Abstract
The formation of mature synaptic connections involves the targeted transport and aggregation of synaptic vesicles, the gathering of presynaptic release sites and the clustering of postsynaptic neurotransmitter receptors and ion channels. Positional cues are required to orient the cytoskeleton in the direction of neuronal outgrowth, and also to direct the juxtaposition of synaptic protein complexes at the pre- and postsynaptic membranes. Both anterograde and retrograde factors are thought to contribute positional information during synaptic differentiation, and recent studies in vertebrates and invertebrates have begun to uncover a new role in this process for proteins that are essential for pattern formation in the early embryo.
Cells use distinct mechanisms for transmitting information to their neighbours, depending on their tissue type and environment. This intercellular communication is vital during the early stages of development, when nascent cells acquire their identities to organize the body plan, and later as mature cells work together to maintain function and lifestyle. Not surprisingly, disruption of intercellular communication leads to various diseases, ranging from cancer to mental retardation, so understanding the fundamental nature of cell-talk remains one of the most important areas of biology.
In the nervous system, the elemental means of communication between cells is synaptic transmission. Chemical synapses are asymmetrical cellular junctions that are involved in the directional transmission of electrical signals. Essential to the high speed and efficiency at which neurons communicate is the exquisite molecular organization of the pre- and postsynaptic apparatus. In the presynaptic compartment, neurotransmitter-laden vesicles are poised at ACTIVE ZONES, ready for immediate release, and the calcium channels that are required to trigger exocytosis are spatially linked to the secretory machinery. At the postsynaptic membrane, neurotransmitter receptors form high-density clusters that are directly apposed to the active zones. The proper development and functioning of synaptic junctions requires positional information, which coordinates the correct placement of pre- and postsynaptic elements.
Once synapses are formed, the strengthening and weakening of synaptic connections is believed to be central to the processes of learning and memory, and also to the regeneration of synaptic connectivity after a traumatic injury. This synaptic plasticity also depends on communication between pre- and postsynaptic compartments1, and it includes both anterograde and retrograde signals. The existence of a retrograde signal has been postulated to account for the adjustment of presynaptic outputs that result from changes in post-synaptic responses. For example, at the fly neuromuscular junction (NMJ), decreased muscle responses to neuronal stimuli can be brought about by manipulation of glutamate receptor (GluR) levels, or by muscle expression of a functional Kir2.1 potassium channel, both of which result in an upregulation of presynaptic transmitter release2–4. Similarly, at mammalian central synapses, several types of retrograde signalling molecules have been described. These include membrane permeant factors, such as nitric oxide, secreted factors, such as neurotrophins and membrane bound proteins, such as neuroligin and neurexin. All of these retrograde signalling molecules modulate the presynaptic release machinery and the cycling of vesicles, and they also trigger other presynaptic cytosolic signals5.
Although several retrograde and anterograde mechanisms have been implicated in the coordinated development of pre- and postsynaptic features, there are still significant gaps in our understanding of these processes. Recent studies, using both mammalian central synapses and the glutamatergic NMJ of Drosophila, have shed light on secreted signals that mediate anterograde and retrograde modulation of synapse development. In this review, we will focus on new evidence that implies that molecules such as Wnt and transforming growth factor-β (TGFβ), which are best known for providing positional information during morphogenesis, also have fundamental roles in promoting the differentiation of synapses. The Wnts and members of the TGFβ family are secreted by either pre- or postsynaptic cells, and they seem to provide crucial anterograde and retrograde signals that regulate the coordinated development of pre- and postsynaptic specializations.
Agrin at the neuromuscular synapse
At the vertebrate NMJ, the nerve-derived HEPARAN SULPHATE proteoglycan Agrin is secreted by the presynaptic terminal, and it has long been thought to provide an early signal to lay down the postsynaptic machinery6. In addition, studies of Agrin knockouts in cultured chick cervical ganglia have shown that preventing normal Agrin function also interferes with presynaptic development in vitro7. So, Agrin has been considered to be one of the secreted signals that coordinates the temporally and spatially synchronized development of pre- and postsynaptic structures. However, recent studies indicate that the postsynaptic machinery can be assembled in an Agrin-independent manner, and that Agrin seems to be necessary to maintain rather than to induce postsynaptic differentiation8–10.
In addition, despite the unquestioned role of Agrin in promoting the development of the NMJ, its function in central synapses has remained unclear11–13. Its expression in the central nervous system (CNS) peaks at times that correlate with periods of synapse formation, as demonstrated both by in situ hybridization and immunocytochemistry. However, complications in the interpretation of these results arise from the fact that the antibodies that were used to study the distribution of Agrin in the CNS recognize both neural and non-neural forms of Agrin.Also, because mRNA is confined largely to the cell body, in situ hybridization studies do not allow one to assess the distribution of Agrin protein at synapses.
Agrin knockout mice die at birth, before the formation of most CNS synapses, so a thorough assessment of its role at central synapses is still lacking. Nevertheless, glutamatergic synapses are still observed in the CNS of Agrin-deficient mice13, and mutant hippocampal and cortical neurons still form glutamatergic and GABA (γ-aminobutyric acid) synapses in culture12. Cell culture experiments have provided disparate results — Serpinskaya et al.12 reported that synapse formation between agrin-mutant hippocampal neurons is normal in cell culture, whereas another study by Ferreira et al.14 indicated that these synapses disappeared if the cultures were maintained for a longer period. Moreover, the latter study showed that ANTISENSE Agrin constructs reduced the number of synapses between cultured hippocampal neurons in short-term cultures. These studies led the authors to conclude that Agrin has a role in synapse formation between hippocampal neurons, but that a compensating activity that is induced in agrin-mutant mice is sufficient to allow the mutant neurons to form synapses in culture, at least for a short period14. In this and another study of cultured hippocampal neurons, antisense knockdown of Agrin expression was shown to inhibit synaptogenesis and selectively disrupt GABA receptor clustering14,15. Recent studies also show impaired sympathetic synapse formation in Agrin-deficient neural cultures, and fewer differentiated synapses were found in vivo in Agrin-deficient mice16.
So, although secreted Agrin plays a pivotal role in neuromuscular synapse formation, its role in the formation of central synapses is still unresolved, implying that additional or alternative factors are required to signal the formation of glutamatergic, cholinergic, and GABA synapses. Many invertebrates, including the fly, use glutamate as a primary neuromuscular transmitter, and their synapses have tantalizing molecular similarities to vertebrate central glutamatergic synapses. However, an Agrin orthologue is not evident in the Drosophila genome. Recent evidence indicates that other sets of secreted molecules, such as EPHRINS17, Wnts18–20 and members of the TGFβ family21–23, might have roles similar to Agrin in the development of glutamatergic synapses, in both vertebrates and invertebrates. For the remainder of this review, we will focus on the role of Wnts and TGFβ in synapse differentiation.
Positional cues for body axis formation
During early embryonic development, a number of secreted proteins and their receptors provide positional information that determines the polarity of body structures.One example of this process is the establishment of the segmental organization of insect embryos, where the secreted glycoprotein wingless (Wg) — a Drosophila Wnt protein — defines anterior-posterior polarity within a body segment.A small group of epithelial cells secretes Wg, and the combined processes of endocytosis and cell division, and perhaps also TRANSCYTOSIS, set up a gradient of Wg protein across every segment in the embryo.This process is repeated during the development of the IMAGINAL DISCS24–28. Dose-dependent sensitivity of cells to the Wg protein concentration initiates differential transcription of target genes, and disparate cell identities are established29,30.
Similarly, during the development of the fly wing, orthogonal gradients of Wg and a member of the TGFβ family of secreted factors, Decapentaplegic (Dpp), determine the shape of the wing31. A variety of antagonists and feedback loops serve to inhibit or promote the action of these pathways32,33. In addition, a dynamic interplay exists between the two signalling cascades, and in some tissues Wg and Dpp have even been shown to exert effects that mutually antagonize each other’s transcription34. So, positive and negative regulation of the Wg and Dpp pathways can act in concert to provide positional information in developing tissues.
In vertebrates, many of the same genes have been implicated in both pattern formation and oncogenesis. For example, the mouse Wg homologue, Wnt1, was first discovered as a tumour-causing proto-oncogene that is activated by the integration of viruses into mammary tissues35. Similarly, TGFβ family members control the development and homeostasis of most tissues in metazoan organisms, and mutations in this pathway cause various forms of cancer and developmental disorders32,36. New evidence is emerging that factors such as Wg and Dpp not only define the body plan, but might also provide essential cues for the proper formation and positioning of synaptic specializations.
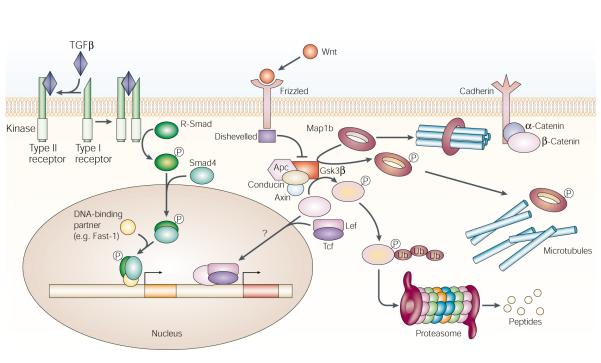
Many aspects of the transduction pathway that underlies Wnt signalling have been defined (FIG. 1). The secreted Wnts bind to heparan sulfate proteoglycans, members of the low density lipoprotein (LDL) receptor-related protein (LRP) family of co-receptors (Arrow in Drosophila), and to the membrane receptor Frizzled (Fz). LRPs are single-pass transmembrane proteins that are proposed to act as essential Wnt co-receptors, along with Fz family members. Fz receptors have a domain profile that is characteristic of G-protein coupled receptors. Indeed, it has recently been suggested thatFz1 is coupled via Go and Gq to the canonical β-catenin-lymphoid enhancer-binding factor/T-cell factor (Lef/Tcf) pathway, and that Fz2 is coupled to this pathway via Gq and Gt (REF. 37). Wnt signal transduction requires the intracellular regions of both LRPs and Fz, and LRP6 in Xenopus has been shown to bind directly to the extracellular domain of Fz, and also to the Wnts38,39.
Figure 1. The Wnt and TGFβ signalling pathways.
Transforming growth factor-β (TGFβ) ligand binding causes heterodimerization of type I and type II receptors. The type II receptor then activates the type I receptor by phosphorylation. The type I receptor then phosphorylates R-Smads, which transduce the signal into the nucleus via a co-Smad, Smad4. The R-Smad/Smad4 complex binds to various developmentally regulated transcription factors to direct gene expression. Wnt binding to the Frizzled receptor leads to phosphorylation of Dishevelled. Phosphorylated Dishevelled prevents the formation of the Apc (adenomatous polyposis coli)-Axin-Conducin-Gsk3β (glycogen synthase kinase 3β) complex, which, in the absence of Wnt, signals the degradation of β-catenin. By contrast, binding of Wnt to Frizzled receptors stabilizes cytoplasmic β-catenin, which complexes with lymphoid enhancer-binding factor/T-cell factor (Lef/Tcf) transcription factors and initiates transcription of Wnt-responsive genes. The Wnt pathway can also modulate cytoskeletal dynamics. In the absence of Wnt signalling, Gsk3β phosphorylates the microtubule-associated protein 1b (Map1b), thereby destabilizing microtubule bundles. Wnt signalling inhibits Gsk3β to maintain a stable microtubular network. Ub, ubiquitination.
In the absence of Wnt signalling, cytoplasmic β-catenin, or its Drosophila homologue Armadillo (Arm), is phosphorylated by casein kinase 1 and glycogen synthase kinase 3β (Gsk3β; Zeste-White/ Shaggy in Drosophila). β-Catenin is then ubiquitinated and subsequently degraded in the proteasome40,41. This process requires the formation of a complex that includes the scaffolding protein Axin and the tumour suppressor protein Apc (adenomatous polyposis coli)42.
By contrast, activation of the Wnt pathway leads to phosphorylation of Dishevelled (Dsh), which prevents β-catenin degradation43. Stabilized β-catenin is required for Lef/Tcf transcription factors to initiate transcription of Wnt-responsive genes. However, surprising new data have challenged the consensus view that the stabilized Arm protein enters the nucleus to form a transcription factor complex with Lef/Tcf. Instead, activation of Wg target genes might be required for either translocation or accumulation of Arm outside the nucleus44.
Wnt pathway activation also modulates microtubule dynamics and levels of cell adhesion.Apc, for example, stabilizes microtubules in vivo and in vitro, and might have a role in cell migration45,46. Wnt7a signalling has been proposed to affect the stability of axonal microtubules during neuronal development by causing a decrease in the level of phosphorylated microtubule-associated protein 1b (Map1b)47. Also, microarray analysis indicates that the gene for NrCAM (neuronal cell adhesion molecule) is one of the genes that is most extensively induced by β- or γ-catenins in a carcinoma cell line48.
TGFβ and related factors regulate gene expression by bringing together two types of receptor serine/threonine kinases — type I and type II receptors (FIG.1)49.Receptors I and II dimerize by TGFβ co-binding, resulting in phosphorylation and activation of the intracellular kinase domain of the type I receptor. Downstream targets for type I kinases are the so-called receptor regulated Smads — R-Smad; sma genes in Caenorhabditis elegans and mad (mothers against dpp) genes in Drosophila50,51. Once phosphorylated, R-Smads associate with co-Smads (for example, Smad4), and translocate into the nucleus, where they activate transcription49.A scan of the completed Drosophila genome sequence reveals the presence of seven TGFβ-type ligands, three type I receptors — Thickveins (Tkv), Saxaphone (Sax) and Baboon (Babo) — two type II receptors — Punt (Put) and Wishful thinking (Wit) — two R-Smads (Mads), one common Smad — Medea (Med) — and one inhibitory Smad — Daughters against Dpp (Dad)52.In addition to their roles during embryonic development, TGFβ superfamily members, which include the bone morphogenetic proteins (BMPs), and their cognate receptors, have been shown to be widely expressed in developing and mature vertebrate nervous systems, and they have a variety of roles in nervous system development53–55.
Wnts induce synaptic differentiation
Recent studies in the mammalian nervous system have shown for the first time that members of the Wnt family might work as signalling factors that induce presynaptic differentiation in the CNS18,19.
In the developing cerebellum, multi-synaptic glomerular rosettes are formed as mossy fibre growth cones establish synaptic interactions with postsynaptic granule cell neurons (GCs). Hall et al. showed that Wnt7a was expressed by GCs as they were contacted by mossy fibres, and that Wnt7a induced axonal remodelling, which was crucial for the formation of glomerular rosettes18. Moreover, in cultures of mossy fibres, Wnt7a induced synapsin I clustering in a Gsk3β-dependent manner. By contrast, wnt7a mutants displayed a delayed accumulation of synapsin I staining. Taken together, these results indicated that Wnt7a influences the development of presynaptic mossy fibres in vivo.Wnt7a was also found to enhance growth cone complexity and to promote microtubule dynamics in culture, and these effects were antagonized by a soluble Wnt inhibitor, secreted Frizzled-related protein (sFrp1). Most remarkably, wnt7a mutant mice displayed a delayed maturation of glomerular rosettes, but this effect was only transient, presumably due to compensation by other Wnt family proteins. These studies provided the first indication that Wnt7a might serve as a retrograde secreted signal that promotes the maturation of the presynaptic cell.
The above observations have recently been extended to another member of the Wnt family, Wnt3 (REF. 19). In the spinal cord, motor neurons that are contacted by neurotrophin-3-sensitive sensory neurons express Wnt3 during synaptogenesis. Furthermore, in spinal cord explants, Wnt3 promoted axonal branching and increased growth cone size, and also promoted synapsin I clustering at synaptic sites. These effects were blocked by sFrp1, and could be mimicked using lithium to inhibit Gsk3β. So, Wnt3, like Wnt7a, also functions as a retrograde signalling factor that controls presynaptic differentiation.
A crucial role for members of the Wnt family in synapse differentiation has recently been demonstrated in Drosophila glutamatergic NMJs20. Muscle fibres of the Drosophila larval body wall are stereotypically innervated by motor neurons that contain a characteristic complement of neurotransmitters, including glutamate, peptides and biogenic amines56. Throughout larval development, wild type motor neuron synapses are challenged by continuously growing target muscles. To maintain synaptic efficacy, the presynaptic arbor continually increases the number and size of synaptic boutons and the overall number of active zones. This process of new synapse formation during the postembryonic period has been referred to as presynaptic expansion or target-dependent new synapse formation, to distinguish it from embryonic synaptogenesis20,56.
A study by Packard et al.20 has shown that at the NMJ, Wg is expressed by the presynaptic motor neurons, and it is secreted by the motor neuron terminals onto the muscle surface (FIG. 2). It interacts with Drosophila Frizzled 2 (DFz2), which is enriched around synaptic boutons. Normally, Wg seems to be endocytosed by the postsynaptic muscles, as blocking endocytosis by genetic manipulation of the Drosophila DYNAMIN gene shibire (shi) led to postsynaptic accumulation of Wg.
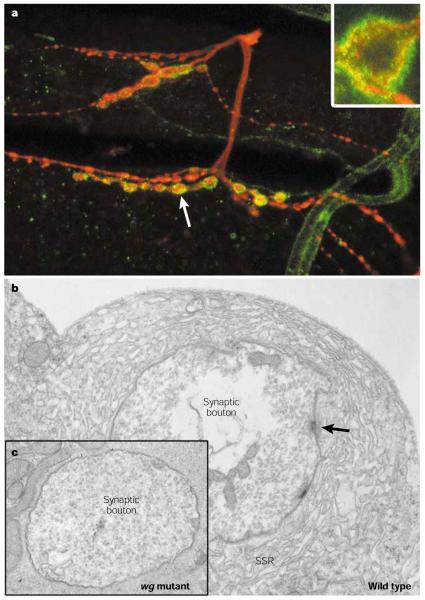
Figure 2. Wingless signalling is essential for the proper formation of pre- and postsynaptic specializations at the fly neuromuscular junction.
a | At the wild type neuromuscular junction (NMJ), Wingless (Wg; green) is secreted by, and localizes to, synaptic boutons (red). Inset: A single bouton at higher magnification, showing that Wg immunostaining (green) is localized both pre- and postsynaptically. Anti-horseradish peroxidase (HRP; red) is the neuronal marker. Arrow points to a synaptic bouton. b and c | At the ultrastructural level, the pre- and postsynaptic specializations seen in the wild type synapse (b) such as the active zone T-bars (arrow) — electron dense structures at Drosophila NMJs that are associated with presynaptic densities, and around which synaptic vesicles cluster — and subsynaptic reticulum (SSR), are completely lacking in the wg mutant (c).
To show that Wg could be secreted by synaptic terminals, we used tissue-specific enhancer sequences (through the use of the GAL4/UAS SYSTEM57) to express Wg selectively in either motor neurons or muscles. Motor neuron Wg expression resulted in accumulation of postsynaptic Wg protein, whereas muscle Wg expression did not. In addition, expression of green fluorescent protein (GFP)-tagged Wg using the Wg promoter (which drives expression in neurons but not muscle) resulted in a GFP signal at postsynaptic sites. The idea that Wg is secreted by synaptic terminals, and that it serves as an anterograde signal, was supported by rescue experiments showing that mutant phenotypes elicited by blocking Wg secretion (see below) are rescued by presynaptic, but not by postsynaptic transgenic Wg expression.
By using a temperature sensitive wg mutation, we were able to ‘freeze’ synapse differentiation. In particular, blocking Wg secretion severely reduced target-dependent formation of new synapses in the larva (FIG. 3). Moreover, the micro-architecture of the localization of proteins such as GluR and Discs large (Dlg), a scaffolding protein of the PSD95 family, was disrupted in the mutants. GluRs at the NMJ are clustered in ‘hot spots’ around synaptic boutons, directly apposed to active zones2. In wg mutants, GluRs were usually still localized to the periphery of synaptic boutons, but their organization into hot spots was lost (FIG. 3e). Similarly, aberrant distribution of Dlg, which is required for the synaptic aggregation of two synaptic proteins, SHAKER K+ CHANNELS58 and the cell adhesion molecule Fasciclin 2 (Fas2)59, was observed in the mutants. So, although Wg is required in the presynaptic cell for secretion, it signals the differentiation of both pre- and postsynaptic surfaces.
Figure 3. Events during new synapse formation at the Drosophila neuromuscular junction, and the role of Wnt and TGFβ pathway in these processes.
a, b | During Drosophila neuromuscular (NMJ) development, precise apposition of the pre- and postsynaptic molecular components is required for bouton maturation and subsequent expansion. New boutons bud out from existing mature boutons to adjust for an increase in target muscle size. c, d | New buds expand and subsequently mature in tune with a synchronous folding of the postsynaptic membrane into the subsynaptic reticulum (SSR). Recent observations at the Drosophila NMJ demonstrate crucial roles for the Wnt and transforming growth factor-β (TGFβ) signal transduction pathways in these processes20–22. e | In the absence of Wingless (Wg) function, fewer synapses form, and those that do form fail to develop pre- and postsynaptic densities, T-bars and postsynaptic SSR. The presynaptic cytoskeletal network appears to be destabilized, and postsynaptic glutamate receptors (GluR) are not correctly localized. f | wit mutant synapses have normal SSR and apparently normal GluR localization. However, they have presynaptic defects such as an increase in the number of T-bars per presynaptic density, abnormal apposition of pre- and postsynaptic densities, and an abnormal appearance of presynaptic T-bars. These studies indicate a presynaptic role for the TGFβ type II receptor, Wit, which functions as a part of a retrograde signalling mechanism to adjust the size of the presynaptic arborization to the postsynaptic muscle size. By contrast, Wg is secreted by presynaptic boutons, and seems to function in an anterograde fashion to control the correct positioning of active zones and postsynaptic SSR, as well as bouton number. Together, they coordinate synapse development by regulating bouton budding, cytoskeletal stability, number of functional active zones, folding of the postsynaptic muscle membrane, and levels of pre- and postsynaptic molecules, including GluRs and cell adhesion molecules. DFz2, Drosophila Frizzled 2; Spin, Spinster.
In mammals, the Wnt pathway has been implicated in the reorganization of the cytoskeleton during axonal growth and growth-cone extension by inhibiting the kinase Gsk3β (REFS 18,47). At the fly NMJ, Gsk3β was found to be enriched in presynaptic arborizations, and examination of the NMJ revealed that a higher proportion of boutons in the mutants contained unbundled microtubule filaments (FIG. 3e).This result indicated that although wg mutants displayed arrested NMJ expansion, the cytoskeleton remained in the type of destabilized state that has been correlated with periods of outgrowth60.
Remarkably, at the ultrastructural level, boutons that developed in the absence of Wg function completely lacked presynaptic densities (FIG. 2b,c). These boutons were also devoid of mitochondria, but they contained large numbers of synaptic vesicles. Postsynaptic specializations, in particular the subsynaptic reticulum (SSR), were also missing. In boutons that developed under partial Wg function, presynaptic densities and SSR developed, but their morphology was markedly abnormal. These studies indicate that in the fly, Wg is required for synapse maturation as well as for the coordinated development of pre- and postsynaptic structures. In contrast to vertebrates, however, the evidence supports a role for Wg as an anterograde signalling factor. Whether this difference represents an evolutionary change in signalling direction, or whether different Wnts in vertebrate and invertebrate species might have specific directionality in their signalling, will need to be resolved by a systematic study of members of this family.
TGFβs in synapse development
Recent studies also indicate that the TGFβ signal transduction pathway forms part of a feedback mechanism during synapse formation at the Drosophila NMJ. In these studies, REVERSE and FORWARD GENETIC approaches were used to show that mutations of the BMP type II receptor gene wit also inhibited target-dependent synapse formation in the larva21,22. This defect could be rescued by presynaptic expression of a wit transgene, implying a presynaptic requirement for this BMP type II receptor.
Consistent with their smaller bouton numbers, wit mutants show defects in neurotransmitter release at the NMJ. In addition, the synaptic levels of Fas2 were decreased in the mutants. At the ultrastructural level, wit mutants, like the wg mutants, contained aberrant pre- and postsynaptic membrane appositions and defective active zones, although unlike in the wg mutants, active zones were still present (FIG. 3f). So, wg and wit mutants share a number of abnormal phenotypes, including their decreased ability to signal new synaptic bouton formation and proper development of active zones. However, the postsynaptic SSR is normal in wit mutants, in striking contrast to wg mutants. The ability of wit mutants to cluster endogenous GluRs has not been thoroughly assayed — although the clustering of an epitope-tagged transgene seemed normal — so a comprehensive analysis of the distribution of pre- and postsynaptic proteins will be required to fully assess the extent to which both genes are required to organize similar subsets of synaptic proteins.
The function of Wit, as well as of other members of the TGFβ family that are involved in synapse formation — in particular, Tkv and Sax — seems to be regulated by
Spinster (Spin), a transmembrane protein that is associated with late endosomal compartments23. Mutations in spin result in over-elaboration of synaptic terminals, but these effects are antagonized by mutations in wit, tkv, or sax. Moreover, enhancement of TGFβ signalling using mutations in an inhibitory Smad (dad)61,62 was sufficient to phenocopy the spin phenotype with respect to bouton number. Although the corresponding BMP ligand has not yet been identified, these studies indicate that Wit acts as a receptor for a retrograde signal that is required to coordinate the changes in the muscle with synaptic bouton formation. As discussed above, in the developing fly, Wnts and BMPs form orthogonal gradients that define the shape of the wing31. An interesting speculation is that, similar to embryo and imaginal tissue development, Wg and Dpp (or other TGFβ members) signal in opposite directions at synapses to specify the correct location of pre- and postsynaptic structures.
Evidence for an involvement of TGFβ family members in synapse development and plasticity has also been provided by studies of the marine mollusc Aplysia63,64. During long-term sensitization of the gilland siphon-withdrawal reflex in this organism, LONG-TERM FACILITATION (LTF) results in the formation of new synaptic connections. When similar sensory and motor neuron connections are reconstituted in culture, LTF and the corresponding increase in synaptic contacts can be mimicked by repeated puffs of serotonin. New synapse formation that is induced by LTF is associated with a downregulation of the cell adhesion molecule apCAM, and activation of a cyclic AMP cascade that rapidly affects synaptic current and later influences gene expression65–67. Interestingly, levels of a TGFβ mRNA, Tbl1, increase in sensory neurons after induction of LTF68, and application of a recombinant human TGFβ homologue, TGFβ1, strengthens the sensory–motor neuron synaptic connections, whereas a TGFβ receptor antagonist blocks this effect64.Recent experiments show that, like serotonin, TGFβ1 stimulates acute changes, such as phosphorylation and subsequent redistribution of synapsin69.
Conclusions and future directions
The studies reviewed here reveal an important and previously uncharacterized role for well-known early developmental genes in synapse differentiation and plasticity.Although synapse dynamics have been extensively studied, some of the key signals that initiate and control them have remained elusive. It was not initially intuitive that pattern-forming molecules such as Wnts and TGFβ could have such a central role at the synapse. However, their identification at the synapse and the subsequent cell biological and genetic analysis of their function opens up new avenues by which one could understand synaptic cross-talk.
The mechanisms by which Wnt and TGFβ control the transmission of messages across the synapse are not yet fully understood, but potentially they could function by altering the number or localization of receptors, or by regulating receptor-ligand complex turnover. Multiple modes of inhibiting the classical Wnt and TGFβ signalling pathways have been demonstrated in a variety of cell types, and it remains to be seen what role these feedback mechanisms might have in synapse development and function. Further analysis of the transduction pathways that are initiated by Wnts and TGFβ-like ligands could shed light on these issues. Particularly intriguing, for example, is the question of whether some of the signalling mechanisms that are mediated by Wnts and TGFβ at synapses involve the regulation of gene expression — as they do in early pattern formation. These emerging roles for Wnt and TGFβ will usher in a new level of investigation into the molecular mechanisms of synaptic plasticity.
Acknowledgements
We would like to thank M. Gorczyca and C. Ruiz-Canada, and to D. Gorczyca for careful reading of the manuscript and helpful discussions.
Glossary
- ACTIVE ZONE
A portion of the presynaptic membrane that faces the postsynaptic density across the synaptic cleft. It constitutes the site of synaptic vesicle clustering, docking and transmitter release.
- HEPARAN SULPHATE
A glycosaminoglycan that consists of repeated units of hexuronic acid and glucosamine residues. It usually attaches to proteins through a xylose residue to form proteoglycans.
- ANTISENSE
A single-stranded RNA molecule whose sequence is complementary to that of the mRNA for a given gene. It can bind to the mRNA, thereby preventing it from being translated.
- EPHRINS
A families of molecules that, by interacting with their Eph receptors, mediate cell-contact-dependent signalling, and are primarily involved in the generation and maintenance of patterns of cellular organization. They accomplish this goal by the control of repulsion at a boundary or gradient, or by upregulating cell adhesion.
- TRANSCYTOSIS
Transport of macromolecules across a cell, consisting of endocytosis of a macromolecule at one side of a monolayer and exocytosis at the other side.
- IMAGINAL DISC
Single-cell layer epithelial structures of the Drosophila larva that give rise to wings, legs and other appendages.
- DYNAMIN
A GTPase that takes part in endocytosis. It seems to be involved in severing the connection between the nascent vesicle and the donor membrane.
- GAL4/UAS SYSTEM
A genetic system for controlling the induction of gene expression.An activator line that expresses the yeast transcriptional activator GAL4 gene under the control of the heat-shock 70 promoter (hsp70) or a tissue-specific promoter is crossed to an effector line that carries the DNA-binding motif of Gal4 (UAS) fused to the gene of interest.As a result, the progeny of this cross expresses the gene of interest in an activator-specific manner.
- SHAKER K+ CHANNEL
A voltage-gated channel, the activation of which leads to the appearance of a transient K+ current. It takes its name from Drosophila with mutations in the gene that encodes this protein. These flies display a violent shaking phenotype when under anaesthesia.
- REVERSE GENETICS
Genetic analysis that proceeds from genotype to phenotype through gene-manipulation techniques.
- FORWARD GENETICS
A genetic analysis that proceeds from phenotype to genotype by positional cloning or candidategene analysis.
- LONG-TERM FACILITATION (LTF)
A lasting increase in the strength of synapses between sensory and motor neurons in Aplysia. LTF is the cellular mechanism that underlies non-associative learning and memory. LTF results largely from presynaptic changes. It is similar to the LTP of the hippocampal mossy fibre pathway, but differs from LTP in other regions of the hippocampus, which are associative.
Footnotes
DATABASES The following terms in this article are linked online to: Entrez: http://www.ncbi.nlm.nih.gov/Entrez/Tbl1
FlyBase: http://flybase.bio.indiana.edu/
Arm ∣ Arr ∣ Babo ∣ Dad ∣ DFz2 ∣ Dlg ∣ Dpp ∣ Dsh ∣ Fas2 ∣ mad ∣ Med ∣ Put ∣ Sax ∣ Shaggy ∣ shi ∣ Spin ∣ Tkv ∣ Wg ∣ Wit
LocusLink: http://www.ncbi.nlm.nih.gov/LocusLink/
Agrin ∣ β-catenin ∣ γ-catenin ∣ Fz1 ∣ Fz2 ∣ Go ∣ Gq ∣ Gsk3β ∣ Lef ∣ LRP ∣ Map1b ∣ NrCAM ∣ sFrp1 ∣ sma ∣ Smad4 ∣ synapsin I ∣ Tcf ∣ TGFβ ∣ Wnt
Swiss-Prot: http://www.expasy.ch/
Apc ∣ Axin ∣ Fz
FURTHER INFORMATION
Encyclopedia of Life Sciences: http://www.els.net/ bone morphogenetic proteins and their receptors ∣ developmental biology and synapse formation ∣ G protein-coupled receptors ∣ G proteins ∣ serotonin ∣ transforming growth factor β
References
- 1.Poo MM. Neurotrophins as synaptic modulators. Nature Rev. Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 2.Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. An important early account of the involvement of postsynaptic neurotransmitter receptors in retrograde signalling at Drosophila synapses. This article established that presynaptic transmitter release could be affected by regulating the size of postsynaptic responses.
- 3.Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- 4.Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 5.Tao HW, Poo M. Retrograde signaling at central synapses. Proc. Natl Acad. Sci. USA. 2001;98:11009–11015. doi: 10.1073/pnas.191351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Rev. Neurosci. 2001;2:791–805. doi: 10.1038/35097557. An excellent review on the initial signals involved in the reorganization of the postsynaptic apparatus at vertebrate cholinergic synapses.
- 7.Gautam M, et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 8.Lin W, et al. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- 9.Yang X, et al. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 10.Pun S, et al. An intrinsic distinction in neuromuscular junction assembly and maintenance in different skeletal muscles. Neuron. 2002;34:357–370. doi: 10.1016/s0896-6273(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith MA, Hilgenberg LG. Agrin in the CNS: a protein in search of a function? Neuroreport. 2002;13:1485–1495. doi: 10.1097/00001756-200208270-00001. [DOI] [PubMed] [Google Scholar]
- 12.Serpinskaya AS, Feng G, Sanes JR, Craig AM. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev. Biol. 1999;205:65–78. doi: 10.1006/dbio.1998.9112. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Hilgenberg LG, O’Dowd DK, Smith MA. Formation of functional synaptic connections between cultured cortical neurons from agrin-deficient mice. J. Neurobiol. 1999;39:547–557. doi: 10.1002/(sici)1097-4695(19990615)39:4<547::aid-neu8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira A. Abnormal synapse formation in agrin-depleted hippocampal neurons. J. Cell Sci. 1999;112:4729–4738. doi: 10.1242/jcs.112.24.4729. [DOI] [PubMed] [Google Scholar]
- 15.Bose CM, et al. Agrin controls synaptic differentiation in hippocampal neurons. J. Neurosci. 2000;20:9086–9095. doi: 10.1523/JNEUROSCI.20-24-09086.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingras J, Rassadi S, Cooper E, Ferns M. Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol. 2002;158:1109–1118. doi: 10.1083/jcb.200203012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalva MB, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 18.Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. This is the first demonstration that a Wnt family member can act as a retrograde synaptogenic signal at mammalian central synapses.
- 19.Krylova O, et al. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. This study demonstrates for the first time that different Wnt family members exhibit tissue specificity in the mammalian central nervous system. Wnt3, but not Wnt7 or other Wnts, was shown to serve as a retrograde signal during formation of specific sensory-motor neuron connections in the mouse spinal cord.
- 20.Packard M, et al. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. Using the glutamatergic Drosophila NMJ system, this study provides important in vivo evidence for the involvement of Wnt signalling in an anterograde manner during synapse formation and differentiation.
- 21.Marques G, et al. The Drosophila BMP type II receptor wishful thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. This study identifies Wit as a BMP type II receptor, and suggests a role for Wit in Drosophila NMJ assembly and function. The authors also report convincing evidence that Wit is required presynaptically, implying that its ligand is probably secreted by the postsynaptic muscle surface.
- 22.Aberle H, et al. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. This study identifies wit as a gene that positively regulates synaptic growth. The study provides crucial genetic evidence for the involvement of TGFβ signalling at this glutamatergic synapse.
- 23.Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster — a late endosomal protein implicated in TGF-β-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 24.Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer S, Alexandre C, Calleja M, Vincent JP. The progeny of wingless-expressing cells deliver the signal at a distance in Drosophila embryos. Curr. Biol. 2000;10:321–324. doi: 10.1016/s0960-9822(00)00381-x. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer S, Ricardo S, Manneville JB, Alexandre C, Vincent JP. Producing cells retain and recycle Wingless in Drosophila embryos. Curr. Biol. 2002;12:957–962. doi: 10.1016/s0960-9822(02)00867-9. [DOI] [PubMed] [Google Scholar]
- 27.Moline MM, Southern C, Bejsovec A. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo. Development. 1999;126:4375–4384. doi: 10.1242/dev.126.19.4375.. This study demonstrates the importance of endocytosis in regulating a normal distribution of Wgacross embryonic epithelia. The authors achieved this by generating a transgene that expressed a dominant negative form of the Drosophila dynamin shibire.
- 28.Dierick H, Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr. Top. Dev. Biol. 1999;43:153–190. doi: 10.1016/s0070-2153(08)60381-6. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence PA. Wingless signalling: more about the Wingless morphogen. Curr. Biol. 2001;11:R638–639. doi: 10.1016/s0960-9822(01)00380-3. [DOI] [PubMed] [Google Scholar]
- 30.Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- 31.Day SJ, Lawrence PA. Measuring dimensions: the regulation of size and shape. Development. 2001;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 32.Massague J. How cells read TGF-β signals. Nature Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 33.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 34.Theisen H, Haerry TE, O’Connor MB, Marsh JL. Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development. 1996;122:3939–3948. doi: 10.1242/dev.122.12.3939. [DOI] [PubMed] [Google Scholar]
- 35.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 36.Katoh M. WNT3-WNT14B and WNT3A-WNT14 gene clusters (Review) Int. J. Mol. Med. 2002;9:579–584. [PubMed] [Google Scholar]
- 37.Liu T, et al. G protein signaling from activated rat frizzled-1 to the β-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 38.Wehrli M, et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 39.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 40.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J. Biol. Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 42.Kitagawa M, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun TQ, et al. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nature Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 44.Chan SK, Struhl G. Evidence that Armadillo transduces Wingless by mediating nuclear export or cytosolic activation of Pangolin. Cell. 2002;111:265–280. doi: 10.1016/s0092-8674(02)01037-1. [DOI] [PubMed] [Google Scholar]
- 45.Nathke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zumbrunn J, Kinoshita K, Hyman AA, Nathke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 β phosphorylation. Curr. Biol. 2001;11:44–49. doi: 10.1016/s0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 47.Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC. Inhibition of GSK-3β leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 48.Conacci-Sorrell ME, et al. Nr-CAM is a target gene of the β-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002;16:2058–2072. doi: 10.1101/gad.227502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massague J. TGF-β signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 50.Raftery LA, Twombly V, Wharton K, Gelbart WM. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics. 1995;139:241–254. doi: 10.1093/genetics/139.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savage C, et al. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor β pathway components. Proc. Natl Acad. Sci. USA. 1996;93:790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newfeld SJ, Wisotzkey RG, Kumar S. Molecular evolution of a developmental pathway: phylogenetic analyses of transforming growth factor-β family ligands, receptors and Smad signal transducers. Genetics. 1999;152:783–795. doi: 10.1093/genetics/152.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorentzon M, Hoffer B, Ebendal T, Olson L, Tomac A. Habrec1, a novel serine/threonine kinase TGF-β type I-like receptor, has a specific cellular expression suggesting function in the developing organism and adult brain. Exp. Neurol. 1996;142:351–360. doi: 10.1006/exnr.1996.0204. [DOI] [PubMed] [Google Scholar]
- 54.Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- 55.Withers GS, Higgins D, Charette M, Banker G. Bone morphogenetic protein-7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. Eur. J. Neurosci. 2000;12:106–116. doi: 10.1046/j.1460-9568.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 56.Koh YH, Gramates LS, Budnik V. Drosophila larval neuromuscular junction: molecular components and mechanisms underlying synaptic plasticity. Microsc. Res. Tech. 2000;49:14–25. doi: 10.1002/(SICI)1097-0029(20000401)49:1<14::AID-JEMT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 57.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 58.Tejedor FJ, et al. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J. Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas U, et al. Synaptic clustering of the cell adhesion molecule Fasciclin II by Discs-Large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 61.Tsuneizumi K, et al. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature. 1997;389:627–631. doi: 10.1038/39362. [DOI] [PubMed] [Google Scholar]
- 62.Inoue H, et al. Interplay of signal mediators of Decapentaplegic (Dpp): molecular characterization of Mothers against dpp, Medea, and Daughters against dpp. Mol. Biol. Cell. 1998;9:2145–2156. doi: 10.1091/mbc.9.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schuman EM. Synapse specificity and long-term information storage. Neuron. 1997;18:339–342. doi: 10.1016/s0896-6273(00)81234-9. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-β in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]
- 65.Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- 66.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu. Rev. Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 67.Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in aplysia. J. Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu QR, et al. A developmental gene (Tolloid/BMP-1) is regulated in Aplysia neurons by treatments that induce long-term sensitization. J. Neurosci. 1997;17:755–764. doi: 10.1523/JNEUROSCI.17-02-00755.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH. Transforming growth factor β1 alters synapsin distribution and modulates synaptic depression in Aplysia. J. Neurosci. 2002;22:RC220. doi: 10.1523/JNEUROSCI.22-09-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]