Abstract
The intercellular distribution of the enzymes and metabolites of assimilatory sulfate reduction and glutathione synthesis was analyzed in maize (Zea mays L. cv LG 9) leaves. Mesophyll cells and strands of bundle-sheath cells from second leaves of 11-d-old maize seedlings were obtained by two different mechanical-isolation methods. Cross-contamination of cell preparations was determined using ribulose bisphosphate carboxylase (EC 4.1.1.39) and nitrate reductase (EC 1.6.6.1) as marker enzymes for bundle-sheath and mesophyll cells, respectively. ATP sulfurylase (EC 2.7.7.4) and adenosine 5′-phosphosulfate sulfotransferase activities were detected almost exclusively in the bundle-sheath cells, whereas GSH synthetase (EC 6.3.2.3) and cyst(e)ine, γ-glutamylcysteine, and glutathione were located predominantly in the mesophyll cells. Feeding experiments using [35S]sulfate with intact leaves indicated that cyst(e)ine was the transport metabolite of reduced sulfur from bundle-sheath to mesophyll cells. This result was corroborated by tracer experiments, which showed that isolated bundle-sheath strands fed with [35S]sulfate secreted radioactive cyst(e)ine as the sole thiol into the resuspending medium. The results presented in this paper show that assimilatory sulfate reduction is restricted to the bundle-sheath cells, whereas the formation of glutathione takes place predominantly in the mesophyll cells, with cyst(e)ine functioning as a transport metabolite between the two cell types.
Maize (Zea mays L.), as a typical C4 plant, is characterized by a compartmentation of carbon assimilation into specific cell types, with CO2 initially being fixed into malate in the MC and then transported into the BSC, where the formation of glycerate 3-phosphate is localized (Black, 1973). Conversely, the reduction of nitrate occurs exclusively in the MC (Moore and Black, 1979). This division of labor is a primary factor contributing to high rates of carbon assimilation (Black, 1973) and nitrogen use efficiency (Brown, 1978) in C4 plants.
The intercellular compartmentation of sulfate assimilation is less well documented. Sulfite reductase (EC 1.8.7.1) and O-acetyl-l-Ser sulfhydrylase (EC 4.2.99.8) activities were found in both cell types at comparable levels (Passera and Ghisi, 1982; Burnell, 1984; Schmutz and Brunold, 1984, 1985). ATPSase (EC 2.7.7.4), the first enzyme in the pathway of assimilatory sulfate reduction, is, however, reported to be predominantly or even exclusively located in the BSC (Gerwick and Black, 1979; Gerwick et al., 1980; Passera and Ghisi, 1982; Burnell, 1984; Schmutz and Brunold, 1984; Ghisi et al., 1986). In addition, the second step in sulfate reduction, catalyzed by APSSTase (Brunold and Rennenberg, 1997), an enzyme that may be identical to 5′-adenylylsulfate reductase recently described by Setya et al. (1996), is essentially restricted to the BSC (Schmutz and Brunold, 1984).
There are no published results concerning the intercellular compartmentation of GSH synthesis. This tripeptide plays an important role in the plant's defense system (Rennenberg and Brunold, 1994). It is involved in various stress situations, such as heavy metal stress (Nussbaum et al., 1988; Rüegsegger and Brunold, 1992; Galli et al., 1996), xenobiotic stress (Farago et al., 1994), and chilling responses (Kocsy et al., 1996). GSH is also an essential factor in the regulation of sulfur nutrition in plants (Brunold and Rennenberg, 1997). Its long-distance transport mediates distribution of reduced sulfur according to the requirements of individual plant organs, and controls sulfate influx into the plant (Rennenberg and Lamoureux, 1990; Herschbach and Rennenberg, 1994; Lappartient and Touraine, 1996). There is also evidence for membrane transport of GSH in an active, carrier-mediated process (Rennenberg and Lamoureux, 1990). These facts, together with the localization of enzymes involved in assimilatory sulfate reduction, raise the question of the nature of thiol compounds transported from BSC to MC in C4 plants.
In this paper we present evidence that cyst(e)ine is the transport metabolite and that GSH synthesis takes place predominantly in MC.
MATERIALS AND METHODS
Fifty maize (Zea mays L. cv LG 9, Limagrain, Ennezat, France) kernels were soaked for 24 h in aerated tap water at room temperature and then transferred to a pot containing 5000 cm3 of moist Perlite (Samen Mauser, Berne, Switzerland). After 3 d in the dark, the seedlings were cultivated in a 16-/8-h photoperiod at 25/20°C with a PPFD of 350 μmol m−2 s−1 and 70% RH. One liter of nutrient solution (Henschel, 1970), modified according to the method of Nussbaum et al. (1988), was added every 3rd d. Second leaves were harvested 10 d after imbibition in the middle of the light period.
Cell Isolation
MC and BSC extracts were obtained by two different mechanical-isolation methods.
Method A Modified after Schmutz and Brunold (1984)
One gram of leaves was cut vertically across the veins in approximately 1-mm segments with a razor blade and transferred to 10 mL of medium containing 2% cellulase and 2% pectinase for protoplast isolation, according to the method of Mills and Joy (1980). The plant material was infiltrated by applying a vacuum and then incubated for 20 min at 30°C. The predigested leaf strips were thoroughly rinsed and then homogenized in 8 mL of 0.1 m Tris-HCl (pH 8.0) containing 20 mm MgCl, 100 mm KCl, and 10 mm dithioerythritol. The extraction was carried out using a Sorvall Omni Mixer for 10 s at 100 V (6,700 rpm) and twice for 15 s at 170 V (11,500 rpm). The homogenate was filtered through a 60-μm nylon net. The filtrate contained the broken MC. The plant material on the nylon net was resuspended in 8 mL of extraction buffer and homogenized again twice for 20 s at 240 V (16,250 rpm), and was then filtered and rinsed. The bundle-sheath strands on the nylon net were collected and broken in 4 mL of extraction buffer using a cooled glass homogenizer.
Method B Using a Roller Device according to the Method of Leegood (1985)
The harvested leaves were cut into 3- to 4-cm sections. Leaf segments, placed on 100 μL of extraction buffer, were rolled once, thus squeezing out MC. The sap from two leaves was collected with a pipette and resuspended in 300 μL of extraction buffer. The rolled leaf laminas were extracted with a cooled glass homogenizer in 4 mL of extraction buffer. The activities of these extracts as well as their thiol content were corrected for pure BSC given that NR is restricted to MC (Moore and Black, 1979; Vaughn and Campbell, 1988).
All extracts were filtered through two layers of 100% viscose fleece (Milette, Migros, Switzerland). Cross-contamination of the cell preparations was determined using Rubisco and NR as marker enzymes for BSC and MC, respectively.
Enzyme Assays
Rubisco activity was determined according to the method of Buchanan and Schürmann (1973) by measuring the nonvolatile radioactivity produced from RuBP and H14CO3− and by following the modifications given by Wyss and Brunold (1979). Incubation was for 10 min at 30°C. NR measurement was carried out according to the method of Neyra and Hagemann (1975) with modifications described by Kast et al. (1995). ATPSase activity was determined by measuring production of ATP from APS and PPi with a luciferin-luciferase system (Schmutz and Brunold, 1982) using a Lumac/3M Biocounter (model M 2010, Lumac, Basel, Switzerland). APSSTase activity was measured as the production of [35S]sulfite, assayed as acid volatile radioactivity formed from [35S]APS (Brunold and Suter, 1990) in the presence of dithioerythritol. GSH synthetase activity was measured as previously reported (Klapheck et al., 1987; Rüegsegger and Brunold, 1992) by the quantification of the reaction product after reverse-phase HPLC separation of its monobromobimane derivative.
The activities of Rubisco, NR, APSSTase, and GSH synthetase were determined immediately after extraction. ATPSase activity, which was stable in the crude extract up to at least 6 h (data not shown), was routinely measured 1 h after the homogenization of the plant material.
Determination of RuBP
RuBP was determined by the incorporation of 14CO2 into an acid-stable product as described by Doulis et al. (1997).
Determination of Cyst(e)ine, γEC, and GSH
Thiols were separated and quantified by reverse-phase HPLC after reduction with NaBH4 and fluorescent labeling with monobromobimane (Newton et al., 1981; Schupp and Rennenberg, 1988) as previously described (Rüegsegger and Brunold, 1992). Recoveries of 88, 90, and 98% were determined for cyst(e)ine, γEC, and GSH, respectively, and were used for calculating the actual amounts of the thiols in the plant material. The measured values were related to average protein content of extracts.
Tracer Experiment
For [35S]sulfate labeling, second leaves were excised and placed into 0.5 mL of nutrient solution containing 75 instead of 750 μm sulfate and 5.55 × 106 Bq of [35S]sulfate. The incubation took place under cultivation conditions. At different times, leaves were immediately extracted with isolation method B. The sections that had been in contact with the nutrient solution were excised and discarded. The experiments were repeated with the method described for determination of thiols, including the acidic extraction.
Measurement of 35S Label in Thiols and Sulfide
The determination of the radioactive sulfide content required an alkaline-extraction method. The extraction buffer consisted of 200 mm 2-(cyclohexyl-amino)-ethanesulfonic acid-NaOH (pH 8.4) containing 1 mm Na2EDTA. Three hundred microliters of extract was reduced on ice with 30 μL of freshly prepared NaBH4 for 30 min. For derivatization, 220 μL of this mixture was added to 20 μL of 15 mm monobromobimane and kept in the dark at room temperature for 15 min. The reaction was stopped with 165 μL of 5% (v/v) acetic acid.
A 50-μL aliquot of each sample was separated by HPLC, as described for the inactive thiols, using a modified gradient of methanol (0–14% methanol in 15 min and then 14–51% methanol in 30 min). Fractions of 0.75 mL were collected in scintillation vials. Two milliliters of Ultima Gold XR scintillation cocktail (Packard, Zürich, Switzerland) was added per fraction, and the radioactivity was counted in a Betamatic V liquid-scintillation counter (Kontron, Zürich, Switzerland).
35S Labeling of Isolated Bundle-Sheath Strands
Two grams of 1-mm leaf segments was blended in 16 mL of medium according to the method of Valle and Heldt (1991) in a polytron with 1-s (20,000 rpm) and 20-s (15,000 rpm) bursts. The bundle-sheath strands were collected on a 280-μm nylon net, washed thoroughly, and resuspended at a level of approximately 50 μg chlorophyll mL−1 resuspending medium (0.3 m sorbitol, 20 mm Tricine-KOH (pH 7.5), 4 mm MgCl2, 10 mm KCl, 2 mm KH2PO4, 1 mm ADP, 10 mm glycerate 3-phosphate, 5 mm glutamate, 5 mm malate, 2 mm O-acetyl-l-Ser, and 0.2 mm K2SO4). The bundle-sheath strands were incubated after the addition of 1.85 × 107 Bq [35S]sulfate mL−1 at 30°C with an irradiance of approximately 1500 μmol m−2 s−1. At different times, supernatants of the suspended bundle-sheath strands were obtained by a 5-min centrifugation at 10,000 rpm at 4°C. The supernatant was treated with 0.3 mm DTT for reducing possibly existing thiols, derivatized, and separated by HPLC as described for cyst(e)ine, γEC, and GSH. The injection volume was 100 μL. Fractions of 0.375 mL were collected and determined as in the tracer experiment with second leaves.
The incubated BSC were washed extensively and extracted in 0.1 n HCl. The determination of 35S-labeled substances was performed as described for the supernatant.
Protein Determination
The protein content of the extracts was measured according to the method of Bradford (1976) with BSA as the standard.
Chlorophyll Determination
Acetone was added to the bundle-sheath strands and the mixture was shaken several times for 30 min in the dark. Chlorophyll was measured in the clear supernatant after centrifugation using the procedure of MacKinney (1941).
Statistical Analysis
The Mann-Whitney rank sum test (SigmaStat for Windows, version 1.0, 1992–1994, Jandel, San Rafael, CA) was used to determine significant differences in the enzyme activities and concentrations of metabolites between extracts of BSC and MC.
Chemicals
Monobromobimane was obtained from Calbiochem and γEC was obtained from Nacalai Tesque (Kyoto, Japan). [35S]APS was prepared according to the method of Li and Schiff (1991), using ATPSase from Sigma and [35S]sulfate from the Radiochemical Centre (Amersham). All other chemicals were purchased from Fluka.
RESULTS
Rubisco was assayed as a marker for BSC and NR was assayed as a marker for MC to determine the level of cross-contamination in the cell preparations. The activity of Rubisco indicated that there was less than 10% of BSC in the mesophyll extracts obtained by method A (Fig. 1A) and less than 5% in those obtained with the roller device (Fig. 1B). Based on the distribution of NR activity, procedure A yielded very pure BSC extracts with a contamination of about 5% (Fig. 1A), whereas the rolled leaf laminas in method B still contained an appreciable amount of MC (Fig. 1B).
Figure 1.
A, Intercellular localization of activities of Rubisco (first panel), NR (second panel), APSSTase (third panel), and ATPSase (fourth panel) in second leaves of 11-d-old maize plants. MC (open bars) and BSC (black bars) extracts were obtained by isolation procedure A. Mean values ± sd of four different experiments are presented. The values of the extracts of the two cell types differ significantly from each other (P ≤ 0.05). B, Intercellular localization of activities of Rubisco (top), NR (middle), and GSH synthetase (bottom) in MC (open bars) and BSC (black bars) extracts obtained by isolation method B. Means ± sd of five independent experiments are presented. The values of the extracts of the two cell types differ significantly from each other (P ≤ 0.05).
The distribution of ATPSase and APSSTase (the two first enzymes of assimilatory sulfate reduction) between BSC and MC is presented in Figure 1A. Both are predominantly located in BSC. The APSSTase activity in the MC extracts was at the same relative level as Rubisco activity, indicating that this enzyme is exclusively active in the BSC. The relative level of ATPSase activity detected in the MC extract is higher than expected from contaminating BSC, indicating that this enzyme of sulfate assimilation is active in both cell types, with more than 90% of total activity in BSC.
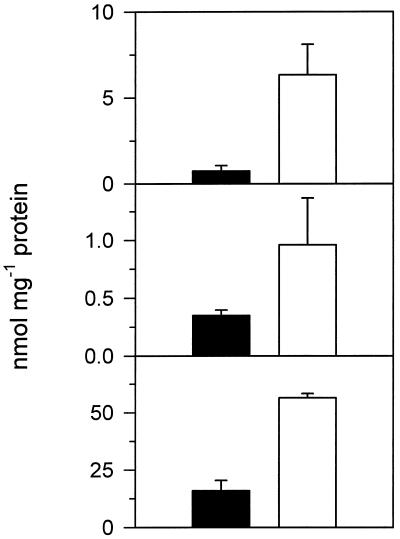
Figures 1B and 2 show the compartmentation of GSH synthetase and thiols. After correction for contamination, only 22% of the total GSH synthetase activity was located in BSC (Fig. 1B). Also, the amounts of the thiols cyst(e)ine, γEC, and GSH in the BSC extracts were significantly lower compared with those in the MC preparations (Fig. 2). The content of RuBP in the MC extract was used to calculate the degree of contamination by low-Mr compounds originating in the BSC (Doulis et al., 1997). The RuBP distribution between extracts of BSC and MC was similar to that of Rubisco (data not shown), indicating that the concentrations of the thiols were not a consequence of leaky plasma membranes.
Figure 2.
Contents of cyst(e)ine (top), γEC (middle), and GSH (bottom) in BSC (black bars) and MC (open bars). Cell types were obtained by isolation procedure B. The values of BSC were corrected for contamination by MC. Means ± sd of four independent experiments are presented. The values of the two cell types differ significantly from each other (P ≤ 0.05).
Mixing of extracts from BSC and MC gave additive activities of all measured enzymes, indicating that no inactivator was present in either cell type and that the activities were comparable to those of the whole leaves, showing that no activity was lost during cell separation (data not shown).
The distribution of the activities of the two first enzymes of assimilatory sulfate reduction indicated an almost exclusive localization of this pathway in BSC, whereas GSH synthesis predominantly took place in MC. This opened the question of intermediates being synthesized in BSC and transported into MC. We addressed this question by feeding excised leaves with [35S]sulfate and analyzing the radioactivity of the thiols in the MC in a time-course experiment. The percentage of 35S-radiolabeled thiols in MC extracts from such an experiment is presented in Figure 3A. Seventy-five percent of the radioactivity of the thiols in MC was detected in cyst(e)ine after a 5-min incubation, whereas after 90 min the label was found at a comparable percentage in GSH. The recovery of cyst(e)ine, γEC, and GSH was 36 to 37% in comparison with the acidic procedure as a standard method for thiol extraction (data not shown). Only negligible amounts of [35S]sulfide were detected at all times, although the recovery rate of sulfide with the alkaline-extraction method was as high as those of the thiols at any time. The repetition of the experiment, including the acidic extraction, resulted in the same time course of radioactivity in the thiols (Fig. 3B). The amount of 35S-radiolabeled thiols in the BSC during the incubation time was very low compared with those in MC (Fig. 3C). After a 90-min incubation, 75% of the total radioactivity in the thiols was measured in GSH of MC. Calculated on the basis of the specific activity of [35S]sulfate fed to the leaves, this percentage corresponded to 0.13 nmol GSH mg−1 protein.
Figure 3.
A, Radioactivity in cyst(e)ine (•), γEC (▴), and GSH (▾) in MC as a percentage of total radioactivity in the three compounds after an alkaline-extraction method. Radioactivity was supplied as [35S]sulfate by incubating 11-d-old second leaves for 5 to 90 min. Values of two independent experiments at each time are presented. B, Radioactivity in cyst(e)ine (•), γEC (▴), and GSH (▾) in MC as a percentage of total radioactivity in the three compounds after an acid-extraction method. Means ± sd of four independent experiments at each time are presented. C, Radioactivity in cyst(e)ine (top), γEC (middle), and GSH (bottom) in BSC (left) and MC (right) as a percentage of the sum of the three compounds in both cell types after acidic extraction. The values of BSC were corrected for contamination by MC. Mean values ± sd of four independent experiments at each time are presented.
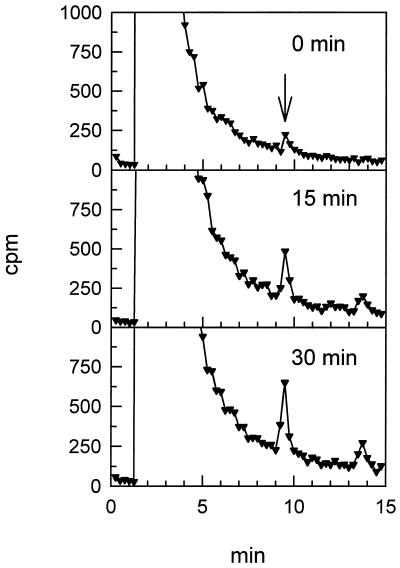
Taken together with the distribution of the enzyme activities involved, these results indicate that cyst(e)ine is the thiol transported from BSC to MC, where it is used for GSH synthesis. To corroborate this result, we incubated isolated bundle-sheath strands in a medium containing [35S]sulfate. These experiments resulted in only one radioactive product detectable in the resuspending medium. Figure 4 shows the HPLC chromatographs of 35S-labeled substances in the resuspending medium for different incubation times. The peak with a retention time of 2.5 min represents the supplied [35S]sulfate, and the signal at 13.5 min resulted from the washing of the column. The only other substance detected with appreciable radioactivity was identified as cyst(e)ine by injecting monobromobimane derivatives of Cys, γEC, and GSH and measuring them with a fluorescence detector. Calculated on the basis of the specific activity of [35S]sulfate fed to the isolated BSC, the amounts of synthesized cyst(e)ine in the 15- and 30-min incubations were 1.2 and 2.4 nmol mg−1 chlorophyll, respectively. The small peak at the beginning of incubation corresponding to 0.5 nmol cyst(e)ine mg−1 chlorophyll can be most likely explained by synthesis of the product during separation of BSC from resuspending medium. In a parallel experiment, in which the BSC were centrifuged first and the resulting supernatant was incubated with [35S]sulfate, no cyst(e)ine was detectable (data not shown).
Figure 4.
Separation of 35S compounds in the incubation medium of isolated bundle-sheath strands by HPLC. Incubation with [35S]sulfate was for 0, 15, and 30 min. The arrow indicates the retention time of the monobromobimane derivative of cyst(e)ine.
To exclude the possibility that the above-mentioned results were due to leaky plasma membranes we measured RuBP as a marker (Doulis et al., 1997). The similar amounts of RuBP that were detected in BSC before and after the incubation indicated that the chloroplasts and plasma membranes of these cells were not leaky for all low-Mr molecules caused by the exposure (data not shown).
DISCUSSION
Because of their lower stability, MC disrupt preferentially in a mechanical isolation procedure. This characteristic makes it possible to obtain very pure MC extracts within seconds using a roller device (Leegood, 1985). The rapid extraction method is, therefore, excellent for short-time tracer experiments and measurements of enzyme activities in MC. If pure extracts of both MC and BSC are of importance, then the leaves should be predigested in a medium for protoplast isolation (Mills and Joy, 1980) before the cell types are separated mechanically (Schmutz and Brunold, 1984).
Bundle-sheath strands consisting of a segment of vascular bundle surrounded by BSC of high functional integrity and metabolic competence can be obtained easily by blending leaf segments in an appropriate medium with a polytron (Valle and Heldt, 1991). The intact plasmodesmata that originally connected the bundle-sheath cytosol with MC are permeable to molecules up to a molecular mass of about 900 D (Valle et al., 1989). This means that the highly intact BSC are accessible to substrates added to the resuspending medium, thus enabling metabolic studies.
The findings presented in this paper suggest a cooperation between BSC and MC in sulfate reduction and GSH synthesis, as shown in Figure 5. The scheme compares the intercellular localization of sulfur assimilation with the compartmentation of reactions involved in carbon and nitrogen assimilation. The distribution of ATPSase and APSSTase activities in maize was consistent with previous results, which indicated a preponderant or even exclusive localization of assimilatory sulfate reduction to BSC (Burnell, 1984; Schmutz and Brunold, 1984, 1985; Ghisi et al., 1986). The high percentage of GSH synthetase activity in MC indicates that this cell type is the main site for synthesis of the tripeptide in maize leaves and correlates with the high levels of GSH determined in MC, as compared with BSC.
Figure 5.
Diagram of the hypothetical intercellular distribution of CO2, nitrate, and sulfate assimilation and GSH synthesis in maize leaves. Cys and Gly exported from BSC and Glu formed in MC are used for GSH synthesis. VB, Vascular bundle.
Information about intermediates of sulfate assimilation transported from BSC to MC was provided by feeding intact leaves with [35S]sulfate and then rapidly extracting the MC. The immediate appearance of 35S-labeled cyst(e)ine in the MC indicated that this amino acid is the molecule transporting reduced sulfur. This hypothesis was corroborated by the finding that isolated bundle-sheath strands released cyst(e)ine into the resuspending medium. Since no cyst(e)ine was detectable in a parallel experiment in which the BSC were centrifuged first, and only the resulting supernatant of the suspension was incubated with [35S]sulfate, we can be sure that the [35S]cyst(e)ine was synthesized in the intact BSC and then transported into the resuspension medium. In a physiological context this cyst(e)ine would be exported into MC.
The compartmentation of CO2 and nitrate assimilation in C4 plants leads to important advantages with regard to carbon (Black, 1973) and nitrogen use efficiency (Brown, 1978). The physiological reason for the cell-type-specific localization of sulfate assimilation and GSH synthesis between BSC and MC is not clear. Sulfate assimilation is located in BSC. These enclose the vascular bundles, which are the source of sulfate originating from the soil. It is plausible that reduced levels of PSII (Sheen and Bogorad, 1988; Pfündel et al., 1996) and correspondingly lower O2 concentrations in BSC compared with MC could prevent autooxidation of the reaction intermediates of assimilatory sulfate reduction, i.e. sulfite and/or sulfide. A second reason might be that Gly decarboxylase and Ser hydroxymethyl transferase (enzymes synthesizing Ser, the precursor of Cys) are localized exclusively in BSC of C4 plants (Gardeström et al., 1978; Ohnishi and Kanai, 1983; Becker et al., 1993). Since Gly is also produced in this cell type (Martin et al., 1983; Farineau et al., 1984; Yamaya and Oaks, 1988), GSH synthesis in the MC could proceed using Cys and Gly formed in BSC and Glu from MC (Fig. 5). A predominant localization of GSH synthesis and correspondingly high levels of GSH in MC would be advantageous for the plant to react against reactive oxygen species (Foyer et al., 1994; Doulis et al., 1997), which are probably produced preferentially in this cell type in various stress situations (Rennenberg and Brunold, 1994) and during pathogen attack (Low and Merida, 1996).
ACKNOWLEDGMENTS
We would like to thank Dr. Stanislav Kopriva and Thomas Imhasly for helpful discussions and Dr. Andrew Fleming for improving the style of the manuscript.
Abbreviations:
- APS
adenosine 5′-phosphosulfate
- APSSTase
adenosine 5′-phosphosulfate sulfotransferase
- ATPSase
adenosine triphosphate sulfurylase
- BSC
bundle-sheath cell(s)
- γEC
γ-glutamylcysteine
- MC
mesophyll cell(s)
- NR
nitrate reductase
- RuBP
ribulose-1,5-bisphosphate
Footnotes
This work was supported by the Swiss National Science Foundation. The term “Cys” is used when it is clear that cystine is not involved; “cyst(e)ine” is used for an undefined mixture of Cys and cystine. The concentrations are expressed in all cases relative to Cys.
LITERATURE CITED
- Becker TW, Perrot-Rechenmann C, Suzuki A, Hirel B. Subcellular and immunocytochemical localization of the enzymes involved in ammonia assimilation in mesophyll and bundle-sheath cells of maize leaves. Planta. 1993;191:129–136. [Google Scholar]
- Black CC. Photosynthetic carbon fixation in relation to net CO2 uptake. Annu Rev Plant Physiol. 1973;24:253–286. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown RH. A difference in N use efficiency in C3 and C4 plants and its implications in adaptation and evolution. Crop Sci. 1978;18:93–98. [Google Scholar]
- Brunold C, Rennenberg H. Regulation of sulfur metabolism in plants: first molecular approaches. Prog Bot. 1997;58:164–186. [Google Scholar]
- Brunold C, Suter M. Adenosine 5′-phosphosulfate sulfotransferase. Methods Plant Biochem. 1990;3:339–343. [Google Scholar]
- Buchanan BB, Schürmann P. Regulation of ribulose 1,5-diphosphate carboxylase in the photosynthetic assimilation of carbon dioxide. J Biol Chem. 1973;248:4956–4964. [PubMed] [Google Scholar]
- Burnell JN. Sulfate assimilation in C4 plants. Plant Physiol. 1984;75:873–875. doi: 10.1104/pp.75.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997;114:1031–1037. doi: 10.1104/pp.114.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago S, Brunold C, Kreuz K. Herbicide safeners and glutathione metabolism. Physiol Plant. 1994;91:537–542. [Google Scholar]
- Farineau J, Lelandais M, Morot-Gaudry JF. Physiol Plant. 1984;60:208–214. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Galli U, Schüepp H, Brunold C. Thiols in cadmium- and copper-treated maize (Zea mays L.) Planta. 1996;198:139–143. [Google Scholar]
- Gardeström P, Edwards GE, Henricson D, Ericson I. The localization of serine hydroxymethyl-transferase in leaves of C3 and C4 species. Plant Cell Physiol. 1978;19:1399–1405. [Google Scholar]
- Gerwick BC, Black CC. Sulfur assimilation in C4 plants. Plant Physiol. 1979;64:590–593. doi: 10.1104/pp.64.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwick BC, Ku SB, Black CC. Initiation of sulfate activation: a variation in C4 photosynthesis plants. Science. 1980;209:513–515. doi: 10.1126/science.209.4455.513. [DOI] [PubMed] [Google Scholar]
- Ghisi R, Anaclerio F, Passera C. Effects of nitrogen deprivation on the ATP-sulphurylase and O-acetylserine sulphydrylase activities of mesophyll protoplasts and bundle sheath strands of maize leaves. Biol Plant. 1986;28:114–119. [Google Scholar]
- Henschel G (1970) Untersuchungen über die Aufnahme von 15N-markiertem Harnstoff bei Phaseolus vulgaris L. PhD thesis. University of Hohenheim, Stuttgart, Germany
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on net uptake of sulphate and sulphate transport in tobacco plants. J Exp Bot. 1994;45:1069–1076. [Google Scholar]
- Kast D, Stalder M, Rüegsegger A, Galli U, Brunold C. Effects of NO2 and nitrate on sulfate assimilation in maize. J Plant Physiol. 1995;147:9–14. [Google Scholar]
- Klapheck S, Latus C, Bergmann L. Localization of glutathione synthetase and distribution of glutathione in leaf cells of Pisum sativum L. J Plant Physiol. 1987;131:123–131. [Google Scholar]
- Kocsy G, Brunner M, Rüegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivities to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulfurylase activity and SO42− uptake in intact canola. Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegood RC. The intercellular compartmentation of metabolites in leaves of Zea mays L. Planta. 1985;164:163–171. doi: 10.1007/BF00396078. [DOI] [PubMed] [Google Scholar]
- Li J, Schiff JA. Purification and properties of adenosine 5′-phosphosulfate sulphotransferase from Euglena. Biochem J. 1991;274:355–360. doi: 10.1042/bj2740355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- MacKinney G. Absorption of light by chlorophyll solutions. Biol Chem. 1941;140:312–322. [Google Scholar]
- Martin F, Winspear MJ, MacFarlane JD, Oaks A. Effect of methionine sulfoximine on the accumulation of ammonia in C3 and C4 leaves. Plant Physiol. 1983;71:177–181. doi: 10.1104/pp.71.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills WR, Joy KW. A method for isolation of purified physiologically active chloroplasts, used to study the intracellular distribution of amino acids in pea leaves. Planta. 1980;148:75–83. doi: 10.1007/BF00385445. [DOI] [PubMed] [Google Scholar]
- Moore RC, Black CC. Nitrogen assimilation pathways in leaf mesophyll and bundle sheath cells of C4 photosynthesis plants formulated from comparative studies with Digitaria sanguinalis (L.) Scop. Plant Physiol. 1979;64:309–313. doi: 10.1104/pp.64.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton GL, Dorian R, Fahey RC. Analysis of biological thiols: derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal Biochem. 1981;114:383–387. doi: 10.1016/0003-2697(81)90498-x. [DOI] [PubMed] [Google Scholar]
- Neyra CA, Hagemann RH. Nitrate uptake and induction of nitrate reductase in excised corn roots. Plant Physiol. 1975;56:692–695. doi: 10.1104/pp.56.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum S, Schmutz D, Brunold C. Regulation of assimilatory sulfate reduction by cadmium in Zea mays L. Plant Physiol. 1988;88:1407–1410. doi: 10.1104/pp.88.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Kanai R. Differentiation of photorespiratory activity between mesophyll and bundle sheath cells of C4 plants. 1. Glycine oxidation by mitochondria. Plant Cell Physiol. 1983;24:1411–1420. [Google Scholar]
- Passera C, Ghisi R. ATP sulphurylase and O-acetylserine sulphydrylase in isolated mesophyll protoplasts and bundle sheath strands of S-deprived maize leaves. J Exp Bot. 1982;33:432–438. [Google Scholar]
- Pfündel E, Nagel E, Meister A. Analyzing the light energy distribution in the photosynthetic apparatus of C4 plants using highly purified mesophyll and bundle-sheath thylakoids. Plant Physiol. 1996;112:1055–1070. doi: 10.1104/pp.112.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H, Brunold C. Significance of glutathione metabolism in plants under stress. Prog Bot. 1994;55:144–156. [Google Scholar]
- Rennenberg H, Lamoureux GL. Physiological processes that modulate the concentration of glutathione in plant cells. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulphur Nutrition and Sulphur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 53–65. [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γglutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz D, Brunold C. Rapid and simple measurement of ATP sulfurylase activity in crude plant extracts using an ATP meter for bioluminescence determination. Anal Biochem. 1982;121:151–155. doi: 10.1016/0003-2697(82)90569-3. [DOI] [PubMed] [Google Scholar]
- Schmutz D, Brunold C. Intercellular localization of assimilatory sulfate reduction in leaves of Zea mays and Triticum aestivum. Plant Physiol. 1984;74:866–870. doi: 10.1104/pp.74.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz D, Brunold C. Localization of nitrite and sulfite reductase in bundle sheath and mesophyll cells of maize leaves. Physiol Plant. 1985;64:523–528. [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione content of spruce needles (Picea abies L.) Plant Sci. 1988;57:113–117. [Google Scholar]
- Setya A, Murillo M, Leustek T. Sulfate reduction in higher plants: molecular evidence for a novel 5′-adenylylsulfate reductase. Proc Natl Acad Sci USA. 1996;93:13383–13388. doi: 10.1073/pnas.93.23.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen JY, Bogorad L. Differential expression in bundle sheath and mesophyll cells of maize of genes for photosystem II components encoded by the plastid genome. Plant Physiol. 1988;86:1020–1026. doi: 10.1104/pp.86.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle EM, Craig S, Hatch MD, Heldt HW. Permeability and ultrastructure of bundle sheath cells isolated from C4 plants: structure-function studies and the role of plasmodesmata. Bot Acta. 1989;102:276–282. [Google Scholar]
- Valle EM, Heldt HW. Alanine synthesis by bundle sheath cells of maize. Plant Physiol. 1991;95:839–845. doi: 10.1104/pp.95.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn KC, Campbell WH. Immunogold localization of nitrate reductase in maize leaves. Plant Physiol. 1988;88:1354–1357. doi: 10.1104/pp.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss HR, Brunold C. Regulation of adenosine 5′-phosphosulfate sulfotransferase activity by H2S and cysteine in primary leaves of Phaseolus vulgaris L. Planta. 1979;147:37–42. doi: 10.1007/BF00384588. [DOI] [PubMed] [Google Scholar]
- Yamaya T, Oaks A. Distribution of two isoforms of glutamine synthetase in bundle sheath and mesophyll cells of corn leaves. Physiol Plant. 1988;72:23–28. [Google Scholar]