Abstract
Objective
Low back pain is associated with lumbar disc degeneration, which is mainly due to genetic predisposition. The objective of this study was to perform a systematic review to evaluate genetic association studies in lumbar disc degeneration as defined on magnetic resonance imaging (MRI) in humans.
Methods
A systematic literature search was conducted in MEDLINE, MEDLINE In-Process, SCOPUS, ISI Web of Science, The Genetic Association Database and The Human Genome Epidemiology Network for information published between 1990–2011 addressing genes and lumbar disc degeneration. Two investigators independently identified studies to determine inclusion, after which they performed data extraction and analysis. The level of cumulative genetic association evidence was analyzed according to The HuGENet Working Group guidelines.
Results
Fifty-two studies were included for review. Forty-eight studies reported at least one positive association between a genetic marker and lumbar disc degeneration. The phenotype definition of lumbar disc degeneration was highly variable between the studies and replications were inconsistent. Most of the associations presented with a weak level of evidence. The level of evidence was moderate for ASPN (D-repeat), COL11A1 (rs1676486), GDF5 (rs143383), SKT (rs16924573), THBS2 (rs9406328) and MMP9 (rs17576).
Conclusions
Based on this first extensive systematic review on the topic, the credibility of reported genetic associations is mostly weak. Clear definition of lumbar disc degeneration phenotypes and large population-based cohorts are needed. An international consortium is needed to standardize genetic association studies in relation to disc degeneration.
Introduction
Low back pain (LBP) is one of the world’s most debilitating conditions, presenting with substantial socio-economic and health-care consequences [1], [2]. LBP can lead to reduced physical activity, lost wages, diminished quality of life, and psychological distress [3]–[6]. Although LBP has numerous determinants, disc degeneration of the lumbar spine is a clear contributing factor [7]–[13].
Disc degeneration is characterized as morphological and biochemical changes of the disc. Magnetic resonance imaging (MRI) is the current gold standard to assess the integrity of the intervertebral disc [14]. Degenerative changes on imaging are generally based on decreased signal intensity (representing loss of hydration), reduced disc height, presence of fissures in the outer layer of the disc or dislocation of disc material outside its normal position [15]–[18]. Disc degeneration is multifaceted, traditionally attributed to age, mechanical loading, gender, trauma, obesity and other factors impairing disc nutrition [11], [19]–[25]. However, since the end of the 20th Century, numerous studies have suggested that heredity is largely responsible for the development of lumbar disc degeneration and that environmental factors play a much smaller role than previously believed [26]–[28]. This has led to the well-justified search for specific genetic risk factors [29]. However, similar to other complex diseases, the genetic associations found in disc degeneration have proven difficult to validate [30]. Only one limited attempt has been made to systematically analyze these studies [31].
The current review is the first systematic assessment focusing specifically on genetic association studies in disc degeneration while including the evaluation of association credibility, which is unique in this field. Published information of genetic factors is growing rapidly and it needs to be approached systematically to identify valid and replicable gene-disease associations [30], [32]. Particularly in disc degeneration, in addition to summing up and critically scrutinizing the existing data, such effort is needed for planning future collaborative studies. As such, the primary objectives of this study were to perform the first systematic analysis of genetic association studies on lumbar disc degeneration, evaluate the quality of the methods used in the studies, and assess the level of evidence [33], [34] in each association. Secondarily, the objectives were to provide a basis on which the field could expand towards more robust evidence and to assess the clinical relevance of the current information. This review succeeded in reaching these objectives.
Methods
Data Sources and Searches
A systematic search was conducted in MEDLINE, MEDLINE In-Process, ISI Web Of Science and SCOPUS from 1990 through to August 2011. On-line association databases, the Genetic Association Database and the Human Genome Epidemiology Network were consulted after a search for any missing studies. The SCI-EXPANDED of ISI Web Of Science was searched from 1990 through to August 2011. Utilizing Boolean operators, different forms (truncation) of the keywords allele, polymorphism and genotype in either title or topic were combined with the words disc, disk, endplate, lumbar, Modic, spondyl(o)arthrosis similarly in either title or topic, and terms noting macular, retinal and ocular were used to exclude conditions not related to the intervertebral disc. A search in SCOPUS was performed in a parallel way using formulations of the words allele, polymorphism and genotype in either title, abstract or article keyword. This search was then combined with the words disc, disk, endplate, lumbar, Modic and spondyl(o)arthrosis similarly in either title, abstract or article keyword with AND Boolean. The NOT operator was used with words macular, retinal, ocular and optic. The MEDLINE and MEDLINE In-Process were searched using the MeSH terms intervertebral disk degeneration and intervertebral disk displacement prior to combining the word endplate and formulations of spondyloarthrosis to the search in all fields -manner. Different formulations of the words allele, polymorphism and genotype were then combined with AND Boolean. Finally the results were limited to humans. The Genetic Association Database (http://geneticassociationdb.nih.gov/) was searched using the words disc and disk in all fields-manner. The Human Genome Epidemiology Network (HuGENet™) was also searched via the HuGE Literature Finder (http://hugenavigator.net/) using the words disc and disk. Reference tracking of included studies was performed after retrieving the full text articles. The citations were handled using the RefWorks–software (ProQuest LLC, both Classic and 2.0 versions were used).
Study Selection
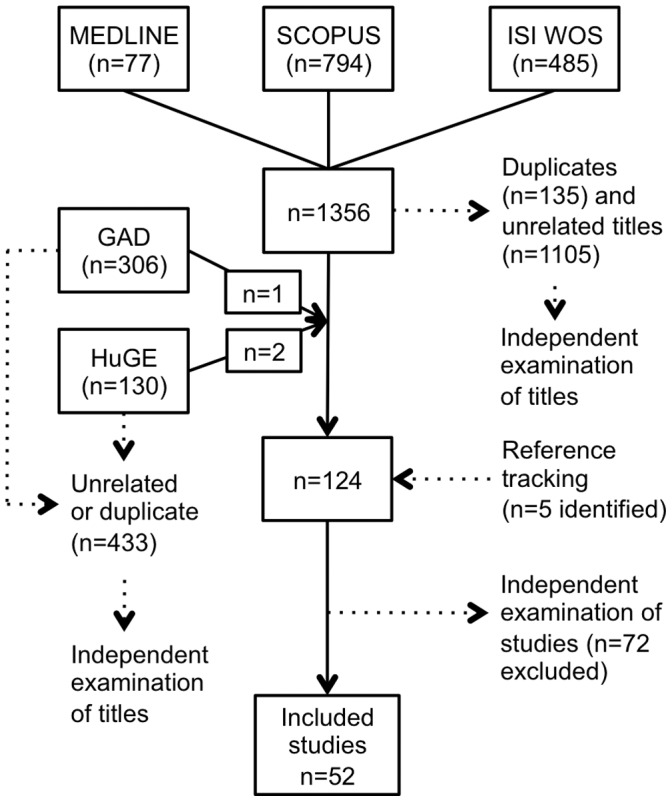
The criteria for considering studies for this review were formalized in an inclusion criteria form (Appendix S1), which was piloted to minimize human error. Two investigators (PE and SL) independently examined the titles and abstracts of the identified studies. If study eligibility was unclear from the abstract, the full text of the article was retrieved and independently assessed by the assessors (Figure 1). Any disagreement was resolved by discussion. Eligible studies included in this review had the following criteria: relevant outcome or disease (intervertebral disc changes, vertebral endplate changes, spondyl(o)arthrosis), reliable definition of outcome (MRI), study subjects not less than fifty, human subjects, and description of specific genetic variant(s). Studies that did not meet one or more of the eligibility criteria were excluded. The studies were not limited to any language.
Figure 1. Study flow diagram.
Data Extraction
Two investigators (PE and SL) independently extracted the data using a standardized form (Appendix S1). The form was pilot-tested on three studies to identify and reduce any potential for misinterpretation (PE, SL and PK). The following topics were recorded from the included studies: study details and sponsorship, population structure, phenotypes and details of the MRI, genotyping details as well as possible biases in selection, performance, detection, attrition and statistical analyses.
Quality Assessment and Data Synthesis
We developed an instrument for methodological quality assessment (Appendix S1). The study quality was based on the information reported in the articles and was simultaneously analyzed with the data extraction phase by two investigators (PE and SL) independently. However, during the data analysis phase, it was noted that the formalized summary score did not fully serve all the needs of the current review [35]. This discrepancy was resolved through discussion (PE and SL), after which the synthesized study quality assessment was noted when estimating protection from bias at the level of evidence analyses. The level of evidence in each genetic variation was analyzed according to the Venice interim guidelines by The HuGENet Working Group [33]. These current guidelines suggest that the level of evidence for genetic association should be assessed at three main levels: amount of evidence, replication and protection from bias. The amount of evidence was graded strong (A) in the case of >1000, moderate (B) in the case of 100–1000 and weak (C) in the case of <100 individuals evaluated in the smallest genetic group of interest. The level of replication was graded strong (A): extensive replication including at least one well-conducted meta-analysis with little between-study inconsistency; moderate (B): well-conducted meta-analysis with some methodological limitations or moderate between-study inconsistency; and weak (C): no association, no independent replication, failed replication, scattered studies, flawed meta-analysis or large inconsistency. Similar tripartite grading was used to analyze the protection from bias: strong (A): bias, if at all present, could affect the magnitude but probably not the presence of the association; moderate (B): no obvious bias that may affect the presence of the association but there is considerable missing information on the generation of evidence; and weak (C): considerable potential for, or demonstrable, bias that can affect even the presence or absence of the association [33], [34]. For a positive replication, both the same phenotype and the same genetic variation were required. The possible additional biological evidence reported in the studies was also acknowledged [33]. Credibility of cumulative epidemiological evidence was recorded for each variation as described in the guidelines (Appendix S1). Two investigators (PE and SL) confirmed the credibility assessments, which were then further reviewed by the other investigators. We examined the studies and extracted data with close scrutiny in order to identify possible multiple association reports from a single study or clear double publications. Multiple association reports from a single study or population were included if they reported on different genetic variations or different disc degeneration phenotypes than those contained in the first report. Multiple association reports were not allowed to inflate the level of association evidence.
Results
The systematic search resulted in 1,356 citations (Figure 1). Duplications and clearly unrelated titles (N = 1240) were removed and the full-text articles of the remaining titles were obtained. Reference tracking, independent evaluation and reviewer discussions found 52 studies eligible for inclusion (Table S1). The list of studies that were excluded after evaluation (N = 72) can be found on-line (Appendix S1). One double publication was identified [36], [37], and the more recent report was excluded.
Methods and Phenotypes in Studies
The genetic association studies identified in this review were published from the year 1998 onwards. All 52 studies included in this review used a candidate gene approach. The number of studied polymorphisms in each study varied between one and 163 [38], [39]. The accuracy of the study methodology and reporting improved from the early to the more recent studies. However, many items still related to error and bias were not consistently reported. Genotyping methodology was generally found to be suitable for each study performed, although methods to validate genotyping, as well as blinding of genotyping towards phenotype or vice versa, were rarely reported. In many cases, the phenotype of disc degeneration varied between the initial and replication studies (Table S1, Table 1) [36], [38]–[84]. The phenotype of disc herniation characterized by sciatica showed the most convincing evidence for association as it was the phenotype in 80% of the studies with moderate evidence of association in the current review (Table 2). The other phenotypes used in the original studies included decrease in disc signal intensity or disc height, disc bulges, disc herniations without specification of symptoms, Modic changes, osteophytes and lumbar spinal stenosis. Different modifications and combinations of these were also used. Despite several studies investigating the same genetic variation, meta-analysis was not feasible due to the clinical and overall heterogeneity of the studies. Additional biological evidence was reported in six studies [50], [52], [69], [78], [85], [86]. References for equivalent rs-numbers [87] for the identified polymorphisms are available on-line as supplementary data (Appendix S1).
Table 1. Initial and replication study phenotypesa.
| Gene | Variation | Initial phenotype | Replication study phenotypes |
| ACAN | VNTR | D | N,D,H,B,S,U,St |
| rs1042631 | D,B | − | |
| ASPN | Allele D14 | S,D | − |
| CASP9 | rs1052576 | H(S) | − |
| CILP | rs2073711 | S | D,S |
| COL1A1 | rs2075555 | D | − |
| COL9A1 | rs696990 | D,B | − |
| COL9A2 | rs137853213 | S | S,D,E |
| COL9A3 | rs61734651 | S | S,D,B,E,∑ |
| COL11A1 | rs1676486 | S | − |
| rs1463035 | B | − | |
| COL11A2 | intron9 | B | − |
| rs2076311 | D | − | |
| rs1799907 | St | D | |
| FAS | rs2234767 | N | − |
| FASLG | rs763110 | N | − |
| GDF5 | rs143383 | X | − |
| IL1A | rs1800587 | B | D,H,E,S |
| rs2071375 | D | − | |
| IL1B | rs1143634 | B | D,E,H,∑ |
| IL1RN | VNTR | H(S) | S |
| IL18RAP | rs1420100 | D | − |
| IL6 | (rs1800795, rs1800796, rs1800796) | S | D |
| IL10 | −1082A/G | D | − |
| −592A/C | D | − | |
| MMP1 | −1607 | D | − |
| MMP2 | −1306C/T | S | − |
| MMP3 | −1171 | X | St |
| MMP9 | rs17576 | S | D |
| −1562C/T | S,N | − | |
| NOS2 | exon22 | H | − |
| NOS3 | −786T/C | H | − |
| SKT | rs16924573 | S | D |
| THBS2 | rs9406328 | S | D |
| VDR | FokI | D | St,U,D,H,E |
| TaqI | D | St,U,D,N,H,E |
Disc degeneration phenotype (D = disc signal changes using different classifications, S = disc herniation with sciatica, N = nonspecific discogenic pain with disc degeneration, B = disc contour change; bulge, H = disc contour change; herniation without specified clinical symptoms, E = endplate changes including Modic changes, U = unspecified degenerative phenotype, X = plain radiograph; MRI for some subjects, St = stenosis, disc signal decrease also recorded, ∑ = combination phenotype).
Table 2. Candidate genes with a moderate level of epidemiological evidence in lumbar disc degenerationa.
| Gene | Variation | Amount of evidence | ReplicationLevel | ProtectionFrom Bias | Additional biological evidenceb | Ethnicity | Phenotype | Reference |
| ASPN | allele D14 | B | B | B | yes | Japanese Chinese | sciatica and disc signal | Song et al 2008 |
| COL11A1 | rs1676486 | B | B | B | yes | Japanese | sciatica | Mio et al 2007 |
| GDF5 | rs143383 | A | B | A | − | Northern European | X-rayc, partially MRI | Williams et al 2011 |
| SKT | rs16924573 | B | B | A | yes | Japanese Finnish | sciatica | Karasugi et al 2009 |
| THBS2 d | rs9406328 | B | B | B | yes | Japanese | sciatica | Hirose et al 2008 |
| MMP9 d | rs17576 | B | B | B | yes | Japanese | sciatica | Hirose et al 2008 |
Based on Venice interim guidelines [33], statistical significance level (p-value) of original association and replication level including also the absence of inconsistent replications. Amount of evidence increases when alleles are contrasted.
Reported in the included studies.
Combined phenotype of disc space narrowing and presence of osteophytes.
One negative replication report [54] in disc signal phenotype.
Level of Association Evidence
None of the genetic variations reached the level of strong evidence for association in the current review. We found a moderate level of evidence for variations from studies investigating asporin (ASPN), collagen XI alpha 1 (COL11A1), growth differentiation factor 5 (GDF5), Sickle tail (SKT); thrombospondin 2 (THBS2) and matrix metalloproteinase 9 (MMP9) genes (Table 2). These studies had at least a moderate amount of evidence, replication in an independent sample (same or independent report), meta-analysis or a combined analysis performed in the initial study (Table 3), sufficient protection from bias, and a high statistical significance level in the initial study. Furthermore, additional biological evidence was reported in the original papers for all these polymorphisms (Table 2). Inadequacy in the number of subjects, lack of an independent replication report or some inconsistency in replications, phenotyping problems or missing information in the report to evaluate protection from bias hindered these associations from reaching the level of strong association evidence (Table 1, Table 2, Table 3).
Table 3. Details of meta-analyses in the identified reports of genes with a moderate level of evidence.
| Gene | Cohort | N | OR | CI | P-value | Meta Analysis Statistics | HeterogeneityMeasure | Statistic test | Software | Reference |
| ASPN | Meta-analysis | 2408 | 1.70 | 1.35–2.20 | 0.000013 | Random-effect model | − | − | MIX | Song et al (2008) |
| Japanese | 1353 | 1.78 | 1.26–2.51 | 0.00087 | ||||||
| Chinese | 1055 | 1.66 | 1.17–2.35 | 0.0039 | ||||||
| COL11A1 | Combined | 1722 | 1.42 | 1.23–1.65 | 0.0000033 | − | − | X2 | − | Mio et al (2007) |
| GDF5 | Meta-analysis | 5259 | 1.72 | 1.15–2.57 | 0.008 | Fixed-effects model | Not significant(Q statistics and I2) | Logistic regression model | R | Williams et al (2011) |
| SKT | Meta-analysis | 2264 | 1.34 | 1.14–1.58 | 0.00040 | Mantel-Haenszel | Japanese+Finnish:p-value = 0.27 | Logistic regression testand X2 | Haploview 4.0 and Microsoft Excel | Karasugi et al (2009) |
| Japanese A+B | 1758 | 1.31 | 1.11–1.55 | 0.0015 | Japanese A+B:p-value = 0.67 | |||||
| Finnish | 506 | 2.81 | 1.09–7.24 | 0.026 | ||||||
| THBS2 | Combined | 1743 | 1.38 | 1.21–1.58 | 0.0000028 | − | − | Kruskal-Wallis and X2 | − | Hirose et al (2008) |
| Japanese A | 1089 | 1.43 | 1.20–1.70 | 0.000040 | ||||||
| Japanese B | 654 | 1.30 | 1.05–1.62 | 0.018 | ||||||
| MMP9 | Combined | 1743 | 1.29 | 1.12–1.48 | 0.00049 | − | − | Kruskal-Wallis and X2 | − | Hirose et al (2008) |
| Japanese A | 1089 | 1.32 | 1.10–1.58 | 0.0023 | ||||||
| Japanese B | 654 | 1.23 | 0.98–1.55 | 0.077 |
CI = 95% confidence interval, N = total number of individuals in the study. Dash (−) indicates missing information in the report.
The association study of ASPN consisted of two independent Asian cohorts of Japanese (N = 1353) and Chinese (N = 1055) origin as reported by Song et al [50]. All Japanese cases had a lumbar disc herniation characterized by sciatica (LDH) confirmed by MRI, while disc signal decreases were also recorded. Association between the presence of at least one D14 allele and LDH was found to be significant, while in the Chinese population the presence of at least one D14 repeat was associated with lumbar disc degeneration. A meta-analysis using the above-mentioned phenotypes showed that individuals carrying the D14 allele had increased odds of LDH or disc degeneration 1.7-fold (Table 3) [50].
A Japanese study found an association between COL11A1 rs1676486 T-allele and LDH characterized by sciatica. The original study consisted of three case-control populations (N = 367, N = 645, N = 710), each independently showing a significant association. When the populations were combined for meta-analysis (N = 1661), the minor allele T was more prevalent among cases compared to controls (Table 3). Additional biological evidence was also reported [69].
A recent multicohort study with Northern European subjects investigated the rs143383 of the GDF5 gene. Out of the total population (N = 5259), one cohort (N = 613) was scanned with MRI, therefore making the study eligible for our review. In the meta-analysis rs143383, a significant association was found among women for combined phenotype of disc space narrowing and osteophytes (Table 3). When only the MRI cohort was investigated, the association was not statistically significant, thus generating some inconsistency in this association [88]. However, as this specific disc degeneration phenotype can be obtained on multiple imaging modalities, such as radiographes, computed tomography or MRI [89], we included the results of the meta-analysis.
Another recent study analyzed the Sickle tail (SKT) gene polymorphisms [86]. Of the 68 SNPs studied, the rs16924573 was the most strongly associated with LDH among Japanese subjects (N = 1758) and the finding was replicated among Finnish subjects (N = 506) (Table 3) [86]. Allele frequencies were different between Finnish and Japanese populations, but the meta-analysis of over 2200 subjects supported the association (Table 3) [85]. A replication study between disc signal decrease and SKT rs16924573 has been recently published (OR 0.27 [95% CI 0.07–0.96], p = 0.024) [56]. The G-allele frequency was higher in the case group of both studies, thus indicating an increased risk. However, the A-allele was rarer in the more recent study and the association was only seen when the GA-genotype was compared to GG-genotype. Therefore the OR in the more recent study is protective [56].
Two thrombospondin genes (thrombospondin-1 and THBS2) were examined in two independent Japanese populations (N = 1089 and N = 654) as candidate genes for LDH. Multiple polymorphisms of the THBS2 were associated with LDH. The polymorphism rs9406328 showed significant association in both populations independently as well as when populations were combined (Table 3) [78]. The same study also reported a significant association between the rs17576 of the MMP9 gene and LDH (Table 3) [78].
In this review, the most studied candidate genes for LDD were vitamin-D receptor (VDR), aggrecan (ACAN), interleukin-1 alpha (IL1A), interleukin-1 beta (IL1B), collagen IX alpha 2 (COL9A2) and collagen IX alpha 3 (COL9A3) (Table S1). However, a large proportion of the association studies investigating disc degeneration had some faults, which weakened the evidence for association. The most common weaknesses were the relatively low number of study subjects and difficulty in replicating the previous association signal. In general, there was a lack of replication; and where replication did exist, studies were often too heterogeneous, leading to inconsistencies and differences in the final phenotype. In some studies, protection from bias seemed to be insufficient. However, due to failure to report the study details properly, it was often very difficult to adequately assess studies. In summary, due to general heterogeneity of studies, replications were inconclusive and meta-analyses were not feasible, thus leading to a weak level of association evidence in many cases.
Protein–protein Interaction Network Analysis
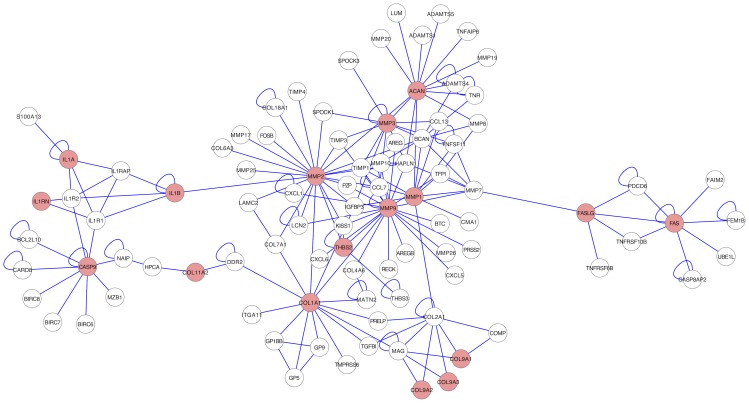
We performed post hoc analysis (Appendix S1) for protein–protein interactions (PPI) network combining all genes from included studies with any positive association as input [90]. This resulted in a significant (p<0.0001) network including 60.7% (17/28) of the genes with positive associations reported in the included studies, and proposed 76 new interaction partners as possible topics of future investigations (Figure 2). The PPI results were not incorporated into the credibility levels of previously identified associations.
Figure 2. PPI-network analysis.
Significant PPI-network (p-value <0.0001) containing 17 proteins associated with disc degeneration (red circles) and 76 interaction partners (white circles).
Discussion
The study of lumbar disc degeneration is clinically relevant. Numerous studies have noted that lumbar disc degeneration is associated with LBP [7]–[13], [91]. Therefore, to determine preventative and therapeutic measures for LBP, it is beneficial to understand the etiology of disc degeneration. However, lumbar disc degeneration is a multifaceted condition, in which hereditary factors play an important role. Our knowledge about the natural history of degenerative disc disease is constantly improving [14], [25]. Still, the complexity of the degenerative process is not fully understood. For example, disc space narrowing, facet disease and spinal stenosis tend to progress slowly over time while disc herniations can occur rapidly [92]–[94]. Imaging techniques have improved significantly [15] since the earliest genetic association reports; however, the definitions of the imaging phenotype, subject selection and study detail reporting are not consistent or standardized in this field.
In this first extensive systematic review on the subject, we identified 52 candidate gene studies that had used MRI for disc degeneration definition and defined a specific genetic polymorphism. The phenotype with the most convincing evidence was disc herniation characterized by sciatica. This phenotype was utilized in 80% (ASPN, COL11A1, SKT, THBS2 and MMP9) of the studies with a moderate level of evidence in the current review.
All the genes in which the variations were found to have a moderate level of evidence are biologically plausible in disc degeneration. The asporin protein, coded by ASPN, is one of the small leucine-rich proteoglycans of the lumbar disc playing an important role in cartilage homeostasis. The specific D14-allele, that we found had a moderate level of evidence, has been reported to decrease collagen type II and aggrecan synthesis via inhibition of transforming growth factor beta 1 (TGF-β), which is a regulator of cartilage metabolism [95]. Type XI collagen is a minor component of the lumbar disc. It is expressed both in the outer and inner parts of the disc, and it has an important function in the interplay of collagens and proteoglycans [96]. The rs1676486 T-allele has been reported to result in decreased synthesis and stability of COL11A1 mRNA suggesting a functional importance in disc degeneration [69]. The growth differentiation factor 5, coded by GDF5, is a cartilage anabolic protein and has been linked with both osteoarthritis and disc degeneration [88], [97]. The rs143383, which was associated with increased risk for disc degeneration, has been reported to have genome-wide significance in multiple musculoskeletal phenotypes such as height and Achilles tendinopathy. However, the modulation of GDF5 expression seems to be intricate [98], [99]. The human SKT is expressed in the human intervertebral disc and the importance of this gene in disc development has been established previously in an animal study [100]. The specific way in which SKT participates in disc homeostasis and the functional importance of the identified risk variation rs16924573 remains to be elucidated [86]. Thrombospondin-2 (THBS2), which is also expressed in the disc, regulates the effective levels of catabolic proteins (matrix metalloproteinase-2 and MMP9) in the extracellular matrix. The variation rs9406328, with a moderate level of association evidence, has been reported to have an effect on THBS2 binding with these catabolic proteins. Furthermore, the variation rs17576 in MMP9 is located within a highly conserved region and possibly has an effect on substrate binding [78].
The earlier non-systematic reviews have suggested that various other genes may be related to the development of lumbar disc degeneration [29], [101]–[107]. However, based on our systematic review, there is only a weak level of evidence for these genes at the most. This is probably due to more strict inclusion criteria applied in the current review; for instance, studies without MRI evaluation, as well as studies reporting success of surgical treatments as the phenotype, have been included in some of the earlier reviews. Moreover, studies with less than 100 subjects in the smallest genetic group, no replication/meta-analysis or with some bias were currently considered as having weak evidence.
Based on our systematic review, the quality of the evaluated studies varied considerably and some recurrent weaknesses were identified. Definitions of imaging phenotypes were not clearly reported and there was some variability in the selection of subjects. Further, there were a few studies where the control group was not evaluated using MRI or subjects with LBP and/or sciatica were included as controls. As such, differences in subject selection and phenotype definition hindered efforts to produce a reasonable meta-analysis. Population-based studies with large study samples with adequate statistical power were rare; in fact, only five studies possessed a sample size greater than 1000. Quality control steps, such as population stratification, Hardy-Weinberg equilibrium testing, and statistical power calculations, were often not consistently reported, even though the reporting improved in more recent publications [108]. Moreover, the publication bias (i.e. the tendency to under-report negative results) was clearly visible in the published data under review, whereby nearly 90% of the included studies reported positive associations between lumbar disc degeneration and specific polymorphisms.
Variation in allele frequencies between different ethnic populations (e.g. Caucasians, Asians) may suggest that different risk alleles may be involved in the development of lumbar disc degeneration in different ethnic groups. Thus, replication studies are needed in study populations of similar and different ethnic/geographic origin to provide a more comprehensive understanding. In the current systematic review, the most consistent evidence (i.e. moderate evidence) was based on studies in Asian populations. However, in one study, the association with moderate evidence (SKT gene and sciatica) was originally reported in Japanese and replicated in Finnish population [56], [86]. Alternatively, in many cases, replications were inconsistent, either due to different phenotypes or different genetic variation examined in the replication study (Table 1).
In lumbar disc degeneration, possibilities to develop an even larger number of distinct phenotypes expand as new imaging techniques, such as high field T1ρ or T2 relaxation mapping, are used more widely [109]–[112]. Although there have been recent suggestions to adapt to differences in phenotypes [113], there is a great risk that without standardization of subject selection (e.g. population-based vs. patient-based), phenotype definition and study detail reporting in this field, such an approach will further contribute to the development of an even more complex state in disseminating the evidence for the association between genetics and lumbar disc degeneration. Afterall, the clinically relevant endpoints of disc degeneration, pain or neurological deficits, are similarly complex entities and it is unlikely that sound progress would be achieved via incorporation of all the phenotypes. Therefore, the cumulative epidemiological evidence described in the current review can be considered ‘early evidence’, as defined by The HuGENet Working Group [33].
Rapid advancements in genetics and bioinformatics have led to the situation where the amount of data under analysis has increased substantially [114]–[119], providing new opportunities to reveal genetic background of complex traits. Over the last 5 years, genome-wide association studies (GWAS) have become a powerful tool for identifying common genetic variants, which have led to the discovery of common risk loci for several complex diseases [120]–[123]. However, to our knowledge, no GWAS has yet been published to address lumbar disc degeneration. On the other hand, the protein-protein interaction analyses, that we also included, are currently considered to be a valuable method in order to deepen our understanding of common complex diseases [117], [124], [125]. These interactions between proteins demonstrate one of the strongest functional relationships between genes. Therefore, by combining the genomic data with available proteomic data, we may gain a more in-depth understanding of common human diseases [117]. The current protein-protein interaction analysis included in this review can act as a starting point to stimulate forthcoming research. For more refined discovery of risk variants for several complex traits, efforts towards incorporating exome or whole-genome sequencing approaches, due to increased capacity and accuracy of next-generation sequencing, are currently being carried out.
In conclusion, our systematic review has noted multiple genetic polymorphisms to be related to the development of lumbar disc degeneration; however, due to variation between study designs, sampling methods, populations, and phenotype definitions, the level of evidence of that association remains weak. As such, our review stresses the limitations of the current status of genetic association studies in relation to lumbar disc degeneration. Collaborative studies with large population-based cohorts and well-defined phenotypes as well as genotype characteristics are necessary for major advances in understanding the genetic component of lumbar disc degeneration. By increasing the understanding of the etiology of lumbar disc degeneration, preventative and therapeutic measures can be designed to address such degenerative changes, which may also translate into decreasing the risk of developing LBP and its consequences. Therefore, a call to action to establish an international consortium is needed to standardize methods and limit variations between genetic studies of lumbar disc degeneration.
Supporting Information
Details of the included studies.
(HTM)
Supporting information about methods; study inclusion criteria, study quality assessment essentials, data extraction form (including formalized summary scoring), categories for the credibility of cumulative epidemiological evidence, protein-protein interaction network analysis methods. Supporting information about results; equivalent rs-numbers, included studies, excluded studies (with reasons for exclusion).
(PDF)
Study flow.
(DOC)
Presubmission checklist.
(DOC)
Funding Statement
This study was supported in part by grants from the Academy of Finland (grants 121620 and 129504, to JK). The Päivikki and Sakari Sohlberg Foundation, the Eemil Aaltonen Foundation, the Finnish-Norwegian Medical Foundation and the Finnish Medical Foundation have supported PE. SL was financially supported by the Academy of Finland (MSDs@Lifecourse Concortium, Responding to Public Health Challenges (SALVE), project no 129364). PK was supported by a grant from the Danish Agency for Science, Technology and Innovation (271-08-0374). This work was further supported by an Area of Excellence grant from the University Grants Committee of Hong Kong (AoE/M-04/04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Andersson GB (1999) Epidemiological features of chronic low-back pain. Lancet 354: 581–585. [DOI] [PubMed] [Google Scholar]
- 2. Katz JN (2006) Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am 88 Suppl 221–24. [DOI] [PubMed] [Google Scholar]
- 3. Deyo RA, Tsui-Wu YJ (1987) Descriptive epidemiology of low-back pain and its related medical care in the united states. Spine (Phila Pa 1976) 12: 264–268. [DOI] [PubMed] [Google Scholar]
- 4. Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 8: 8–20. [DOI] [PubMed] [Google Scholar]
- 5. Ekman M, Johnell O, Lidgren L (2005) The economic cost of low back pain in sweden in 2001. Acta Orthop 76: 275–284. [DOI] [PubMed] [Google Scholar]
- 6. Wieser S, Horisberger B, Schmidhauser S, Eisenring C, Brugger U, et al. (2011) Cost of low back pain in Switzerland in 2005. Eur J Health Econ 12: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou D, Samartzis D, Bellabarba C, Patel A, Luk KDK, et al. (2011) Degenerative magnetic resonance imaging changes in patients with chronic low back pain: A systematic review. Spine (Phila Pa 1976) 36: 43–53. [DOI] [PubMed] [Google Scholar]
- 8. de Schepper EIT, Damen J, van Meurs JBJ, Ginai AZ, Popham M, et al. (2010) The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976) 35: 531–536. [DOI] [PubMed] [Google Scholar]
- 9. Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T (2005) Magnetic resonance imaging and low back pain in adults: A diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976) 30: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 10. Paajanen H, Erkintalo M, Kuusela T, Dahlstrom S, Kormano M (1989) Magnetic resonance study of disc degeneration in young low-back pain patients. Spine (Phila Pa 1976) 14: 982–985. [DOI] [PubMed] [Google Scholar]
- 11. Samartzis D, Karppinen J, Mok F, Fong DYT, Luk KDK, et al. (2011) A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am 93: 662–670. [DOI] [PubMed] [Google Scholar]
- 12. Takatalo J, Karppinen J, Niinimaki J, Taimela S, Nayha S, et al. (2011) Does lumbar disc degeneration on MRI associate with low back symptom severity in young Finnish adults? Spine (Phila Pa 1976) 36: 2180–2189. [DOI] [PubMed] [Google Scholar]
- 13. Visuri T, Ulaska J, Eskelin M, Pulkkinen P (2005) Narrowing of lumbar spinal canal predicts chronic low back pain more accurately than intervertebral disc degeneration: A magnetic resonance imaging study in young Finnish male conscripts. Mil Med 170: 926–930. [DOI] [PubMed] [Google Scholar]
- 14. Modic MT, Ross JS (2007) Lumbar degenerative disk disease. Radiology 245: 43–61. [DOI] [PubMed] [Google Scholar]
- 15. Haughton V (2006) Imaging intervertebral disc degeneration. J Bone Joint Surg Am 88 Suppl 215–20. [DOI] [PubMed] [Google Scholar]
- 16. Fardon DF, Milette PC (2001) Nomenclature and classification of lumbar disc pathology. Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976) 26: 93–93. [DOI] [PubMed] [Google Scholar]
- 17. Jarvik JG, Deyo RA (2002) Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 137: 586–597. [DOI] [PubMed] [Google Scholar]
- 18. Zou J, Yang H, Miyazaki M, Morishita Y, Wei F, et al. (2009) Dynamic bulging of intervertebral discs in the degenerative lumbar spine. Spine (Phila Pa 1976) 34: 2545–2550. [DOI] [PubMed] [Google Scholar]
- 19. Cheung KMC, Samartzis D, Karppinen J, Mok FPS, Ho DWH, et al. (2010) Intervertebral disc degeneration: New insights based on “skipped” level disc pathology. Arthritis Rheum 62: 2392–2400. [DOI] [PubMed] [Google Scholar]
- 20. Battie MC, Videman T, Gill K, Moneta GB, Nyman R, et al. (1991) 1991 Volvo award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: An MRI study of identical twins. Spine (Phila Pa 1976) 16: 1015–1021. [PubMed] [Google Scholar]
- 21. Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P (2000) Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 25: 1625–1636. [DOI] [PubMed] [Google Scholar]
- 22. Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, et al. (2005) Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 29: 903–908. [DOI] [PubMed] [Google Scholar]
- 23. Rivinoja AE, Paananen MV, Taimela SP, Solovieva S, Okuloff A, et al. (2011) Sports, smoking, and overweight during adolescence as predictors of sciatica in adulthood: A 28-year follow-up study of a birth cohort. Am J Epidemiol 173: 890–897. [DOI] [PubMed] [Google Scholar]
- 24. Urban JPG, Smith S, Fairbank JCT (2004) Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 29: 2700–2709. [DOI] [PubMed] [Google Scholar]
- 25. Chan WCW, Sze KL, Samartzis D, Leung VYL, Chan D (2011) Structure and biology of the intervertebral disk in health and disease. Orthop Clin North Am 42: 447–464. [DOI] [PubMed] [Google Scholar]
- 26. Varlotta GP, Brown MD, Kelsey JL, Golden AL (1991) Familial predisposition for herniation of a lumbar disc in patients who are less than twenty-one years old. J Bone Joint Surg Am 73: 124–128. [PubMed] [Google Scholar]
- 27. Matsui H, Terahata N, Tsuji H, Hirano N, Naruse Y (1992) Familial predisposition and clustering for juvenile lumbar disc herniation Spine (Phila Pa 1976). 17: 1323–1328. [DOI] [PubMed] [Google Scholar]
- 28. Battie MC, Videman T, Gibbons LE, Fisher LD, Manninen H, et al. (1995) 1995 Volvo award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine (Phila Pa 1976) 20: 2601–2612. [PubMed] [Google Scholar]
- 29. Battie MC, Videman T, Kaprio J, Gibbons LE, Gill K, et al. (2009) The twin spine study: Contributions to a changing view of disc degeneration. Spine J 9: 47–59. [DOI] [PubMed] [Google Scholar]
- 30. Bracken MB (2005) Genomic epidemiology of complex disease: The need for an electronic evidence-based approach to research synthesis. Am J Epidemiol 162: 297–301. [DOI] [PubMed] [Google Scholar]
- 31. Ryder JJ, Garrison K, Song F, Hooper L, Skinner J, et al. (2008) Genetic associations in peripheral joint osteoarthritis and spinal degenerative disease: A systematic review. Ann Rheum Dis 67: 584–591. [DOI] [PubMed] [Google Scholar]
- 32. Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001) Replication validity of genetic association studies. Nat Genet 29: 306–309. [DOI] [PubMed] [Google Scholar]
- 33. Ioannidis JP, Boffetta P, Little J, O’Brien TR, Uitterlinden AG, et al. (2008) Assessment of cumulative evidence on genetic associations: Interim guidelines. Int J Epidemiol 37: 120–132. [DOI] [PubMed] [Google Scholar]
- 34. Munafo MR (2010) Credible genetic associations? Int J Mol Epidemiol Genet 1: 31–34. [PMC free article] [PubMed] [Google Scholar]
- 35. Juni P, Witschi A, Bloch R, Egger M (1999) The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282: 1054–1060. [DOI] [PubMed] [Google Scholar]
- 36. Paz Aparicio J, López-Anglada Fernández E, Montes Prieto H, Pena Vázquez J, Fernández Bances I, et al. (2010) Association between the genetic polymorphism of interleukin-1β (3953 T/C) and symptomatic lumbar herniated disc. Revista Espanola De Cirugia Ortopedica y Traumatologia 54: 227–233. [Google Scholar]
- 37. Paz Aparicio J, Fernandez Bances I, Lopez-Anglada Fernandez E, Montes AH, Paz Aparicio A, et al. (2011) The IL-1β (+3953 T/C) gene polymorphism associates to symptomatic lumbar disc herniation. Eur Spine J 20 Suppl 3383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bei T, Tilkeridis C, Garantziotis S, Boikos S, Kazakos K, et al. (2008) A novel, non-functional, COL1A1 polymorphism is not associated with lumbar disk disease in young male Greek subjects unlike that of the Sp1 site. Hormones (Athens) 7: 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Videman T, Saarela J, Kaprio J, Nakki A, Levalahti E, et al. (2009) Associations of 25 structural, degradative, and inflammatory candidate genes with lumbar disc desiccation, bulging, and height narrowing. Arthritis Rheum 60: 470–481. [DOI] [PubMed] [Google Scholar]
- 40. Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, et al. (1999) Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine (Phila Pa 1976) 24: 2456–2460. [DOI] [PubMed] [Google Scholar]
- 41. Eser O, Eser B, Cosar M, Erdogan MO, Aslan A, et al. (2011) Short aggrecan gene repetitive alleles associated with lumbar degenerative disc disease in Turkish patients. Genet Mol Res 10: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 42. Eser B, Cora T, Eser O, Kalkan E, Haktanir A, et al. (2010) Association of the polymorphisms of vitamin D receptor and aggrecan genes with degenerative disc disease. Genet Test Mol Biomarkers 14: 313–317. [DOI] [PubMed] [Google Scholar]
- 43. Solovieva S, Noponen N, Mannikko M, Leino-Arjas P, Luoma K, et al. (2007) Association between the aggrecan gene variable number of tandem repeats polymorphism and intervertebral disc degeneration. Spine (Phila Pa 1976) 32: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 44. Cong L, Pang H, Xuan D, Tu G (2010) The interaction between aggrecan gene VNTR polymorphism and cigarette smoking in predicting incident symptomatic intervertebral disc degeneration. Connect Tissue Res 51: 397–403. [DOI] [PubMed] [Google Scholar]
- 45. Cong L, Pang H, Xuan D, Tu GJ (2010) Association between the expression of aggrecan and the distribution of aggrecan gene variable number of tandem repeats with symptomatic lumbar disc herniation in Chinese Han of northern China. Spine (Phila Pa 1976) 35: 1371–1376. [DOI] [PubMed] [Google Scholar]
- 46. Mashayekhi F, Shafiee G, Kazemi M, Dolati P (2010) Lumbar disk degeneration disease and aggrecan gene polymorphism in northern iran. Biochem Genet 48: 684–689. [DOI] [PubMed] [Google Scholar]
- 47. Noponen-Hietala N, Kyllonen E, Mannikko M, Ilkko E, Karppinen J, et al. (2003) Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis 62: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, et al. (2006) The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater 11: 1–7. [PubMed] [Google Scholar]
- 49. Kim NK, Shin DA, Han IB, Yoo EH, Kim SH, et al. (2011) The association of aggrecan gene polymorphism with the risk of intervertebral disc degeneration. Acta Neurochir 153: 129–133. [DOI] [PubMed] [Google Scholar]
- 50. Song Y, Cheung KMC, Ho DWH, Poon SCS, Chiba K, et al. (2008) Association of the asporin D14 allele with lumbar-disc degeneration in Asians. Am J Hum Genet 82: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun Z, Ling M, Huo Y, Chang Y, Li Y, et al. (2011) Caspase 9 gene polymorphism and susceptibility to lumbar disc disease in the han population in northern china. Connect Tissue Res 52: 198–202. [DOI] [PubMed] [Google Scholar]
- 52. Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, et al. (2005) A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet 37: 607–612. [DOI] [PubMed] [Google Scholar]
- 53. Virtanen IM, Song YQ, Cheung KMC, Ala-Kokko L, Karppinen J, et al. (2007) Phenotypic and population differences in the association between CILP and lumbar disc disease. J Med Genet 44: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Min SK, Nakazato K, Okada T, Ochi E, Hiranuma K (2009) The cartilage intermediate layer protein gene is associated with lumbar disc degeneration in collegiate judokas. Int J Sports Med 30: 691–694. [DOI] [PubMed] [Google Scholar]
- 55. Min S, Nakazato K, Yamamoto Y, Gushiken K, Fujimoto H, et al. (2010) Cartilage intermediate layer protein gene is associated with lumbar disc degeneration in male, but not female, collegiate athletes. Am J Sports Med 38: 2552–2557. [DOI] [PubMed] [Google Scholar]
- 56. Kelempisioti A, Eskola PJ, Okuloff A, Karjalainen U, Takatalo J, et al. (2011) Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC Med Genet 12: 153–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Annunen S, Paassilta P, Lohiniva J, Perala M, Pihlajamaa T, et al. (1999) An allele of COL9A2 associated with intervertebral disc disease. Science 285: 409–412. [DOI] [PubMed] [Google Scholar]
- 58. Higashino K, Matsui Y, Yagi S, Takata Y, Goto T, et al. (2007) The alpha2 type IX collagen tryptophan polymorphism is associated with the severity of disc degeneration in younger patients with herniated nucleus pulposus of the lumbar spine. Int Orthop 31: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jim JJ, Noponen-Hietala N, Cheung KM, Ott J, Karppinen J, et al. (2005) The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine (Phila Pa 1976) 30: 2735–2742. [DOI] [PubMed] [Google Scholar]
- 60. Seki S, Kawaguchi Y, Mori M, Mio F, Chiba K, et al. (2006) Association study of COL9A2 with lumbar disc disease in the Japanese population. J Hum Genet 51: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 61. Kales SN, Linos A, Chatzis C, Sai Y, Halla M, et al. (2004) The role of collagen IX tryptophan polymorphisms in symptomatic intervertebral disc disease in southern European patients. Spine (Phila Pa 1976) 29: 1266–1270. [DOI] [PubMed] [Google Scholar]
- 62. Karppinen J, Paakko E, Raina S, Tervonen O, Kurunlahti M, et al. (2002) Magnetic resonance imaging findings in relation to the COL9A2 tryptophan allele among patients with sciatica. Spine (Phila Pa 1976) 27: 78–83. [DOI] [PubMed] [Google Scholar]
- 63. Karppinen J, Paakko E, Paassilta P, Lohiniva J, Kurunlahti M, et al. (2003) Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology 227: 143–148. [DOI] [PubMed] [Google Scholar]
- 64. Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, et al. (2001) Identification of a novel common genetic risk factor for lumbar disk disease. JAMA 285: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 65. Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, et al. (2006) Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur Spine J 15: 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, et al. (2002) COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: Evidence of gene-environment interaction. Spine (Phila Pa 1976) 27: 2691–2696. [DOI] [PubMed] [Google Scholar]
- 67. Karppinen J, Solovieva S, Luoma K, Raininko R, Leino-Arjas P, et al. (2009) Modic changes and interleukin 1 gene locus polymorphisms in occupational cohort of middle-aged men. Eur Spine J 18: 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karppinen J, Daavittila I, Solovieva S, Kuisma M, Taimela S, et al. (2008) Genetic factors are associated with Modic changes in endplates of lumbar vertebral bodies. Spine (Phila Pa 1976) 33: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 69. Mio F, Chiba K, Hirose Y, Kawaguchi Y, Mikami Y, et al. (2007) A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am J Hum Genet 81: 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eskola PJ, Kjaer P, Daavittila IM, Solovieva S, Okuloff A, et al. (2010) Genetic risk factors of disc degeneration among 12–14-year-old Danish children: A population study Int J Mol Epidemiol Genet. 1: 158–165. [PMC free article] [PubMed] [Google Scholar]
- 71. Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, et al. (2005) Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain 114: 186–194. [DOI] [PubMed] [Google Scholar]
- 72. Solovieva S, Kouhia S, Leino-Arjas P, Ala-Kokko L, Luoma K, et al. (2004) Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology 15: 626–633. [DOI] [PubMed] [Google Scholar]
- 73. Kim D, Lee S, Kim K, Yu S (2010) Association of interleukin-1 receptor antagonist gene polymorphism with response to conservative treatment of lumbar herniated nucleus pulposus. Spine (Phila Pa 1976) 35: 1527–1531. [DOI] [PubMed] [Google Scholar]
- 74. Lin WP, Lin JH, Chen XW, Wu CY, Zhang LQ, et al. (2011) Interleukin-10 promoter polymorphisms associated with susceptibility to lumbar disc degeneration in a Chinese cohort. Genet Mol Res 10: 1719–1727. [DOI] [PubMed] [Google Scholar]
- 75. Song Y, Ho DWH, Karppinen J, Kao PYP, Fan B, et al. (2008) Association between promoter -1607 polymorphism of MMP1 and lumbar disc disease in southern Chinese. BMC Med Genet 9: 38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dong DM, Yao M, Liu B, Sun CY, Jiang YQ, et al. (2007) Association between the -1306C/T polymorphism of matrix metalloproteinase-2 gene and lumbar disc disease in Chinese young adults. Eur Spine J 16: 1958–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, et al. (2001) The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br 83: 491–495. [DOI] [PubMed] [Google Scholar]
- 78. Hirose Y, Chiba K, Karasugi T, Nakajima M, Kawaguchi Y, et al. (2008) A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation. Am J Hum Genet 82: 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun ZM, Miao L, Zhang YG, Ming L (2009) Association between the -1562 C/T polymorphism of matrix metalloproteinase-9 gene and lumbar disc disease in the young adult population in north China. Connect Tissue Res 50: 181–185. [DOI] [PubMed] [Google Scholar]
- 80. Nunes FTB, Conforti-Froes NDT, Negrelli WF, Souza DRS (2007) Genetic and environmental factors involved on intervertebral disc degeneration. Acta Ortopedica Brasileira 15: 9–13. [Google Scholar]
- 81. Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, et al. (1998) Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine (Phila Pa 1976) 23: 2477–2485. [DOI] [PubMed] [Google Scholar]
- 82. Cheung KMC, Chan D, Karppinen J, Chen Y, Jim JJT, et al. (2006) Association of the Taq I allele in vitamin D receptor with degenerative disc disease and disc bulge in a Chinese population. Spine (Phila Pa 1976) 31: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 83. Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, et al. (2002) The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am 84-A: 2022–2028. [DOI] [PubMed] [Google Scholar]
- 84. Oishi Y, Shimizu K, Katoh T, Nakao H, Yamaura M, et al. (2003) Lack of association between lumbar disc degeneration and osteophyte formation in elderly Japanese women with back pain. Bone 32: 405–411. [DOI] [PubMed] [Google Scholar]
- 85. Zhu G, Jiang X, Xia C, Sun Y, Zeng Q, et al. (2011) Association of FAS and FAS ligand polymorphisms with the susceptibility and severity of lumbar disc degeneration in Chinese han population. Biomarkers 16: 485–90. [DOI] [PubMed] [Google Scholar]
- 86. Karasugi T, Semba K, Hirose Y, Kelempisioti A, Nakajima M, et al. (2009) Association of the tag SNPs in the human SKT gene (KIAA1217) with lumbar disc herniation. J Bone Miner Res 24: 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, et al. (2001) dbSNP: The NCBI database of genetic variation. Nucleic Acids Res 29: 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williams FM, Popham M, Hart DJ, de Schepper E, Bierma-Zeinstra S, et al. (2011) GDF5 single-nucleotide polymorphism rs143383 is associated with lumbar disc degeneration in northern European women. Arthritis Rheum 63: 708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Williams FMK, Bansal AT, van Meurs JB, Bell JT, Meulenbelt I, et al. (2012) Novel genetic variants associated with lumbar disc degeneration in northern europeans: A meta-analysis of 4600 subjects. Ann Rheum Dis 63: 708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lage K, Karlberg EO, Storling ZM, Olason PI, Pedersen AG, et al. (2007) A human phenome-interactome network of protein complexes implicated in genetic disorders. Nat Biotechnol 25: 309–316. [DOI] [PubMed] [Google Scholar]
- 91. Cheung KM, Samartzis D, Karppinen J, Luk KD (2012) Are “patterns” of lumbar disc degeneration associated with low back pain?: new insights based on skipped level disc pathology. Spine (Phila Pa 1976) 37: E430–8. [DOI] [PubMed] [Google Scholar]
- 92. Saal JA, Saal JS, Herzog RJ (1990) The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976) 15: 683–686. [DOI] [PubMed] [Google Scholar]
- 93. Modic MT, Ross JS, Obuchowski NA, Browning KH, Cianflocco AJ, et al. (1995) Contrast-enhanced MR imaging in acute lumbar radiculopathy: A pilot study of the natural history. Radiology 195: 429–435. [DOI] [PubMed] [Google Scholar]
- 94. Modic MT, Obuchowski NA, Ross JS, Brant-Zawadzki MN, Grooff PN, et al. (2005) Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology 237: 597–604. [DOI] [PubMed] [Google Scholar]
- 95. Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, et al. (2005) An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet 37: 138–144. [DOI] [PubMed] [Google Scholar]
- 96. Smith GN, Williams JM, Brandt KD (1985) Interaction of proteoglycans with the pericellular (1 alpha, 2 alpha, 3 alpha) collagens of cartilage. J Biol Chem 260: 10761–10767. [PubMed] [Google Scholar]
- 97. Evangelou E, Chapman K, Meulenbelt I, Karassa FB, Loughlin J, et al. (2009) Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum 60: 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reynard LN, Bui C, Canty-Laird EG, Young DA, Loughlin J (2011) Expression of the osteoarthritis-associated gene GDF5 is modulated epigenetically by DNA methylation. Hum Mol Genet 20: 3450–3460. [DOI] [PubMed] [Google Scholar]
- 99.Dodd AW, Syddall CM, Loughlin J (2012) A rare variant in the osteoarthritis-associated locus GDF5 is functional and reveals a site that can be manipulated to modulate GDF5 expression. Eur J Hum Genet [Epub ahead of print] doi: 10.1038/ejhg.2012.197. [DOI] [PMC free article] [PubMed]
- 100. Semba K, Araki K, Li Z, Matsumoto K, Suzuki M, et al. (2006) A novel murine gene, sickle tail, linked to the danforth’s short tail locus, is required for normal development of the intervertebral disc. Genetics 172: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ala-Kokko L (2002) Genetic risk factors for lumbar disc disease. Ann Med 34: 42–47. [DOI] [PubMed] [Google Scholar]
- 102. Chan D, Song Y, Sham P, Cheung KMC (2006) Genetics of disc degeneration. Eur Spine J 15 Suppl 3317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Battie MC, Videman T (2006) Lumbar disc degeneration: Epidemiology and genetics. J Bone Joint Surg Am 88 Suppl 23–9. [DOI] [PubMed] [Google Scholar]
- 104. Zhang Y, Sun Z, Liu J, Guo X (2008) Advances in susceptibility genetics of intervertebral degenerative disc disease. Int J Biol Sci 4: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kalichman L, Hunter DJ (2008) The genetics of intervertebral disc degeneration. associated genes. Joint Bone Spine 75: 388–396. [DOI] [PubMed] [Google Scholar]
- 106. Song YQ, Sham P, Cheung KMC, Chan D (2008) Genetics of disc degeneration. Current Orthopaedics 22: 259–266. [Google Scholar]
- 107. Kalb S, Martirosyan NL, Kalani MYS, Broc GG, Theodore N (2012) Genetics of the degenerated intervertebral disc. World Neurosurg 77: 491–501. [DOI] [PubMed] [Google Scholar]
- 108. Yesupriya A, Evangelou E, Kavvoura FK, Patsopoulos NA, Clyne M, et al. (2008) Reporting of human genome epidemiology (HuGE) association studies: An empirical assessment. BMC Med Res Methodol 8: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Borthakur A, Maurer PM, Fenty M, Wang C, Berger R, et al. (2011) T1ρ magnetic resonance imaging and discography pressure as novel biomarkers for disc degeneration and low back pain. Spine (Phila Pa 1976) 36: 2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Blumenkrantz G, Zuo J, Li X, Kornak J, Link TM, et al. (2010) In vivo 3.0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn Reson Med 63: 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Johannessen W, Auerbach JD, Wheaton AJ, Kurji A, Borthakur A, et al. (2006) Assessment of human disc degeneration and proteoglycan content using T1rho-weighted magnetic resonance imaging. Spine (Phila Pa 1976) 31: 1253–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zuo J, Saadat E, Romero A, Loo K, Li X, et al. (2009) Assessment of intervertebral disc degeneration with magnetic resonance single-voxel spectroscopy. Magn Reson Med 62: 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chen Y, Jiang T, Jiang R (2011) Uncover disease genes by maximizing information flow in the phenome-interactome network. Bioinformatics 27: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Niedringhaus TP, Milanova D, Kerby MB, Snyder MP, Barron AE (2011) Landscape of next-generation sequencing technologies. Anal Chem 83: 4327–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Montgomery SB, Dermitzakis ET (2011) From expression QTLs to personalized transcriptomics. Nat Rev Genet 12: 277–282. [DOI] [PubMed] [Google Scholar]
- 116. Sethumadhavan R, Doss CGP, Rajasekaran R (2011) In silico searching for disease-associated functional DNA variants. Methods Mol Biol 760: 239–250. [DOI] [PubMed] [Google Scholar]
- 117. Pattin KA, Moore JH (2009) Role for protein-protein interaction databases in human genetics. Expert Rev Proteomics 6: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kere J (2010) Genetics of complex disorders. Biochem Biophys Res Commun 396: 143–146. [DOI] [PubMed] [Google Scholar]
- 119. Mechanic LE, Chen H, Amos CI, Chatterjee N, Cox NJ, et al. (2011) Next generation analytic tools for large scale genetic epidemiology studies of complex diseases. Genet Epidemiol 36: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, et al. (2007) Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet 39: 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, et al. (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885. [DOI] [PubMed] [Google Scholar]
- 122. Day-Williams AG, Southam L, Panoutsopoulou K, Rayner NW, Esko T, et al. (2011) A variant in MCF2L is associated with osteoarthritis. Am J Hum Genet 89: 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wellcome Trust Case Control Consortium (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447: 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang PI, Marcotte EM (2010) It’s the machine that matters: Predicting gene function and phenotype from protein networks. J Proteomics 73: 2277–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang W, Chen Y, Sun F, Jiang R (2011) DomainRBF: A bayesian regression approach to the prioritization of candidate domains for complex diseases. BMC Syst Biol 5: 55–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the included studies.
(HTM)
Supporting information about methods; study inclusion criteria, study quality assessment essentials, data extraction form (including formalized summary scoring), categories for the credibility of cumulative epidemiological evidence, protein-protein interaction network analysis methods. Supporting information about results; equivalent rs-numbers, included studies, excluded studies (with reasons for exclusion).
(PDF)
Study flow.
(DOC)
Presubmission checklist.
(DOC)