Abstract
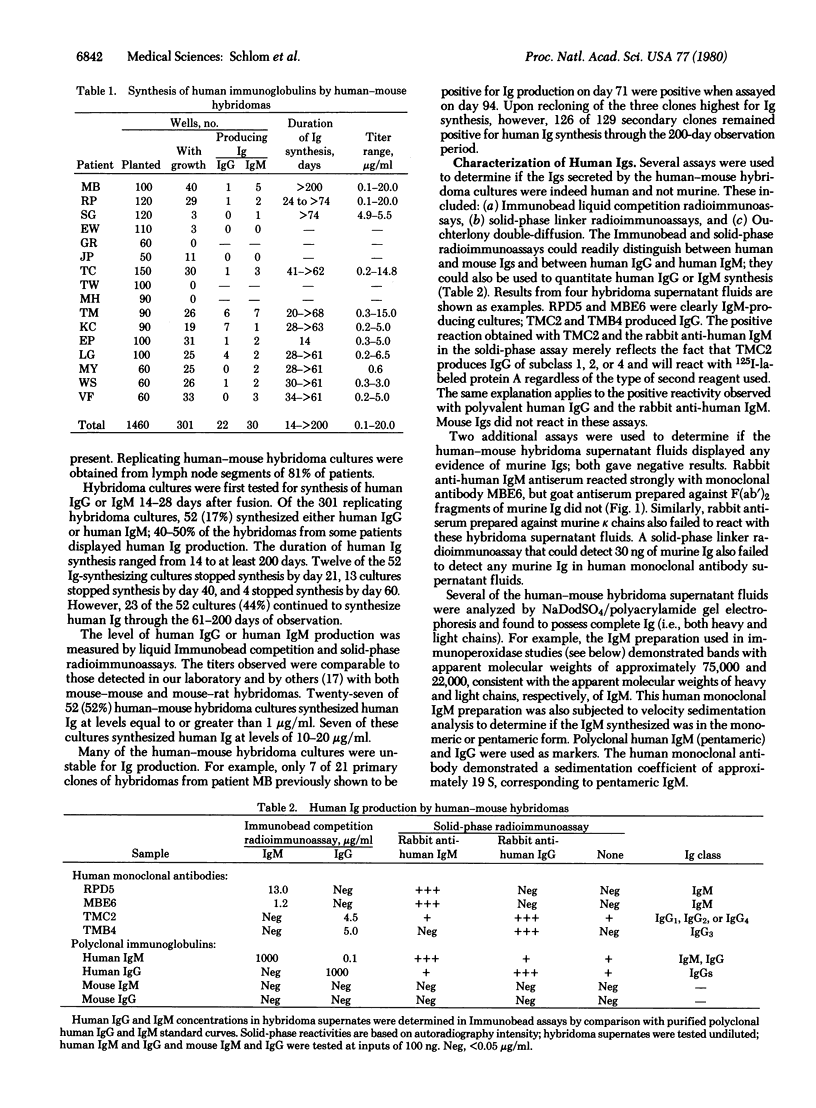
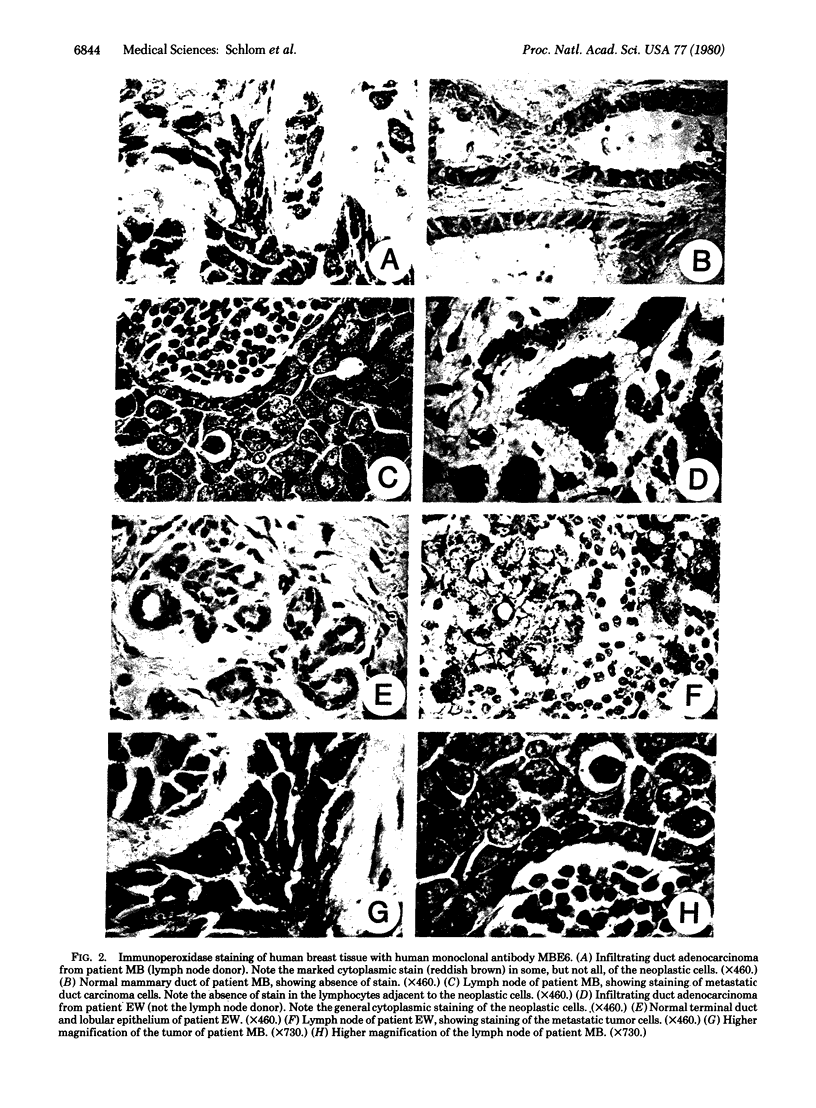
Lymphocytes from lymph nodes obtained at mastectomy in breast cancer patients have been fused with murine nonproducer myeloma cells to obtain human-mouse hybridoma cultures that synthesize human monoclonal antibodies. To date, 52 hybridoma cultures synthesizing either human IgG or human IgM have been obtained from lymph nodes of 13 patients. Ig production was stable in many of these cloned cultures through 60-200 days of observation. Levels of human Ig synthesis ranged from 0.1 to 20 microgram/ml of supernatant fluid. The immunological reactivities of the human Igs were assayed on tissue sections by using the immunoperoxidase technique. Several of the human monoclonal antibodies demonstrated preferential binding to human mammary tumor cells. One human IgM monoclonal antibody was used to discriminate between mammary carcinoma cells (from 55 of 59 patients) and normal mammary epithelial cells, stroma, or lymphocytes of the same breast. Decreased binding to some of the benign breast tumors tested and to selected non-breast adenocarcinomas was also observed. This same human monoclonal antibody, however, reacted significantly with metastatic mammary carcinoma cells in lymph nodes of breast cancer patients, with no binding to normal lymphocytes or to stroma of the same node. These studies demonstrate that stable clones of human-mouse hybridomas can be generated by using lymph nodes of mastectomy patients and that clones can be selected which synthesize human monoclonal antibodies reactive with human mammary carcinoma cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avis F., Avis I., Newsome J. F., Haughton G. Antigenic cross-reactivity between adenocarcinoma of the breast and fibrocystic disease of the breast. J Natl Cancer Inst. 1976 Jan;56(1):17–25. doi: 10.1093/jnci/56.1.17. [DOI] [PubMed] [Google Scholar]
- Black M. M., Zachrau R. E., Shore B., Dion A. S., Leis H. P., Jr Cellular immunity to autologous breast cancer and RIII-murine mammary tumor virus preparations. Cancer Res. 1978 Jul;38(7):2068–2076. [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Hollinshead A. C., Jaffurs W. T., Alpert L. K., Harris J. E., Herberman R. B. Isolation and identification of soluble skin-reactive membrane antigens of malignant and normal human breast cells. Cancer Res. 1974 Nov;34(11):2961–2968. [PubMed] [Google Scholar]
- Howard D. R., Taylor C. R. A method for distinguishing benign from malignant breast lesions utilizing antibody present in normal human sera. Cancer. 1979 Jun;43(6):2279–2287. doi: 10.1002/1097-0142(197906)43:6<2279::aid-cncr2820430618>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Leung J. P., Bordin G. M., Nakamura R. M., DeHeer D. H., Edgington T. S. Frequency of association of mammary tumor glycoprotein antigen and other markers with human breast tumors. Cancer Res. 1979 Jun;39(6 Pt 1):2057–2061. [PubMed] [Google Scholar]
- Levy R., Dilley J. Rescue of immunoglobulin secretion from human neoplastic lymphoid cells by somatic cell hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2411–2415. doi: 10.1073/pnas.75.5.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa-Tejada R., Keydar I., Ramanarayanan M., Ohno T., Fenoglio C., Spiegelman S. Detection in human breast carcinomas of an antigen immunologically related to a group-specific antigen of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1529–1533. doi: 10.1073/pnas.75.3.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. R., Heppner G. H. Immunologic heterogeneity of tumor cell subpopulations from a single mouse mammary tumor. J Natl Cancer Inst. 1979 Dec;63(6):1457–1463. [PubMed] [Google Scholar]
- Schwaber J. Immunoglobulin production by a human-mouse somatic cell hybrid. Exp Cell Res. 1975 Jul;93(2):343–354. doi: 10.1016/0014-4827(75)90459-0. [DOI] [PubMed] [Google Scholar]
- Sheikh K. M., Quismorio F. P., Friou G. J., Lee Y. T. Ductular carcinoma of the breast: serum antibodies to tumor-associated antigens. Cancer. 1979 Dec;44(6):2083–2089. doi: 10.1002/1097-0142(197912)44:6<2083::aid-cncr2820440619>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Smith M., Hirschhorn K. Location of the genes for human heavy chain immunoglobulin to chromosome 6. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3367–3371. doi: 10.1073/pnas.75.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer G. F., Desai P. R., Murthy M. S., Scanlon E. F. Human carcinoma-associated precursor antigens of the NM blood group system. J Surg Oncol. 1979;11(2):95–106. doi: 10.1002/jso.2930110204. [DOI] [PubMed] [Google Scholar]
- St George J. A., Cardiff R. D., Young L. J., Faulkin L. J. Immunocytochemical distribution of mouse mammary tumor virus antigens in BALB/cfC3H mammary epithelium. J Natl Cancer Inst. 1979 Sep;63(3):813–820. doi: 10.1093/jnci/63.3.813. [DOI] [PubMed] [Google Scholar]











