Abstract
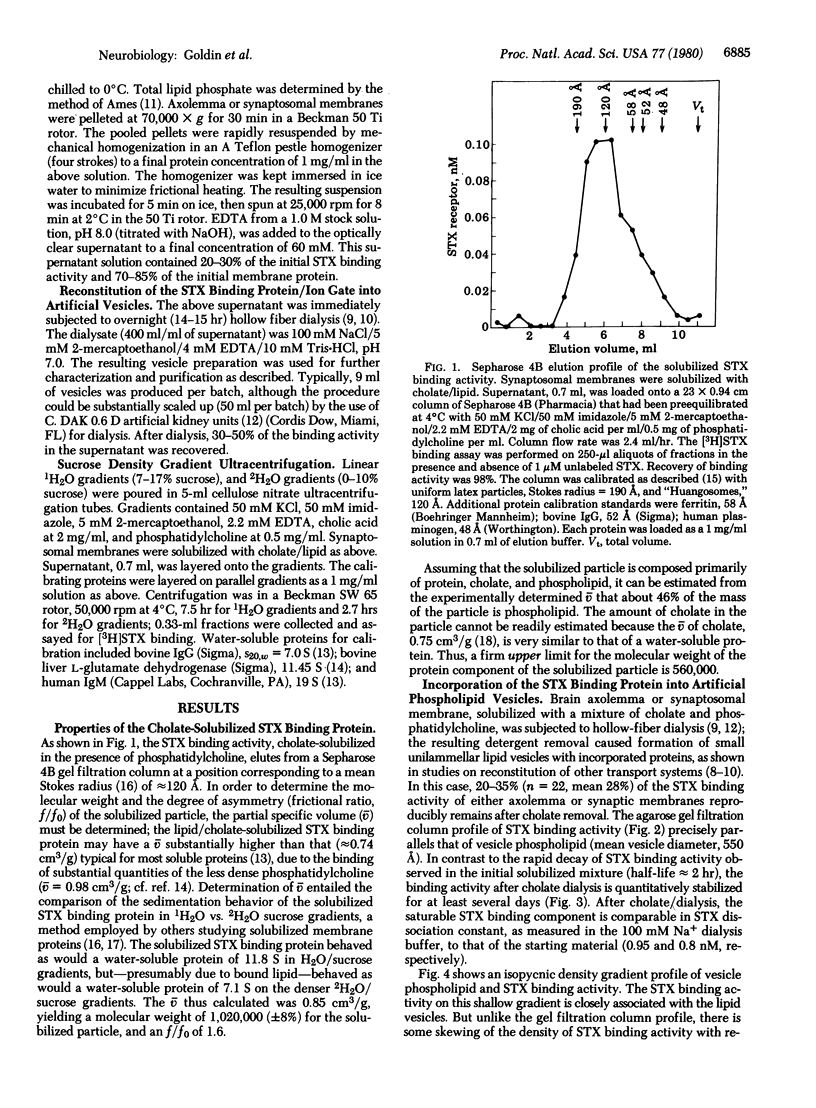
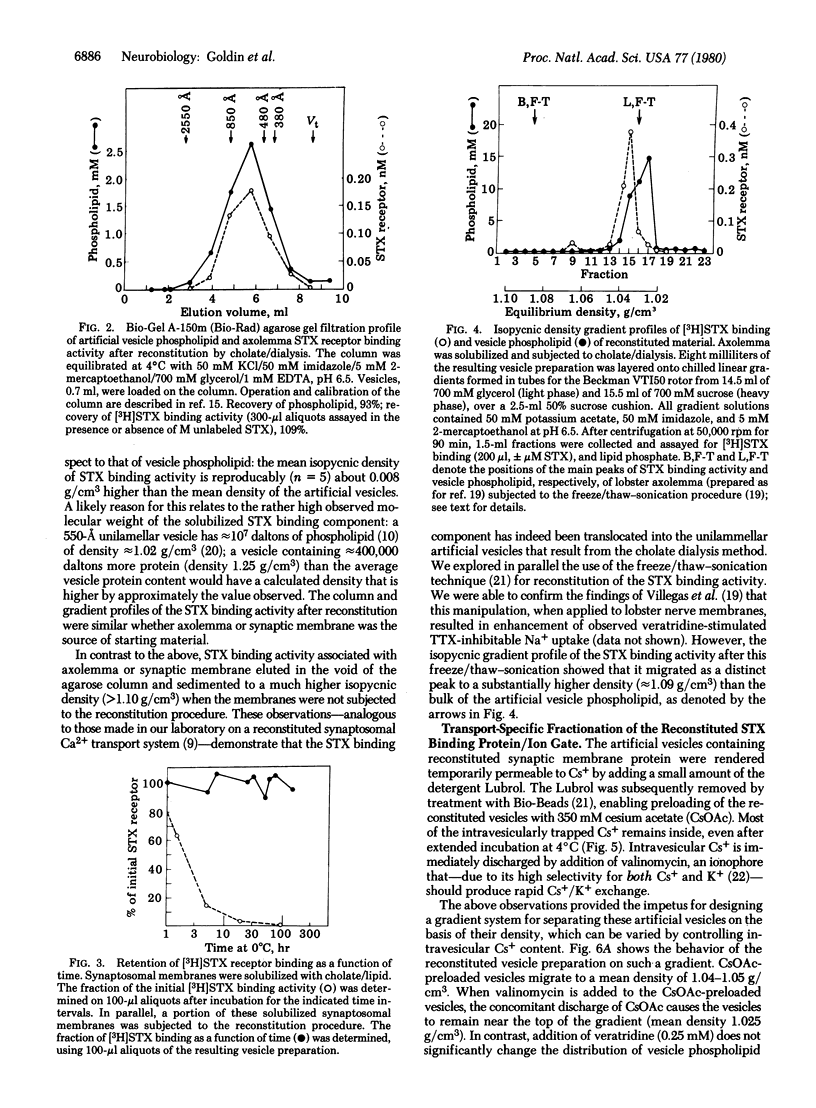
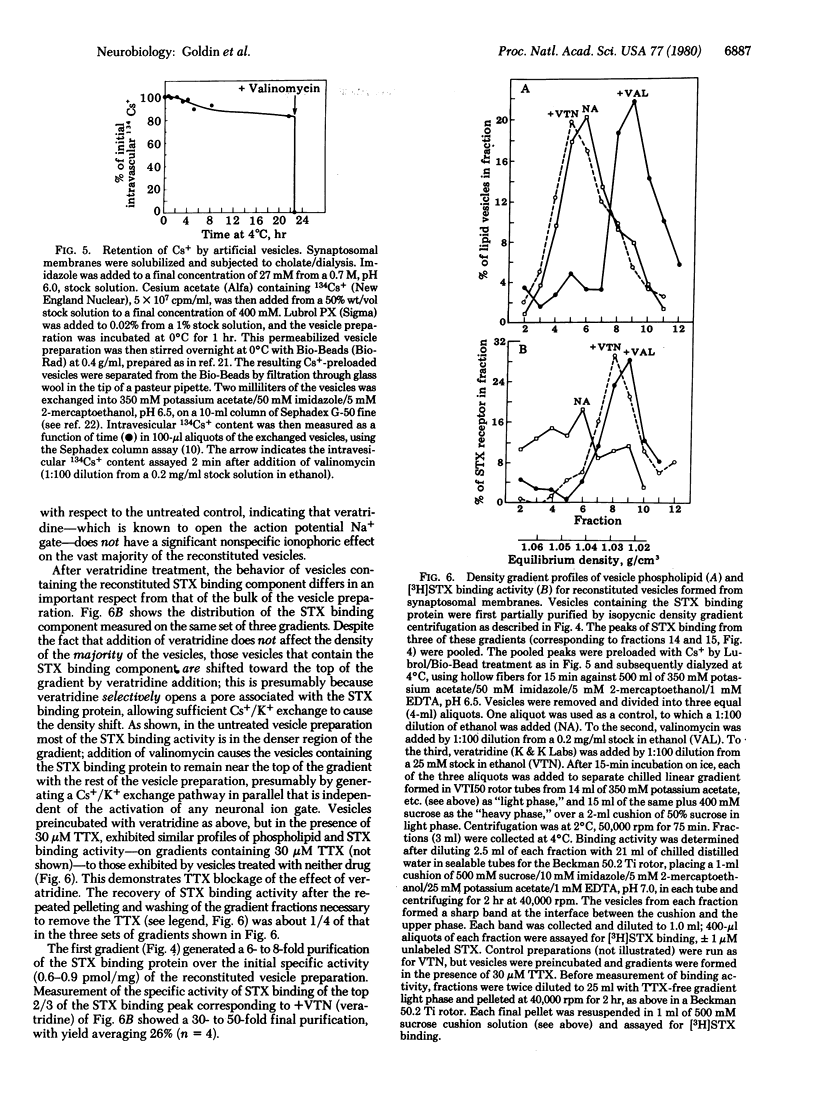
The saxitoxin (STX) binding protein has been solubilized by sodium cholate, both from axolemma and from synaptosomal membranes of mammalian brain. On the basis of agarose gel filtration and sedimentation properties in H2O and 2H2O, the solubilized particle has the following molecular properties: Stokes radius, 120 A; partial specific volume, 0.85 cm3/g; mass, 1,020,000 daltons; frictional ratio f/fo, 1.6. The solubilized STX binding protein was incorporated into unilamellar (approximately 550-A) artificial phosphatidylcholine vesicles. Based on the expectation that the STX binding protein contains functional monovalent cation gating activity ("action potential Na+ gate") that can be activated by veratridine and inhibited by tetrodotoxin, a strategy was devised for partial purification of the reconstituted sodium gate/STX binding protein by "transport-specific fractionation." When the entire vesicle population was preloaded with 0.4 M cesium ion, addition of veratridine allowed Cs+ efflux from specifically those vesicles containing the ion gate; the concomitant reduction in intravesicular density permitted the ion gate/STX binding protein to be fractionated on density gradients. These observations demonstrate functional reconstitution and partial (30- to 50-fold) purification of the STX binding protein/Na+ gate of mammalian brain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi R. L., Cohen S. A., Murphy L. E. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin S. M. Active transport of sodium and potassium ions by the sodium and potassium ion-activated adenosine triphosphatase from renal medulla. Reconstitution of the purified enzyme into a well defined in vitro transport system. J Biol Chem. 1977 Aug 25;252(16):5630–5642. [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Hinkle P. C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J Biol Chem. 1977 Oct 25;252(20):7384–7390. [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Changeux J. P. Studies on the cholinergic receptor protein from Electrophorus electricus. Effect of detergents on some hydrodynamic properties of the receptor protein in solution. FEBS Lett. 1972 Jul 15;24(1):63–68. doi: 10.1016/0014-5793(72)80827-5. [DOI] [PubMed] [Google Scholar]
- Neer E. J. The size of adenylate cyclase. J Biol Chem. 1974 Oct 25;249(20):6527–6531. [PubMed] [Google Scholar]
- Papazian D., Rahamimoff H., Goldin S. M. Reconstitution and purification by "transport specificity fractionation" of an ATP-dependent calcium transport component from synaptosome-derived vesicles. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3708–3712. doi: 10.1073/pnas.76.8.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisler E., Pouyet J., Eisenberg H. Molecular weights, association, and frictional resistance of bovine liver glutamate dehydrogenase at low concentrations. Equilibrium and velocity sedimentation, light-scattering studies, and settling experiments with macroscopic models of the enzyme oligomer. Biochemistry. 1970 Jul 21;9(15):3095–3102. doi: 10.1021/bi00817a600. [DOI] [PubMed] [Google Scholar]
- Rhoden V. A., Goldin S. M. The binding of saxitoxin to axolemma of mammalian brain. Cooperative competition between saxitoxin and sodium ion. J Biol Chem. 1979 Nov 25;254(22):11199–11201. [PubMed] [Google Scholar]
- Rhoden V., Goldin S. M. Formation of unilamellar lipid vesicles of controllable dimensions by detergent dialysis. Biochemistry. 1979 Sep 18;18(19):4173–4176. doi: 10.1021/bi00586a020. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Villegas R., Villegas G. M., Barnola F. V., Racker E. Incorporation of the sodium channel of lobster nerve into artificial liposomes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):210–217. doi: 10.1016/0006-291x(77)90082-1. [DOI] [PubMed] [Google Scholar]


