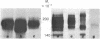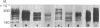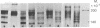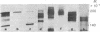Abstract
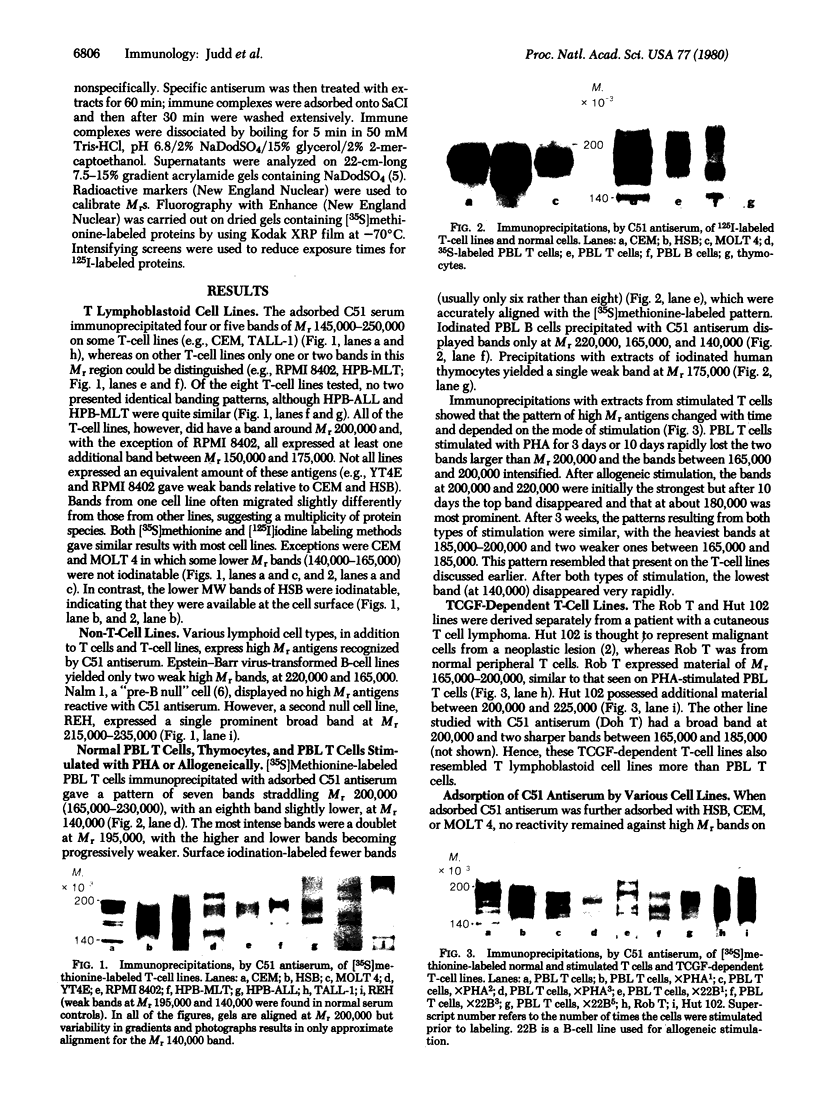
A series of eight high molecular weight (140,000-220,000) glycoproteins on human peripheral T cells were recognized by radioimmunoprecipitation with a rabbit antiserum. The pattern of antigens present on each of eight human T cell lines studied was unique, and no line displayed the range of antigens present on peripheral T cells. The pattern of bands on peripheral T cells changed after allogeneic or lectin stimulation. Adsorption/elution experiments with antiserum showed that some of these proteins were antigenically related, and at least three different groups of proteins were present. Two of these groups could be partially distinguished by their ability to bind to ricin or lentil lectin and by their reactivity with two additional rabbit antisera. On some cell lines, it was found that proteins bound by lentil lectin but not ricin were precursors of higher molecular weight material recognized by ricin. Taken together, the data suggest that these proteins may be the products of a multigenic or multiallelic system, probably equivalent to the murine Ly 5 antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. K., Metzgar R. S. Detection and partial characterization of human T and B lymphocyte membrane antigens with antisera to HSB and SB cell lines. J Immunol. 1978 Jan;120(1):262–271. [PubMed] [Google Scholar]
- Anderson J. K., Metzgar R. S. Further characterization of a human T lymphocyte associated antigen. Clin Exp Immunol. 1979 Aug;37(2):339–347. [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Gahmberg C. G., Kimura A. K., Wigzell H. Activated human T lymphocytes display new surface glycoproteins. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3455–3458. doi: 10.1073/pnas.75.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Karhi K. K., Gahmberg C. G., Rodt H. Molecular identification of T cell-specific antigens on human T lymphocytes and thymocytes. Eur J Immunol. 1980 May;10(5):359–362. doi: 10.1002/eji.1830100508. [DOI] [PubMed] [Google Scholar]
- Dunlap B., Bach F. H., Bach M. L. Cell surface changes in alloantigen activated T lymphocytes. Nature. 1978 Jan 19;271(5642):253–255. doi: 10.1038/271253a0. [DOI] [PubMed] [Google Scholar]
- Ferrara G. B. Identification of new cell surface markers in man: the problem of immunogenicity. Transplant Proc. 1979 Mar;11(1):715–718. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minowada J., Koshiba H., Janossy G., Greaves M. F., Bollum F. J. A Philadelphia chromosome positive human leukaemia cell line (NALM-1) with pre-B characteristics. Leuk Res. 1979;3(4):261–266. doi: 10.1016/0145-2126(79)90050-x. [DOI] [PubMed] [Google Scholar]
- Niaudet P., Greaves M. Diversity of cell surface polypeptides that are recognized by heteroantisera to human T cells. J Immunol. 1980 Mar;124(3):1203–1208. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Scheid M. P. T200 cell surface glycoprotein of the mouse. Polymorphism defined by the Ly-5 system of alloantigens. J Exp Med. 1980 May 1;151(5):1311–1316. doi: 10.1084/jem.151.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. Cell surface polypeptides of murine T-cell clones expressing cytolytic or amplifier activity. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1111–1115. doi: 10.1073/pnas.77.2.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen A. Alloantibodies that react with subsets of human T cells. Tissue Antigens. 1979 Nov;14(5):437–443. doi: 10.1111/j.1399-0039.1979.tb00872.x. [DOI] [PubMed] [Google Scholar]