shorter F-actin-containing structures than do normal cells. Two-color fluorescence microscopy of single cells reveals that F-actin staining by Fl-phalloidin picks out the cytoskeletal cables more sharply than does antibody to actin, due to a reduced intracellular background fluorescence. This improved resolution permits sorting of cellular Fl-phalloidin patterns into four classes ranging in organization from 90% of the cytoplasm occupied by large cables to the absence of detectable cables. Reproducible differences in pattern distributions between normal and transformed cell lines have been quantitated. Fl-phalloidin together with rhodamine-based indirect antibody to simian virus 40 tumor antigen reveals a direct relationship between the degree of pattern change and simian virus 40 nuclear antigen expression in intermediate transformed 3T3 cell lines [Risser, R. & Pollack, R. (1974) Virology 59, 477-489].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Burridge K. Changes in cellular glycoproteins after transformation: identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4457–4461. doi: 10.1073/pnas.73.12.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Schäfer A. J., Weckauf M. The dissociation of the phalloidin-actin complex. Hoppe Seylers Z Physiol Chem. 1977 Feb;358(2):181–184. doi: 10.1515/bchm2.1977.358.1.181. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Burridge K. A rapid purification of alpha-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980 Feb 10;255(3):1194–1199. [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Yerna M. J., Schloss J. A. Localization and organization of microfilaments and related proteins in normal and virus-transformed cells. J Supramol Struct. 1976;5(2):155–183. doi: 10.1002/jss.400050206. [DOI] [PubMed] [Google Scholar]
- Herman I. M., Pollard T. D. Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol. 1979 Mar;80(3):509–520. doi: 10.1083/jcb.80.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Lengsfeld A. M., Löw I., Wieland T., Dancker P., Hasselbach W. Interaction of phalloidin with actin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2803–2807. doi: 10.1073/pnas.71.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N. K., Ryan W. L. Effect of 3-methylcholanthrene and dimethylnitrosamine on anchorage dependence of rat fibroblasts chronically infected with Rauscher leukemia virus. Int J Cancer. 1973 Jan 15;11(1):123–130. doi: 10.1002/ijc.2910110114. [DOI] [PubMed] [Google Scholar]
- Osborn M., Born T., Koitsch H. J., Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978 Jul;14(3):477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E., Kopelovich L. The cytoskeleton in cultured cells: coordinate in vitro regulation of cell growth and shape. Methods Achiev Exp Pathol. 1979;9:207–230. [PubMed] [Google Scholar]
- Pollack R., Lo A., Steinberg B., Smith K., Shure H., Blanck G., Verderame M. SV40 and cellular gene expression in the maintenance of the tumorigenic syndrome. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):681–688. doi: 10.1101/sqb.1980.044.01.072. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel I., Levinthal C., Macagno E. R. Special techniques for the automatic computer reconstruction of neuronal structures. Annu Rev Biophys Bioeng. 1980;9:347–362. doi: 10.1146/annurev.bb.09.060180.002023. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Steinberg B. M., Pollack R. Anchorage independence: analysis of factors affecting the growth and colony formation of wild-type and dl 54/59 mutant SV40-transformed lines. Virology. 1979 Dec;99(2):302–311. doi: 10.1016/0042-6822(79)90009-6. [DOI] [PubMed] [Google Scholar]
- Steinberg B. M., Rifkin D., Shin S., Boone C., Pollack R. Tumorigenicity of revertant from an SV40-transformed line. J Supramol Struct. 1979;11(4):539–546. doi: 10.1002/jss.400110412. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Steinberg B., Pollack R., Topp W., Botchan M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell. 1978 Jan;13(1):19–32. doi: 10.1016/0092-8674(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Sanford K. K., Frankel R. Tubulin and actin in paired nonneoplastic and spontaneously transformed neoplastic cell lines in vitro: fluorescent antibody studies. Cell. 1978 Apr;13(4):629–642. doi: 10.1016/0092-8674(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]