Abstract
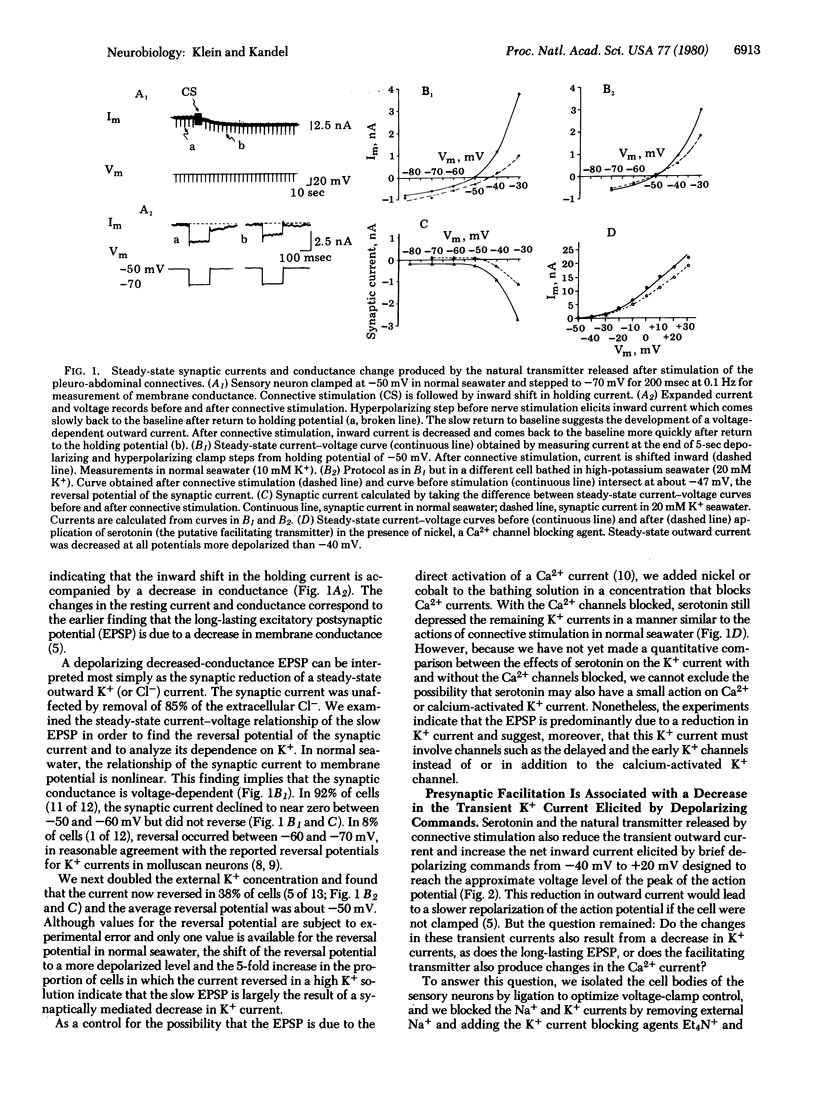
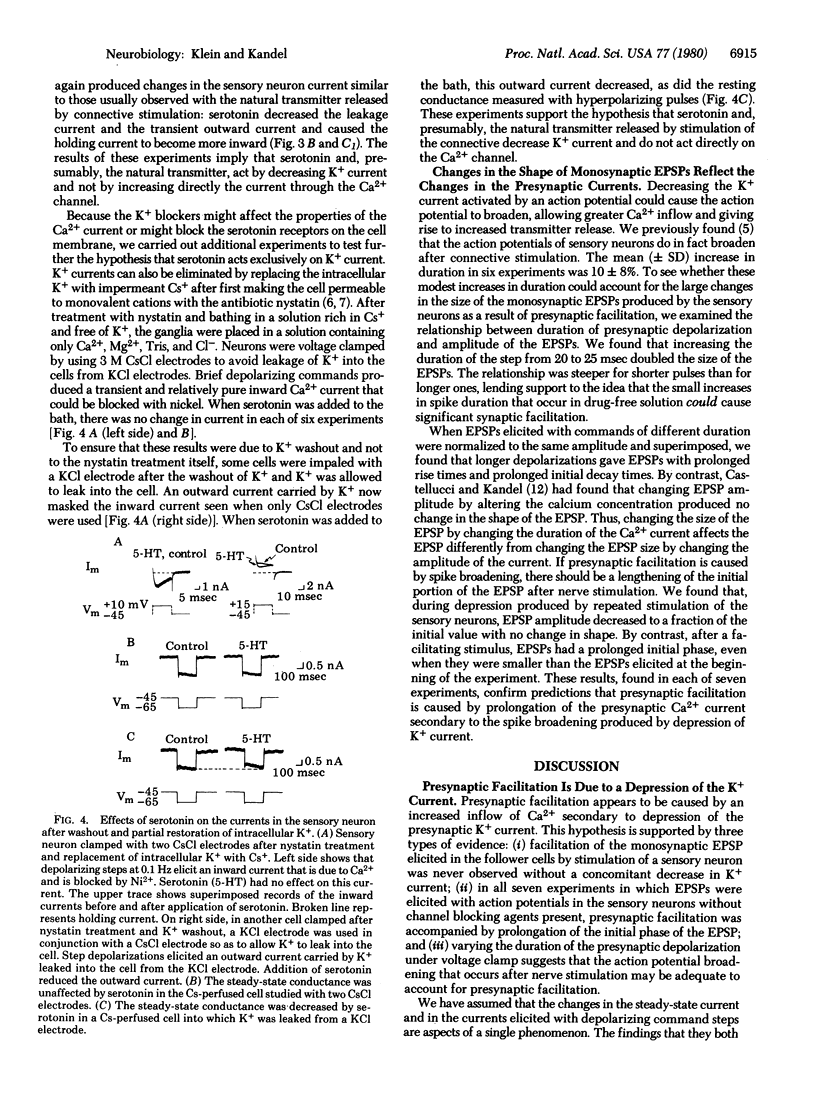
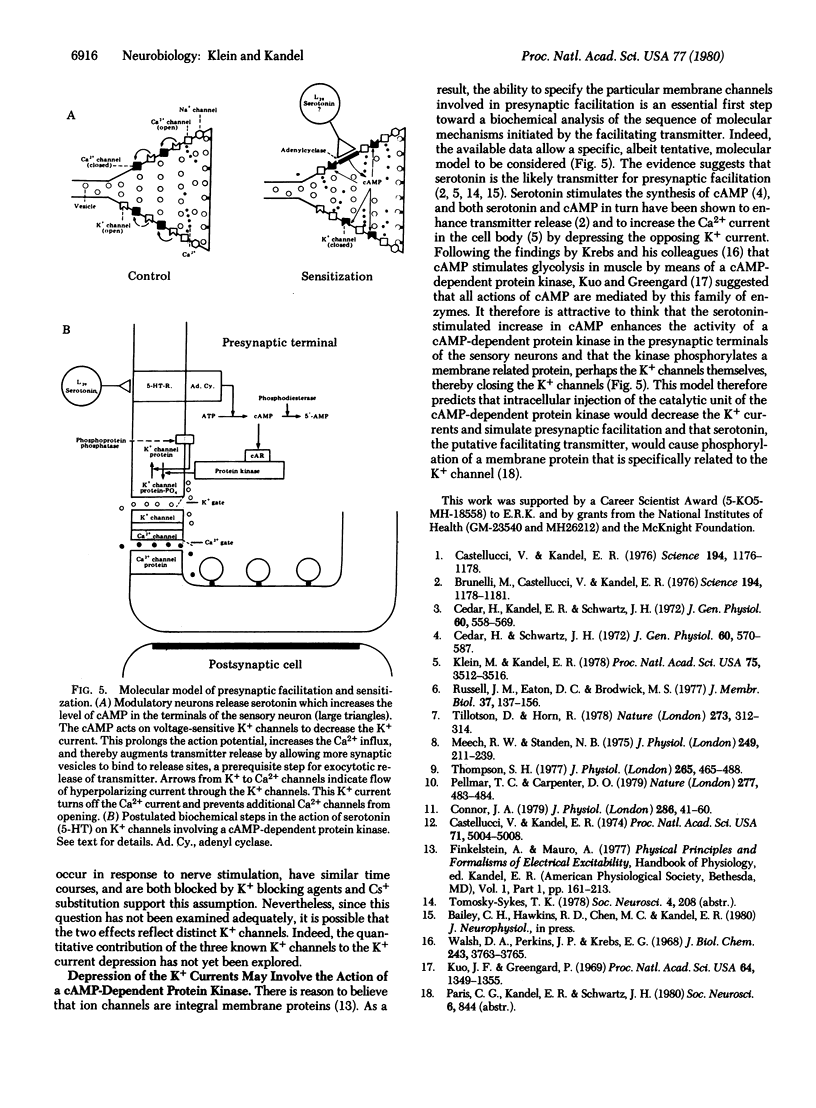
Behavioral sensitization of the gill-withdrawal reflex of Aplysia is caused by presynaptic facilitation at the synapses of the mechanoreceptor sensory neurons of the reflex onto the motor neurons and interneurons. The presynaptic facilitation has been shown to be simulated by serotonin (the putative presynaptic facilitatory transmitter) and by cyclic AMP and to be accompanied by an increase in the Ca2+ current of sensory neuron cell bodies exposed to tetraethylammonium. This increase in the Ca2+ current could result from either a direct action on the Ca2+ channel or an action on an opposing K+ current. Here we report voltage clamp experiments which indicate that the increase in Ca2+ current associated with presynaptic facilitation results from a decrease in a K+ current. Stimulation of the connective (the pathway that mediates sensitization) or application of serotonin causes a decrease in a voltage-sensitive, steady-state outward current measured under voltage clamp as well as an increase in the transient net inward and a decrease in the transient outward currents elicited by brief depolarizing command steps. The reversal potential of the steady-state synaptic current is sensitive to extracellular K+ concentration, and both the steady-state synaptic current and the changes in the transient currents are blocked by K+ current blocking agents and by washout of K+. These results suggest that serotonin and the natural transmitter released by connective stimulation act to decrease a voltage-sensitive K+ current. The decrease in K+ current prolongs the action potential, and this in turn increases the duration of the inward Ca2+ current and thereby enhances transmitter release.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Cedar H., Kandel E. R., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. I. Increased synthesis in response to synaptic stimulation. J Gen Physiol. 1972 Nov;60(5):558–569. doi: 10.1085/jgp.60.5.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Cyclic adenosine monophosphate in the nervous system of Aplysia californica. II. Effect of serotonin and dopamine. J Gen Physiol. 1972 Nov;60(5):570–587. doi: 10.1085/jgp.60.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Voltage-dependent calcium current induced by serotonin. Nature. 1979 Feb 8;277(5696):483–484. doi: 10.1038/277483a0. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Eaton D. C., Brodwick M. S. Effects of nystatin on membrane conductance and internal ion activities in Aplysia neurons. J Membr Biol. 1977 Oct;37(2):137–156. doi: 10.1007/BF01940929. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]


