Abstract
Cytochrome P4501A1 (CYP1A1), an important drug metabolizing enzyme, is expressed in human placenta throughout gestation as well as in fetal liver. Obesity, a chronic inflammatory condition, is known to alter CYP enzyme expression in non-placental tissues. In the present study, we test the hypothesis that maternal obesity alters the distribution of CYP1A1 activity in feto-placental unit. Placentas were collected from non-obese (BMI<30) and obese (BMI>30) women at term. Livers were collected from gestation day 130 fetuses of non-human primates fed either control diet or high-fat diet (HFD). Cytosol and microsomes were collected using differential centrifugation, and incubated with 7-Ethoxyresorufin. The CYP1A1 specific activity (pmoles of resorufin formed/min/mg of protein) was measured at excitation/emission wavelength of 530/590nm. Placentas of obese women had significantly reduced microsomal CYP1A1 activity compared to non-obese women (0.046 vs. 0.082; p<0.05); however no such effect was observed on cytosolic activity. Similarly, fetal liver from HFD fed mothers had significantly reduced microsomal CYP1A1 activity (0.44±0.04 vs. 0.20±0.10; p<0.05), with no significant difference in cytosolic CYP1A1 activity (control, 1.23±0.20; HFD, 0.80±0.40). Interestingly, multiple linear regression analyses of placental efficiency indicates cytosolic CYP1A1 activity is a main effect (5.67±2.32 (β±SEM); p=0.022) along with BMI (−0.57±0.26; p=0.037), fetal gender (1.07±0.26; p<0.001), and maternal age (0.07±0.03; p=0.011). In summary, while maternal obesity affects microsomal CYP1A1 activity alone, cytosolic activity along with maternal BMI is an important determinant of placental efficiency. Together, these data suggest that maternal lifestyle could have a significant impact on CYP1A1 activity, and hints at a possible role for CYP1A1 in feto-placental growth and thereby well-being of fetus.
Keywords: Human placenta, Maternal Obesity, Cytochrome P4501A, Cytosol, Microsomes
Introduction
More than 90% of pregnant women take prescription, non-prescription (over the counter), social (tobacco, alcohol), or illicit drugs during pregnancy [1]. While some of these drugs are essential, the use of drugs during pregnancy is discouraged by the medical community due to the potential harmful effects of exogenous chemicals on the embryo and fetus. Structural defects (teratogenicity), preterm birth, and altered birth weight are well described detrimental outcomes that are associated with maternal use of drugs [2, 3]. There is increasing evidence that the use of drugs can lead to pathophysiological changes that increase vulnerability for diseases later in life [4, 5].
The feto-placental cytochrome P450 (CYP) enzymes are of particular interest as they are involved in the detoxification of drugs, as well as biosynthesis and degradation of endogenous agents such as sex hormones that are vital for the rapidly growing fetus. The human placenta represents a first line of defense from xenobiotics for the fetus. This is facilitated by selective permeability mediated by drug transporters, and metabolism/ biotransformation by drug metabolizing enzymes [6, 7]. The CYP enzyme expression in human placenta is detected in early pregnancy [8], and the expression decreases with the progression of gestation [6]. The overall abundance of placental CYPs is quite low compared to the maternal liver profile [6], leading to the question of whether placental CYP enzymes are involved in xenobiotic detoxification.
CYP1A1 is interesting because of its role in bioactivation of procarcinogens into carcinogens. Smoking during pregnancy induces CYP1A1 enzyme activity in the placenta [9], and up-regulates a plethora of CYP genes [10]. Increased levels of CYP enzyme, particularly CYP1A1, could lead to poor outcomes at birth, during neonatal & postnatal life due to their ability to biotransform xenobiotics like polycyclic aromatic hydrocarbon into reactive intermediates [11]. Placental and fetal liver CYP1A1, unlike other members of the CYP superfamily, is expressed throughout pregnancy and is consistently detectable using mRNA, protein, and specific activity measurements [12, 13].
While the expression of CYPs in the feto-placental unit of women with a normal body mass index (BMI; 20–25 kg/m2) is well studied, such vital information is missing in obese/over-weight women (BMI >25 kg/m2) who constitute half of women of child bearing age [14, 15]. Obesity, a chronic inflammatory condition, is known to alter CYP enzyme expression in non-placental tissues [16]. However, no studies have reported the effect of maternal obesity on placental or fetal CYP enzyme expression. In this study, we have focused on a select CYP isozyme- CYP1A1 - which plays a crucial role in pharmacokinetics and pharmacodynamics of xenobiotics and toxins [17]. Our hypothesis is that maternal obesity is associated with decreased CYP1A1 expression in the feto-placental unit. Thus, the objective of this study is to determine CYP1A1 expression in (a) full term placenta of non-obese and obese women, and (b) fetal liver of a non-human primate model of maternal obesity. Most studies have used the microsomal subcellular fraction consisting of the endoplasmic reticulum to estimate in vitro specific activity of CYP enzymes. However, it has been demonstrated that the kidney and brain have detectable CYP1A1 activity in the cytosolic subcellular fraction that is equal to or greater than that found in microsomes [18, 19]. Hence, in this study, we tested our hypothesis in both microsomal and cytosolic fractions.
CYP1A1 also plays an important role in metabolism of physiological substrates including oestrogens which have a potent effect on the angiogenesis of placenta [20]. Poor vascularization in the placenta can give rise to hypoxia, oxidative stress, and decreased nutrient transport which are associated with intrauterine growth restriction and preeclampsia [21]. Placental efficiency, measured as a ratio of birth weight to placenta weight, is a useful metric for estimating the conceptual cost of fetal growth [22, 23]. As a secondary analysis, we used multiple linear regression to determine the degree to which CYP1A1 activity is correlated with placenta efficiency.
Methods & Materials
Reagents
Rabbit polyclonal antibodies raised against E. coli expressed human CYP1A1 (Xenotech LLC, Lenexa, KS), protein assay dye reagent concentrate (Bio-Rad, Hercules, CA), 10x phosphate buffer saline (LiCor Biosciences, Lincoln, Nebraska), and disodium salt of EDTA, potassium dibasic phosphate (Fisher Scientific, Fairlawn, NJ) were purchased. All other reagents were obtained from Sigma Aldrich, St. Louis, MO.
Human Placental tissue collection
Placental tissue was collected with written, informed consent from subjects and approved by the Oregon Health & Science University (OHSU) Institutional Review Board. Thirty-eight women who were 18 years of age or older, at term (≥37 weeks gestation) and had uncomplicated pregnancies (no gestational diabetes, preeclampsia or hypertension) were consented upon admission to OHSU Labor & Delivery for scheduled caesarean section. The placenta was collected within 20 min of delivery. Placental villous tissue was snap frozen in liquid nitrogen and stored at −80�C until further analysis. Various maternal parameters (BMI, age, parity, and smoking status) and fetal parameters (gender, birth weight, and placental weight) were collected. The study subjects were not taking any chronic drugs except for prenatal vitamins.
Non-human primate fetal liver collection
Detailed description was described elsewhere [24]. Briefly, animal procedures were conducted as per the guidelines of Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC) and Oregon Health & Science University. Japanese macaques were matched for age and weight, and randomly assigned to two dietary groups: Control diet (13% of calories from fat; Monkey Diet no. 5052, Lab Diet, Richmond, IN, USA) or high-fat diet (HFD; 35.2% of calories from fat; Custom Diet 5A1F, Test Diet, Richmond, IN, USA). The animals were on the HFD for a minimum of 3 years, and the body weight increased by 25% compared to chow-fed controls.
The animals were group housed and had ad libitum access to food and water. Each maternal group was housed with two males so that pregnancies would occur during the yearly breeding season (November–February). Singleton pregnancies at gestation day 130, as determined by ultrasound, were terminated by cesarean section by ONPRC veterinarians. After cesarean section, fetuses were deeply anesthetized with sodium pentobarbital (>30 mg/kg i.v.) and exsanguinated. All peripheral tissues, including livers, were removed, weighed and stored at −80°C for subsequent analyses.
Isolation of Subcellular fractions
Placenta tissue (1–4 grams; n= 38 ) and NHP fetal liver (n=3–4) was homogenized with a motor powered Potter-Elvehjem Tissue grinder in 12mL of buffer (50 mM Tris, 0.15 M KCl, pH 7.4). Homogenate was spun at 600×g for 10 minutes at 4°C. Supernatant was spun at 10,000×g for 20 minutes at 4°C. The resulting supernatant (S9) was spun at 100,000×g for 60 minutes at 4°C to yield cytosol (supernatant) and microsomes (pellet). The microsomal pellet was resuspended in storage buffer (50mM Tris, 0.25M Sucrose, pH 7.4), and cytosolic supernatant was stored as such at −80°C. Protein content of the subfractions was measured according to the Bradford method (Bradford, 1976).
To examine whether the final centrifugal force (100,000×g) was adequate for separation of cytosol and microsomal fractions, a portion of S9 supernatant from three placental samples were spun at a higher centrifugal force (200,000×g).
CYP1A1 activity assay
The CYP1A1 activity was measured using a widely published method (Burke, 1985). Briefly, microsomes (30–300ug) or cytosol (100–600ug) fractions from human placenta or non-human primate fetal livers were pre-incubated in potassium phosphate buffer (100mM buffer and 1mM EDTA, pH 7.4) with an NADPH regenerating system (2U of glucose-6-phosphate dehydrogenase, 120mM MgCl2, 200mM glucose-6-phosphate, 40mM NADP+) at 37°C for 10 minutes. The reaction was initiated by adding 7-Ethoxyresorufin to obtain a final concentration of 5µM. Fluorescence was measured at excitation / emission wavelength of 530nm / 590nm in a kinetic mode. The microsomal and cytosolic CYP1A1 activity, measured as pmoles/min/mg of protein, was based on a standard curve of the product (resorufin).
Inhibition assays
To confirm the presence of CYP1A1, the specific activity was measured in cytosolic subfractions of four placenta samples in the presence of either α-naphthoflavone or rabbit polyclonal antibodies raised against E. coli expressed human CYP1A1. For chemical inhibition studies, 10µl of DMSO (vehicle) or 1, 5, or 10 mM α-napthoflavone solution were added to achieve final concentration of 0, 10, 50, and 100 µM concentrations of inhibitor in 1mL of incubation buffer. Control incubations did not contain either DMSO or α-napthoflavone. For antibody inhibition studies, 20 µl of CYP1A1 antibodies were added to 1mL of incubation buffer. The CYP1A1 activity assay was carried out as described above.
Cytochrome P450 Reductase (CPR) assay
Using three placenta samples, CPR activity was measured in both cytosol and microsomes. Each subcellular fraction (125 µg/mL) was incubated with 100uM Cytochrome c (oxidized form) and 100 µM NADPH in a buffer (3mM MgCl2, 1mM Na2EDTA, 100uM KCN, and 330mM PO4), and absorbance was measured at 550nm over five minutes. The specific activity (amount of Cytochrome c reduced/min/mg of protein) was calculated using an extinction coefficient of 19.6mM−1cm−1 for Cytochrome c reduced [25, 26].
Western blotting
To further confirm the presence of CYP1A1, western blotting of subcellular fractions was performed. Briefly, 18 µg of total protein from both cytosolic and microsomal fractions were loaded onto a 10% SDS-polyacrylamide gel and electrophoresed before being transferred to a nitrocellulose membrane. The membrane was blocked with a 1:1 solution of LiCor Biosciences (Lincoln, Nebraska) Odyssey® Blocking Buffer and 1x phosphate buffer saline before blotting for human CYP1A1 with rabbit anti-E. coli primary antibody and goat anti-rabbit secondary antibody (680nm IRDye®) from LiCor Biosciences. Imaging was performed on LiCor Biosciences Odyssey® imaging system at 700nm and images were edited using NIH ImageJ software. All samples were run in duplicate on a separate blot.
Data analysis
Paired t-test or Wilcoxon signed rank test was used to examine differences between cytosolic and microsomal fractions in CYP1A1 and cytochrome P450 reductase activities. Student’s t-test or one-way ANOVA followed by Holm-Sidak post hoc test was used to detect statistical differences in the degree of antibody and chemical inhibition of CYP1A1 activity, respectively. The effect of smoking status on CYP1A1 activity on both microsomal and cytosolic subcellular fractions was tested using Mann-Whitney rank sum test. Smokers were excluded from all other analyses.
Placentas were grouped based on clinic-measured maternal early second trimester BMI: obese (BMI > 30; n=16) and non-obese (BMI <30; n=14). Outliers (outside of 2 S.D.) were excluded in the data analysis. Student’s t-test was used to detect differences in cytosolic and microsomal CYP1A1 activities with respect to BMI in human placenta, and in NHP fetal livers with respect to diet. Multiple linear regression was employed to estimate the influence of CYP1A1 specific activity and various maternal parameters (BMI, age, parity, etc) on placental efficiency (birth weight (g)/placenta weight (g)). Predictor variables were entered stepwise. All statistical tests were conducted at p < 0.05 (Sigma Stat v11.0, San Jose, CA).
Results
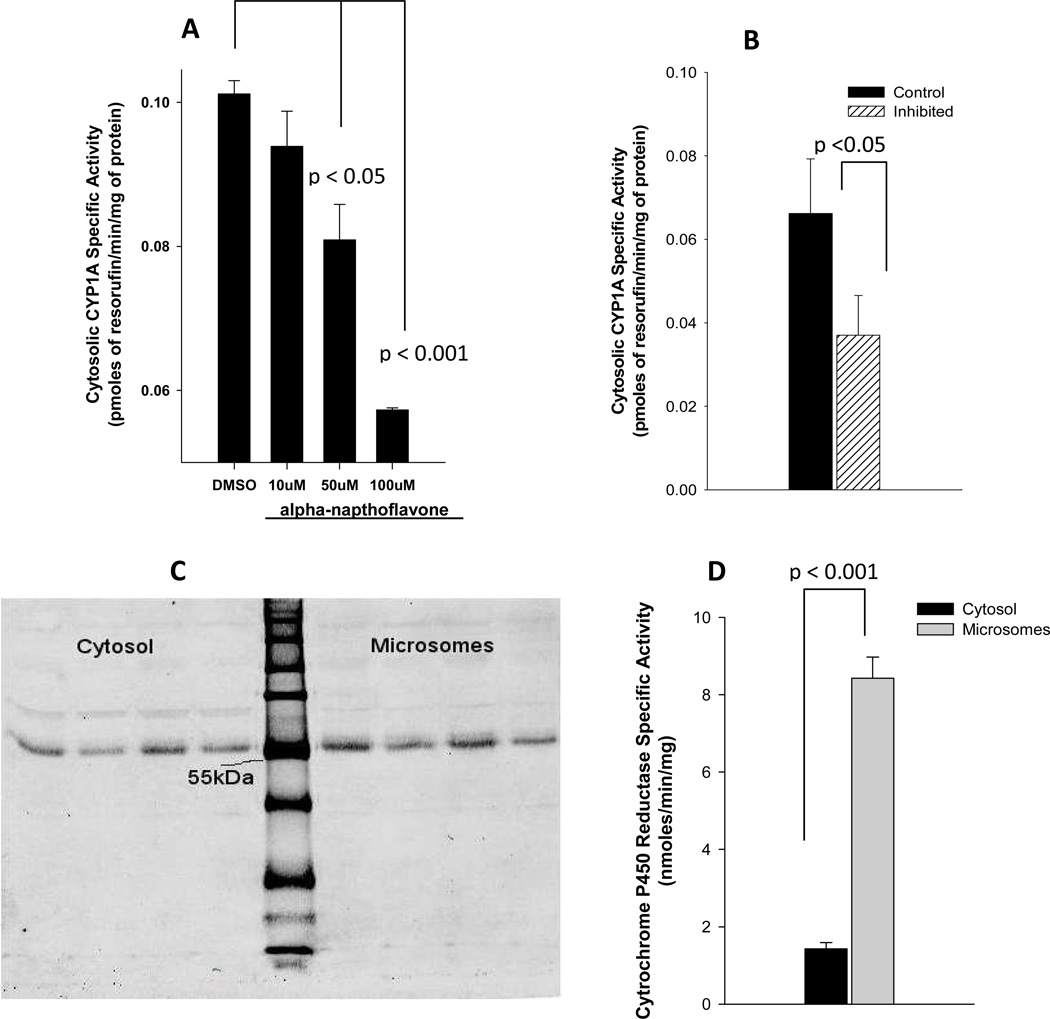
Typically, CYP enzyme activities are measured in microsomal fractions of the tissue. However, CYP1A1 activity was reported in cytosolic fractions of kidney and brain. Therefore, we suspected that the cytosolic fraction of placental tissue also expresses CYP1A1. To characterize the cytosolic CYP1A1 activity, we undertook chemical and antibody inhibition assays. CYP1A1 specific chemical inhibitor, α-napthoflavone, inhibited cytosolic CYP1A1 activity in a dose dependent fashion (Figure 1A). Compared to vehicle (DMSO) treatment, cytosolic CYP1A1 activity was reduced to 93%, 80%, and 57% by 10, 50, and 100µM α-napthoflavone, respectively. The inhibition of CYP1A1 activity by 50 and 100 µM α-napthoflavone was statistically significant (p<0.05). Similarly, CYP1A1 activity was reduced by approximately 50% upon antibody inhibition (p<0.05) (Figure 1B). Western blot analysis demonstrated similar intensity of CYP1A1 band at ~55KDa in both sub-cellular fractions (Figure 1C).
Figure 1. Cytosolic CYP1A1 in human placenta.
(A) Placental cytosolic fractions from two pooled samples (n=3 placentas in each pooled sample) were incubated in duplicate with 10µl of DMSO (vehicle) or α-napthoflavone (final concentrations of 10, 50, or 100 µM). Reaction was started by adding ethoxyresorufin, and the CYP1A specific activity was estimated as amount of the product (resorufin) formed/min/mg of protein. A one-way analysis of variance was performed to test for differences among treatments; multiple comparisons versus controls (DMSO) were carried out using one-way analysis of variance with a Holm-Sidak test for pair-wise comparisons. (B) Cytosolic fractions (n=4) were incubated with 20µl of rabbit polyclonal E. coli expressed against human CYP1A1. Average CYP1A1 activity with and without these antibodies are shown above. A paired t-test was used to compare control and antibody-inhibited incubations. (C) Western blots of cytosolic and microsomal fractions (n=4) are shown to the left and right, respectively, of the molecular weight marker (LiCor Odyssey® Two-Color Protein Molecular Weight Marker). Strong signal was detected at ~55kDa. (D) Placental cytosolic and microsomal fractions were incubated with oxidized form of Cytochrome c, and the specific activity of Cytochrome P450 Reductase was estimates as the amount of the product (reduced form of Cytochrome c) formed/min/mg of protein. A paired t-test was used to compare microsomal activity to cytosolic activity (n=3).
The cytochrome P450 reductase activity was measured to determine if the observed cytosolic CYP1A1 activity was a result of contamination of cytosolic subcellular fractionation with microsomes. The microsomal fractions demonstrated ~ 6-fold higher CPR activity than the cytosolic fractions (8.43±0.52 vs. 1.43±0.18 nmoles/min/mg) (P<0.001) (Figure 1D). Additionally, CYP1A1 activity in subcellular fractions obtained from higher centrifugal force spin (200,000×g) was compared to that of routinely used centrifugal force (100,000xg) as another assessment of contamination of subcellular fractions. There were no differences in CYP1A1 activity in cytosolic or microsomal fractions prepared by either centrifugal force (data not shown).
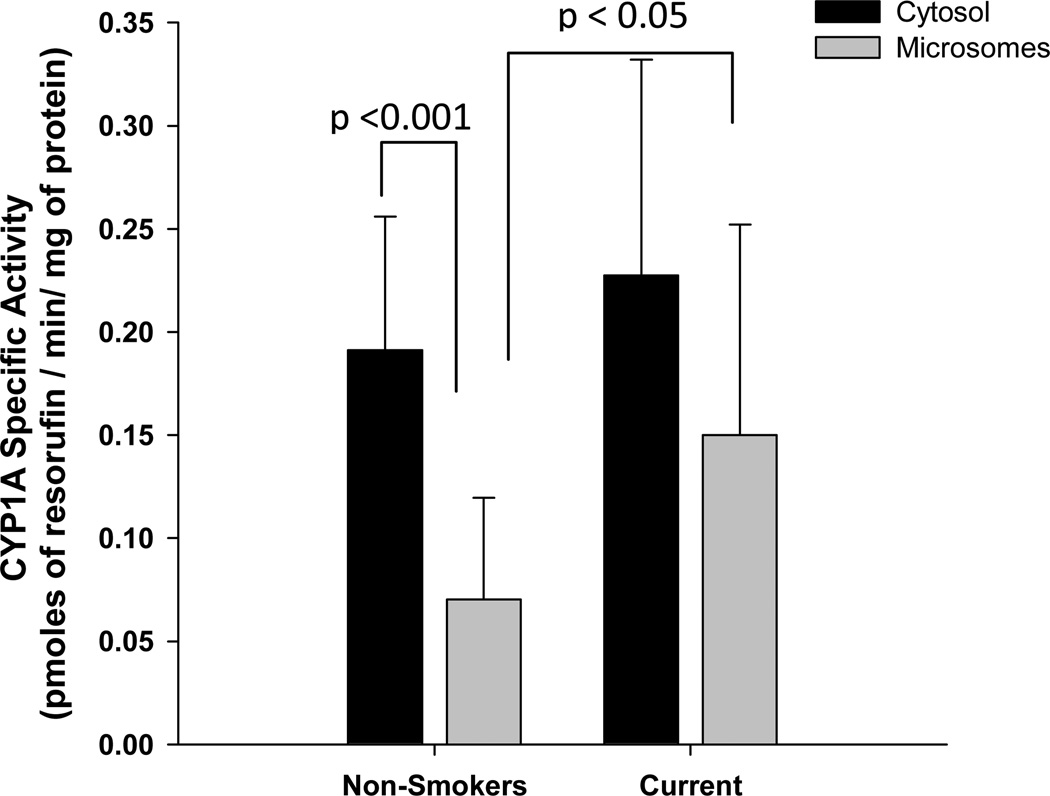
Smoking is a known inducer of CYP1A1 expression. Therefore, the effect of maternal smoking status on CYP1A1 in vitro specific activities in the microsomal and cytosolic subcellular fractions of the placenta were compared (Figure 2). Both cytosolic and microsomal CYP1A1 specific activity in one of the 6 smokers was ~ 30–40 fold higher than the rest of the smokers, and hence considered an outlier for the data analysis. The cytosolic CYP1A1 activity (0.191±0.065 pmoles/min/mg of protein, n = 32; mean ± S.D.) was 2.5-fold greater than that of microsomal CYP1A1 activity (0.070±0.049 pmoles/min/mg of protein, n = 32) among non-smokers (P <0.001). There was no difference between microsomal and cytosolic CYP1A1 specific activities in current smokers. However, as expected, microsomal CYP1A1 activity in current smokers (0.150±0.102 pmoles/min/mg of protein, n = 5) was approximately 2-fold greater compared to non-smokers (p<0.05). There were no differences between the cytosolic CYP1A1 activities of non-smokers and current smokers.
Figure 2. Maternal smoking and CYP1A activity in microsomal and cytosolic subcellular fractions of placenta.
Both microsomal and cytosolic proteins were incubated with ethoxyresorufin, and the CYP1A specific activity was estimated as amount of the product (resorufin) formed/min/mg of protein. Statistical differences in CYP1A specific activity between sub-cellular fraction among non-smokers (n=32) and current-smokers (n=5) were tested using Mann-Whitney rank sum test. Statistical difference between subcellular fractions (microsomes vs. cytosol) among non-smokers was tested using Wilcoxon signed rank test.
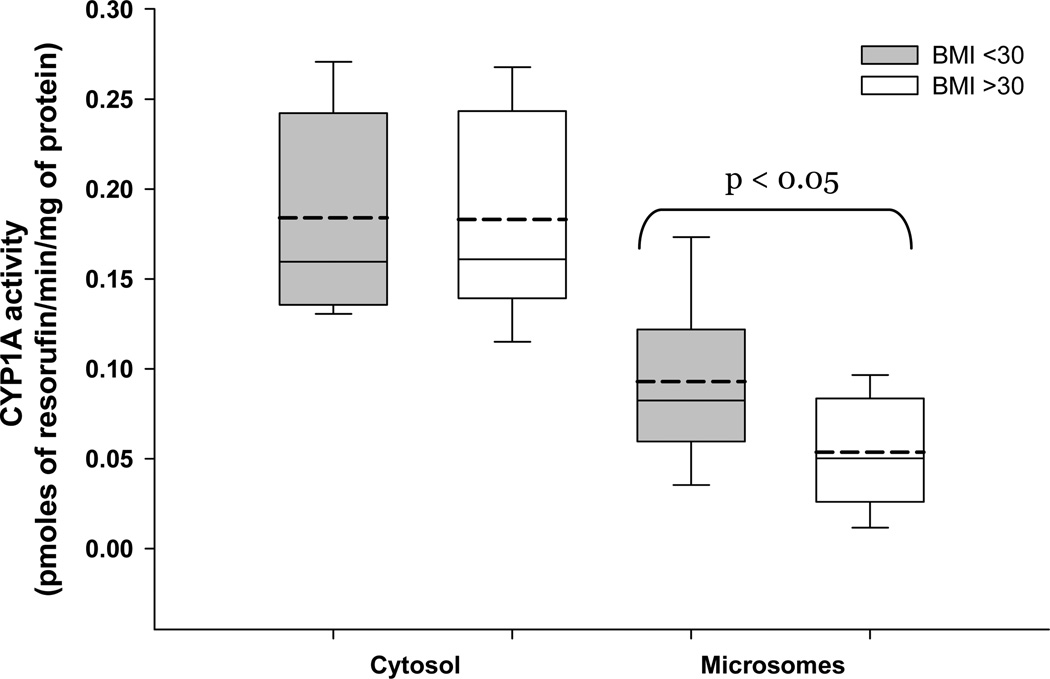
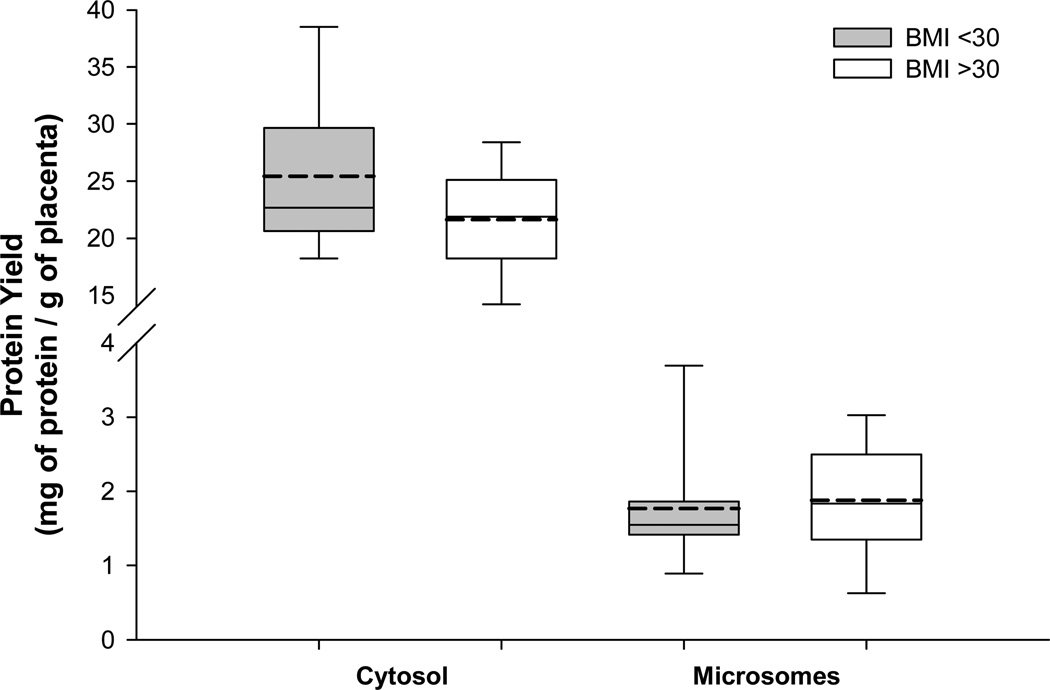
The activity of CYP1A1 was measured in both microsomal and cytosolic fractions of placenta. The microsomal CYP1A1 activity, compared to non-obese women, was significantly lower in placentas of obese women (0.082 vs. 0.046 pmoles/min/mg of protein; p<0.05). We found no effect of maternal BMI on cytosolic CYP1A1 activity (figure 3). The effect of maternal obesity on CYP1A, or the lack of it, is observed in the absence of differences in the protein yields of either sub-cellular fraction between BMI groups (figure 4).
Figure 3. Maternal obesity and placental CYP1A1.
Placental CYP1A1 activity in both cytosolic and microsomal sub-cellular fractions was measured and the activity was normalized to amount of protein. All placentas were obtained from non-smoking mothers with a BMI <30 (n=14) or BMI >30 (n=16). Each box represents inter quartile range with mean and median depicted as dotted and solid line, respectively. Whiskers represent 95% confidence interval. Statistical differences were tested using student’s t-test.
Figure 4. Maternal obesity and total protein yield in placental tissue.
Cytosolic and microsomal sub-cellular fractions were isolated using differential centrifugation. The protein concentrations were estimated using Bradford method. All placentas were obtained from non-smoking mothers with a BMI <30 (n=14) or BMI >30 (n=16). Each box represents inter quartile range with mean and median depicted as dotted and solid line, respectively. Whiskers represent 95% confidence interval. Statistical differences were tested using student’s t-test.
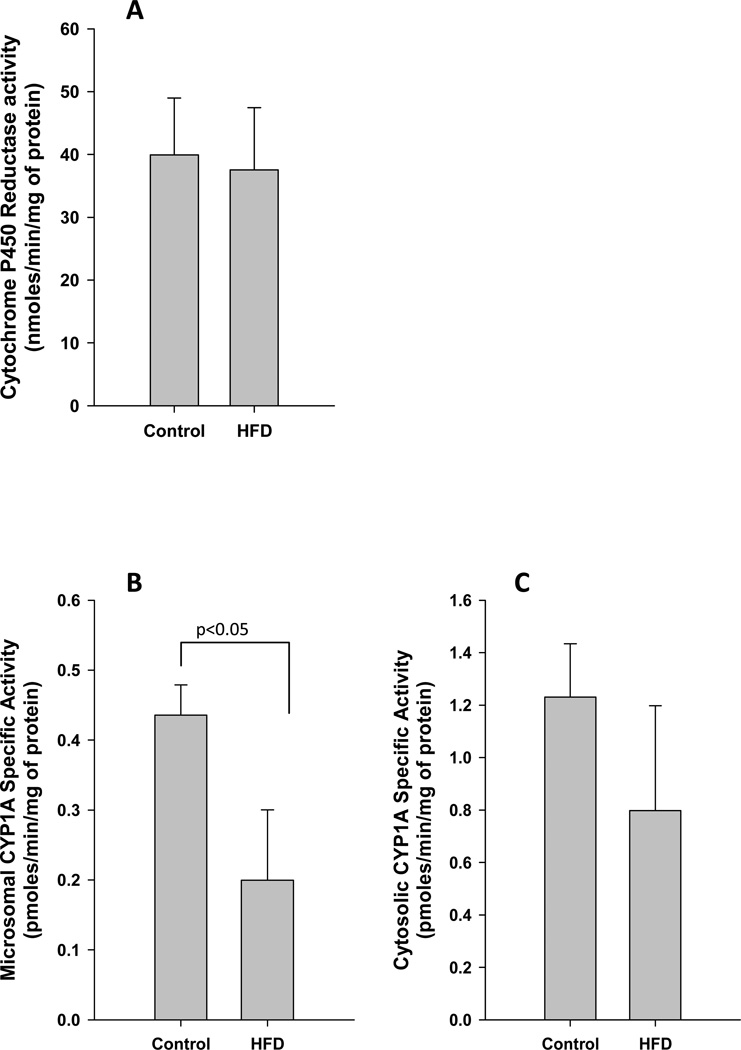
The effect of maternal high fat diet during gestation on fetal liver CYP1A1 is shown in (Figure 5). While placental microsomal CYP1A1 activity was significantly reduced in the high fat diet group offspring (0.44±0.04 vs. 0.20±0.10 pmoles/min/mg of protein; p<0.05; Figure 5B), there was no significant difference in cytosolic CYP1A1 activity (control, 1.23±0.20; HFD, 0.80±0.40 pmoles/min/mg of protein; Figure 5C). It is noteworthy that the above changes in CYP1A1 were observed in the absence of significant differences in the rate limiting coenzyme- CYP reductase (Figure 5A).
Figure 5. Maternal obesity and fetal liver CYP1A1.
The cytochrome P450 reductase (A), microsomal CYP1A1 (B), and cytosolic CYP1A1 (C) activities were measured in fetal livers of non-human primate mothers who were fed either control (n=3) or HFD (n=4) throughout pregnancy. Statistical differences were tested using student’s t-test.
Multiple linear regression was used to identify relationships between CYP1A1 activity and placental efficiency (Table 1). Cytosolic CYP1A1 specific activity (5.67 ± 2.32 (coefficient ± SEM), p=0.022) and maternal BMI (−0.57 ± 0.26, p=0.037) were related to efficiency. The other predictors are age (0.07 ±0.03 p=0.011), gender (1.07 ±0.27 p<0.001), and cytosolic protein yield (0.09 ±0.02 p<0.001). On the other hand, microsomal CYP1A1 activity did not predict placental efficiency (data not shown).
Table 1.
Multiple linear regression model for placental efficiency
| Predictor | Coefficient | Standard error of mean |
P value | Adjusted Regression |
|---|---|---|---|---|
| Constant | 1.304 | 1.415 | 0.366 | 0.576 |
| Gender of fetus1 | 1.074 | 0.260 | <0.001 | |
| Maternal age | 0.073 | 0.027 | 0.011 | |
| BMI factor2 | −0.568 | 0.257 | 0.037 | |
| Cytosolic CYP1A1 activity | 5.668 | 2.323 | 0.022 | |
| Cytosolic protein yield | 0.091 | 0.024 | <0.001 |
Gender of the fetus (female = 0, male = 1).
BMI factor – factorized maternal BMI (non-obese = 0, obese = 1).
Discussion
Drug metabolizing enzymes expressed in the feto-placental unit play a vital role in minimizing fetal exposure to teratogenic agents. In this study, we have shown that maternal obesity alters the feto-placental expression of CYP1A1, an important drug metabolizing enzyme, in the microsomal compartment. Furthermore, this study demonstrated that the cytosolic CYP1A1 along with maternal obesity is an important predictor of placental efficiency.
Obesity is a pro-inflammatory condition associated with higher circulating cytokines which can affect the expression of various CYP enzymes [27]. The degree to which maternal cytokines affect placental function is not known but the placenta does express cytokines which have been shown to be at higher levels in placentas of obese women [28–30]. Consistent with our hypothesis, we found a ~45% decrease in CYP1A specific activity among obese women. The effect of maternal obesity on CYP1A1 is observed in the absence of inherent differences between BMI groups in total protein yields of sub-cellular fractions. This provides further evidence that the finding in the current study is not an artifact, but rather an effect of maternal obesity.
Fetuses of non-human primates fed a HFD were used to assess the effect of maternal obesity on fetal liver CYP1A1. Non-human primate tissue was used because normal human fetal liver tissue cannot be ethically obtained. The HFD-fed non-human primate model of maternal obesity has been well characterized and offspring of obese mothers display many features reported in human offspring of obese women [31, 32]. Furthermore, CYP enzymes exhibit high degree of homology between humans and non-human primates, and hence easily extrapolated to humans [33]. In this study a HFD in the maternal non-human primate was associated with a ~55% decrease in fetal liver expression of microsomal CYP1A1 as seen in human placenta of obese women. The authors speculate that a HFD (which is associated with obesity [34]) could trigger the chain of events leading to placental cytokine induction, resulting in lower expression of CYP1A1, under the influence of excess deleterious fatty acids.
We have demonstrated for the first time that CYP1A1 is expressed robustly in the cytosolic fraction of term human placenta and represents a larger contributor to overall placental CYP1A1 activity than the microsomal fraction. Given the traditional view that CYP1A1/2 is associated with endoplasmic reticulum membrane, it is quite puzzling that we find activity in the cytosolic fraction. We first suspected that our cytosolic fraction was contaminated with microsomes. Cytochrome P450 reductase, a membrane bound enzyme found exclusively in the endoplasmic reticulum, is a common biomarker to detect contamination of cytosolic fraction with microsomes [19]. We found cytochrome P450 reductase activity is almost non-existent (83% less) in the cytosol compared to the microsomal fraction. This would suggest that contamination of the cytosolic fraction with microsomal membrane bound proteins was minimal. CYP1A1 activity measured in both cytosolic and microsomal fractions obtained from a high centrifugal force spin were similar to those obtained from usually employed protocol (data not shown). This additional evidence further confirms that the cytosolic fraction is free of microsomal protein.
We methodically investigated whether the observed cytosolic activity is attributable to CYP1A1 in placenta. It has been shown that other soluble cytosolic enzymes, such as NAD(P)H:quinine oxidoreductase1 (DT-diaphorase) and myeloperoxidase can metabolize 7-ethoxyresorufin using NADPH as a cofactor[19]. We estimated the specificity of 7-ethoxyresorufin towards cytosolic CYP1A1 expressed in placenta, using chemical and antibody inhibition assays. The chemical inhibition studies using α-naphthoflavone reduced CYP1A1 activity by ~50%. The antibody inhibition studies indicate a similar ~50% decrease in CYP1A1 activity. Taken together these indicate a significant portion of the observed placental activity is attributable to CYP1A1.
Western blots for CYP1A1 in placental microsomes and cytosol did not show significant differences in CYP1A1 band intensities. This suggests similar expression of CYP1A1 protein per unit of total protein in both sub-cellular fractions. While this contradicts our finding that there is 2.5-fold greater CYP1A1 activity in cytosolic fraction, both chemical and antibody inhibition studies suggest that only roughly half of cytosolic specific activity is attributable to CYP1A1. Therefore, we deduce that the cytosolic CYP1A1 specific activity is similar to (to be precise ~70–80% of) microsomal CYP1A1.
Though we have demonstrated the presence of functionally active CYP1A1 in cytosol, the physiological relevance of this observation is unclear. While maternal obesity is related inversely to placental microsomal CYP1A1 activity, cytosolic CYP1A1 activity is not associated with maternal BMI. Nevertheless, both cytosolic CYP1A1 specific activity and maternal obesity are related to placental efficiency. Maternal obesity has a positive effect on placental weight and birth-weight respectively, but an overall negative effect on placental efficiency. On the contrary, cytosolic CYP1A1 activity is positively related to placenta efficiency. The speculated role of cytosolic CYP1A1 in placental efficiency is interesting. The placental growth is influenced by oestrogens whose physiological concentrations are a function of CYP1A1, an oestrogen metabolizing enzyme, and as well as CYP19A1 (aromatase), an enzyme responsible for oestrogen biosynthesis. Hence, further studies to evaluate status of aromatase in placenta are required to better understand the role of cytosolic CYP1A1 on placental efficiency.
The association of maternal obesity with microsomal CYP1A1, but not cytosolic CYP1A1, is puzzling. The inhibition studies, both antibody and chemical, suggest that about 50% of the measured CYP1A1 activity in cytosol is attributable to non-specific protein. Therefore, one possible explanation is that the effect of maternal obesity on CYP1A1 is confounded by the opposing effect of non-specific protein. Measurement of CYP1A1 activity using a substrate that is highly selective for cytosolic CYP1A enzyme would be helpful to better understand the status of CYP1A1 in cytosol. Another possible explanation is that the CYP1A1 apoprotein is distributed to a different degree between microsomal and cytosolic; the processes that direct soluble apoprotein to membranes are possibly affected by maternal obesity and thus altering the sub-cellular distribution.
Feto-placental metabolism is one of four important determinants of drug transfer to fetus from the mother [35]. The other determinants are lipophilicity, molecular weight, and protein binding of drugs. Obesity-induced changes in plasma protein concentrations could potentially alter binding of drugs to plasma proteins. The effect of obesity on plasma proteins is unclear. While some studies report lack of effect [36], others have reported a decrease in albumin, and increases in alpha-acid glycoprotein [37] and sex-hormone binding globulin [38]. Therefore, obesity-related alterations in feto-placental drug metabolism, such as CYP1A shown in the current study, should be considered along with changes to plasma protein binding to assess the overall outcome on drug transfer to fetus.
In conclusion, this study describes maternal obesity-induced decreases in the activity of CYP1A1 in the feto-placental unit. Altered CYP1A expression could place a rapidly developing fetus at higher risk of exposure to teratogenic agents, and increase the fetus’ susceptibility to diseases during adulthood. Further studies are needed to elucidate the mechanisms underlying obesity-induced alterations in feto-placental CYP1A1 activity. Additionally, it will be important to determine the role of diminished CYP1A1 activity in the poor outcomes associated with offspring of obese women.
Acknowledgements
Financial support from intramural funds from College of Pharmacy, Oregon State University, Medical Research Foundation of Oregon Early Clincal Investigator Award (O’Tierney), The Gerber Foundation (O’Tierney) and the Edwards Foundation (Thornburg) . Dr. O’Tierney is supported by NIH-5K99HD062841-02. Dr. Friedman is supported by NIH- 5R24DK090964.
Abbreviations
- CYP
Cytochrome P450
- CPR
Cytochrome P450 Reductase
- HFD
High fat diet
- BMI
Body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barent N. DuBois, College of Pharmacy, Oregon State University / Oregon Health & Science University, Portland, OR. 97239. dubois@ohsu.edu
Perrie O’Tierney, School of Medicine, Oregon Health & Science University, Portland, OR. 97239. otierney@ohsu.edu
Jacob Pearson, College of Pharmacy, Oregon State University / Oregon Health & Science University, Portland, OR. 97239. pearson@ohsu.edu
Jacob E. Friedman, Dept of Pediatrics, Biochemistry & Molecular Genetics, University of Colorado School of Medicine, Aurora, CO 80045. Jed.friedman@ucdenver.edu
Kent Thornburg, School of Medicine, Oregon Health & Science University, Portland, OR. 97239. thornbur@ohsu.edu
Ganesh Cherala, College of Pharmacy, Oregon State University / Oregon Health & Science University, Portland, OR. 97239. Cheralag@ohsu.edu
References
- 1.Foley MR. Drug Use During Pregnancy. In: Porter RS, editor. The Merck Manuals. Whitehouse Station, NJ, U.S.A.: Merck Sharp & Dohme Corp; [Google Scholar]
- 2.Blomberg MI, Kallen B. Maternal obesity and morbid obesity: the risk for birth defects in the offspring. Birth Defects Res A Clin Mol Teratol. 2010;88:35–40. doi: 10.1002/bdra.20620. [DOI] [PubMed] [Google Scholar]
- 3.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–1158. [PubMed] [Google Scholar]
- 4.Meyer K, Lubo Z. Fetal programming of cardiac function and disease. Reprod Sci. 2007;14:209–216. doi: 10.1177/1933719107302324. [DOI] [PubMed] [Google Scholar]
- 5.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 6.Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43:487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]
- 7.Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158:665–678. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasanen M. The expression and regulation of drug metabolism in human placenta. Adv Drug Deliv Rev. 1999;38:81–97. doi: 10.1016/s0169-409x(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 9.Hakkola J, Pasanen M, Hukkanen J, Pelkonen O, Maenpaa J, Edwards RJ, et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human full-term placenta. Biochem Pharmacol. 1996;51:403–411. doi: 10.1016/0006-2952(95)02184-1. [DOI] [PubMed] [Google Scholar]
- 10.Bruchova H, Vasikova A, Merkerova M, Milcova A, Topinka J, Balascak I, et al. Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta. 2009;31:186–191. doi: 10.1016/j.placenta.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59:1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 13.Pavek P, Dvorak Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr Drug Metab. 2008;9:129–143. doi: 10.2174/138920008783571774. [DOI] [PubMed] [Google Scholar]
- 14.Mojtabai R. Body mass index and serum folate in childbearing age women. Eur J Epidemiol. 2004;19:1029–1036. doi: 10.1007/s10654-004-2253-z. [DOI] [PubMed] [Google Scholar]
- 15.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–273. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–1693. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Audus KL. Controlling drug delivery across the placenta. Eur J Pharm Sci. 1999;8:161–165. doi: 10.1016/s0928-0987(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 18.Meyer RP, Lindberg RL, Hoffmann F, Meyer UA. Cytosolic persistence of mouse brain CYP1A1 in chronic heme deficiency. Biol Chem. 2005;386:1157–1164. doi: 10.1515/BC.2005.132. [DOI] [PubMed] [Google Scholar]
- 19.Meyer RP, Podvinec M, Meyer UA. Cytochrome P450 CYP1A1 accumulates in the cytosol of kidney and brain and is activated by heme. Mol Pharmacol. 2002;62:1061–1067. doi: 10.1124/mol.62.5.1061. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa Y, Fujimoto J, Tamaya T. Placental growth by the estrogen-dependent angiogenic factors, vascular endothelial growth factor and basic fibroblast growth factor, throughout gestation. Gynecol Endocrinol. 2004;19:259–266. doi: 10.1080/09513590400016201. [DOI] [PubMed] [Google Scholar]
- 21.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, et al. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl A):S98–S102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: endocrine regulation. J Physiol. 2009;587:3459–3472. doi: 10.1113/jphysiol.2009.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One. 2011;6:e17261. doi: 10.1371/journal.pone.0017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962;237:2652–2660. [PubMed] [Google Scholar]
- 26.Vermilion JL, Coon MJ. Purified liver microsomal NADPH-cytochrome P-450 reductase. Spectral characterization of oxidation-reduction states. J Biol Chem. 1978;253:2694–2704. [PubMed] [Google Scholar]
- 27.Muntane-Relat J, Ourlin JC, Domergue J, Maurel P. Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology. 1995;22:1143–1153. [PubMed] [Google Scholar]
- 28.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–807. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- 29.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008;29:274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bo S, Menato G, Lezo A, Signorile A, Bardelli C, De Michieli F, et al. Dietary fat and gestational hyperglycaemia. Diabetologia. 2001;44:972–978. doi: 10.1007/s001250100590. [DOI] [PubMed] [Google Scholar]
- 32.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwasaki K, Uno Y. Cynomolgus monkey CYPs: a comparison with human CYPs. Xenobiotica. 2009;39:578–581. doi: 10.1080/00498250903003135. [DOI] [PubMed] [Google Scholar]
- 34.West DB, York B. Dietary fat, genetic predisposition, and obesity: lessons from animal models. Am J Clin Nutr. 1998;67:505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]
- 35.Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- 36.Cheymol G, Poirier JM, Barre J, Pradalier A, Dry J. Comparative pharmacokinetics of intravenous propranolol in obese and normal volunteers. J Clin Pharmacol. 1987;27:874–879. doi: 10.1002/j.1552-4604.1987.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 37.Benedek IH, Blouin RA, McNamara PJ. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br J Clin Pharmacol. 1984;18:941–946. doi: 10.1111/j.1365-2125.1984.tb02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchernof A, Despres JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 2000;32:526–536. doi: 10.1055/s-2007-978681. [DOI] [PubMed] [Google Scholar]