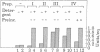Abstract
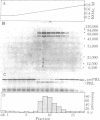
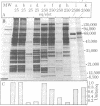
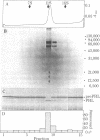
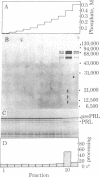
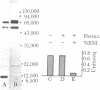
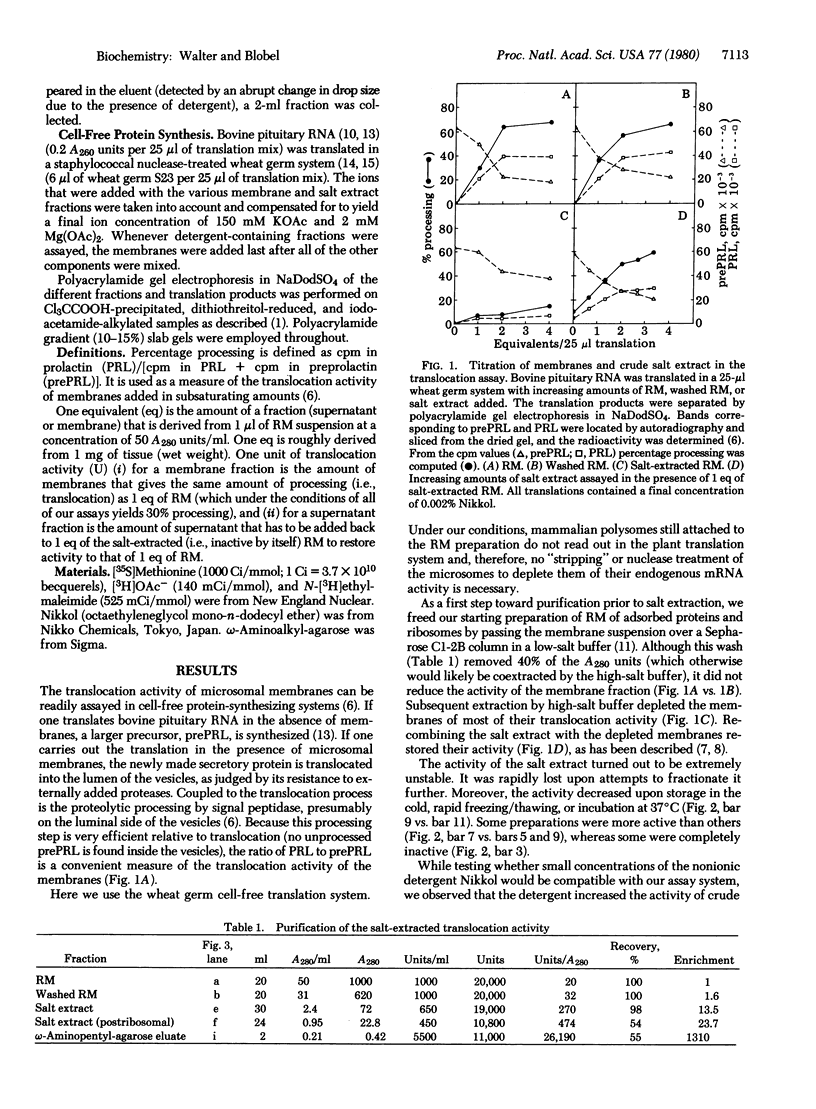
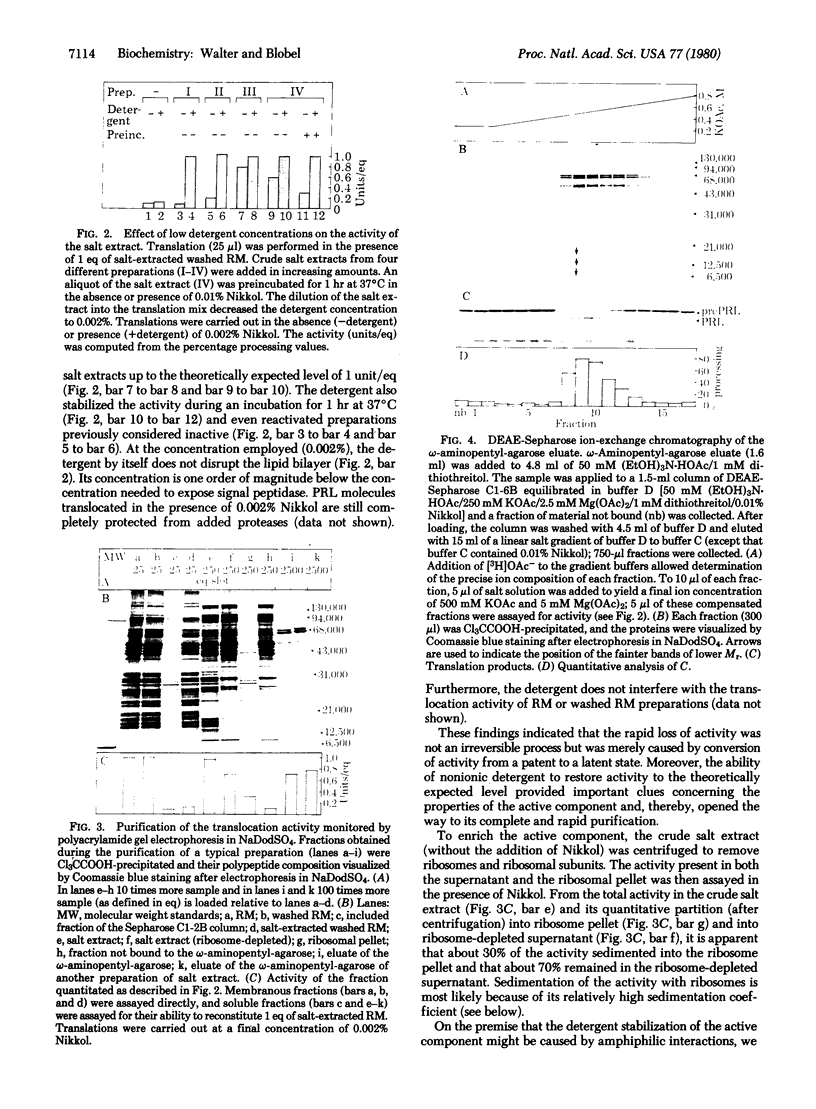
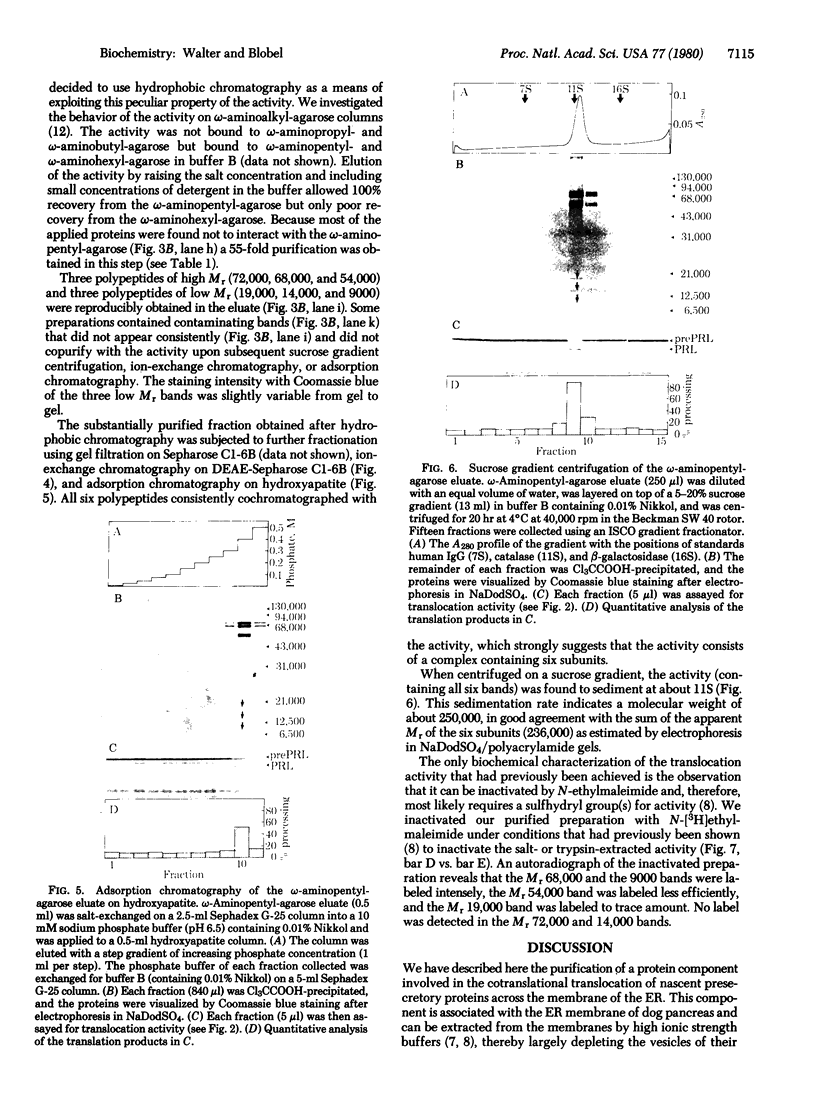
The capacity of microsomal membranes to translocate nascent presecretory proteins across their lipid bilayer can be largely abolished by extracting them with high ionic strength buffers. It can be reconstituted by adding the salt extract back to the depleted membranes [Warren, G. & Doberstein, B. (1978) Nature (London) 273, 569-571]. Utilizing hydrophobic chromatography, we purified to homogeneity a protein component of the salt extract that reconstitutes the translocation activity of the extracted membranes. This component behaves as a homogeneous species upon gel filtration, ion-exchange chromatography, adsorption chromatography, and sucrose-gradient centrifugation. When examined by polyacrylamide gel electrophoresis in NaDodSO4, six polypeptides with apparent Mr of 72,000, 68,000, 54,000, 19,000, 14,000, and 9000 are observed in about equal and constant stoichiometry, suggesting that they are subunits of a complex. The sedimentation coefficient of 11S is in good agreement with the sum of the Mr of the subunits. The Mr 68,000 and 9000 subunits label intensely with N-[3H]ethylmaleimide. Thus, the reported sulfhydryl group requirement of the translocation activity in the unfractionated extract [Jackson, R. C., Walter, P. & Blobel, G. (1980) Nature (London), 286, 174-176] may be localized to either or both the Mr 68,000 and 9000 subunits of the purified complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Hawkins H. C., Freedman R. B. A gel filtration approach to the study of ribosome-membrane interactions. Biochim Biophys Acta. 1979 Nov 16;558(1):85–98. doi: 10.1016/0005-2736(79)90317-1. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Walter P., Blobel G. Secretion requires a cytoplasmically disposed sulphydryl of the RER membrane. Nature. 1980 Jul 10;286(5769):174–176. doi: 10.1038/286174a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G., Dobberstein B., Blobel G. Transfer of proteins across membranes, Biosynthesis in vitro of pretrypsinogen and trypsinogen by cell fractions of canine pancreas. Eur J Biochem. 1978 Jan 16;82(2):593–599. doi: 10.1111/j.1432-1033.1978.tb12055.x. [DOI] [PubMed] [Google Scholar]
- Shaltiel S. Hydrophobic chromatography. Methods Enzymol. 1974;34:126–140. doi: 10.1016/s0076-6879(74)34012-8. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Jackson R. C., Marcus M. M., Lingappa V. R., Blobel G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1795–1799. doi: 10.1073/pnas.76.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Dobberstein B. Protein transfer across microsomal membranes reassembled from separated membrane components. Nature. 1978 Jun 15;273(5663):569–571. doi: 10.1038/273569a0. [DOI] [PubMed] [Google Scholar]