Abstract
Functional neuroimaging studies have converged to suggest that cortico-striatal-thalamo-cortical (CSTC) circuit dysfunction is a core pathophysiolologic feature of obsessive-compulsive disorder (OCD). Now, complementary approaches examining regional neurochemistry are beginning to yield additional insights regarding the neurobiology of aberrant CSTC circuitry in OCD. In particular, proton magnetic resonance spectroscopy (1H-MRS), which allows for the in vivo quantification of various neurochemicals in the CSTC circuit and other brain regions, has recently been used extensively in studies of OCD patients. In this review, we summarize the diverse and often seemingly inconsistent findings of these studies, consider methodological factors that may help to explain these inconsistencies, and discuss several convergent findings that tentatively appear to be emerging. We conclude with suggestions for possible future 1H-MRS studies in OCD.
Keywords: obsessive-compulsive disorder, OCD, magnetic resonance spectroscopy, N-acetylaspartate, glutamate, choline
INTRODUCTION
Obsessive-compulsive disorder (OCD) is an often-debilitating psychiatric illness with an estimated lifetime prevalence of 2.3% in the United States (1). First-line treatments such as selective serotonin reuptake inhibitors (SSRIs), cognitive-behavioral therapy (CBT), or their combination may benefit OCD, but many patients remain partially or completely refractory to treatment (2). Notably, 30-50% of patients develop OCD starting in childhood (3), and early-onset OCD may represent a more severe developmental subtype of the disorder (4). Thus, it is critical to better elucidate the neurobiology of OCD, characterize its subtypes, and improve treatment strategies.
Functional neuroimaging studies have converged to suggest that cortico-striatal-thalamo-cortical (CSTC) circuit dysfunction is a core pathophysiolologic feature of OCD (5). In OCD patients, the key nodes of this circuit, including orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and striatum, exhibit apparent hyperactivity during neutral or resting states (6–8), which is accentuated during symptom provocation (9–11) and attenuated following successful treatment (12–15). Although some emerging data implicate other brain regions in OCD (e.g., amygdala (16), hippocampus (17), etc.), the CSTC circuit remains the prime focus of research.
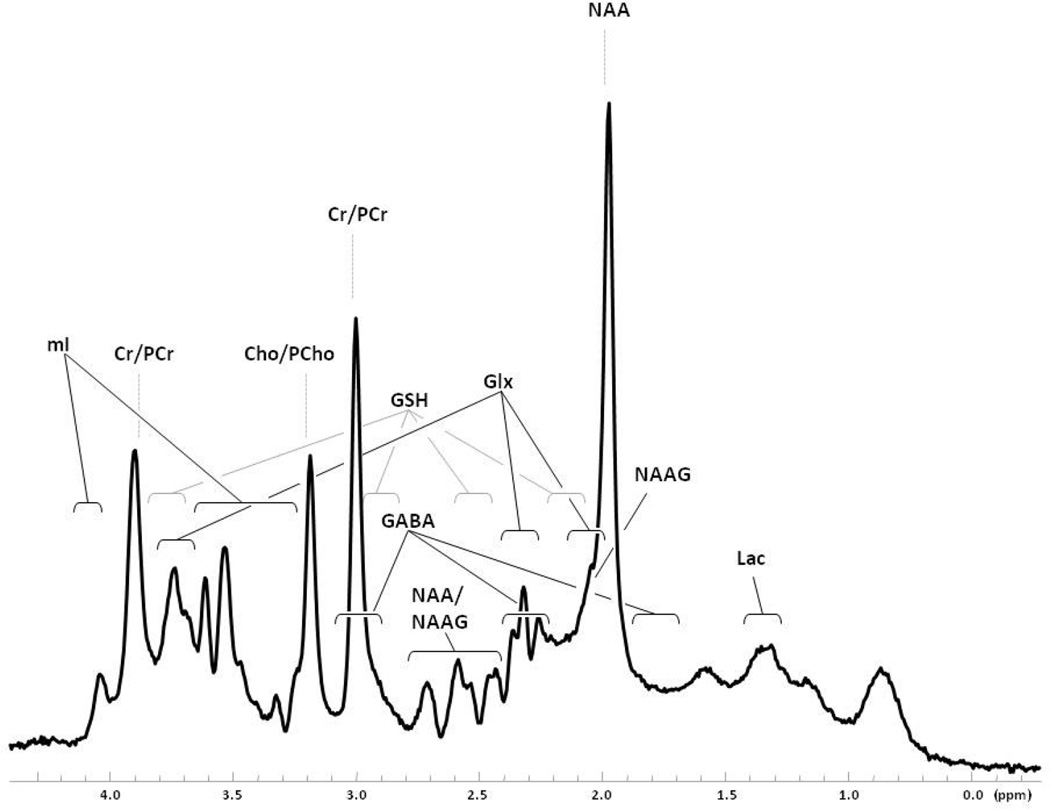
Complementary approaches examining regional neurochemistry now promise additional insights into the neurobiology of OCD. In particular, proton magnetic resonance spectroscopy (1H-MRS) permits in vivo quantification of specific neurochemicals in various brain regions. Using a magnetic field and a brief, tuned radio-frequency pulse, 1H-MRS generates resonance signals from hydrogen nuclei (protons) in neurochemical molecules, yielding a magnetic resonance spectrum with peaks unique to each molecule (Figure 1), and where the strength of each resonance reflects the molecule's concentration.
Figure 1.
In vivo brain 1H-MRS spectrum acquired with 30 ms-TE PRESS acquisition at 4 Tesla. Spectrum is from a 2.5 × 2.5 × 2.5 cm voxel in the parieto-occipital cortex of a healthy adult. Spectral regions denoted with brackets indicate multiplet resonance structures for each featured neurochemical. Spectrum is displayed without any filtering. Cho, choline; Cr, creatine; GABA, γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; GSH, glutathione; Lac, lactate; mI, myo-inositol; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; PCho, phosphorylcholine; PCr, phosphocreatine.
The most commonly reported resonances in the 1H-MRS spectrum are N-acetylaspartate (NAA); N-acetylaspartylglutamate (NAAG); creatine + phosphocreatine (total Cr or “tCr”); choline-containing compounds including choline, phosphorylcholine, and glycerophosphorylcholine (total Cho or “tCho”); myo-inositol (mI); glutathione (GSH); lactate (Lac); and the amino acids glutamate (Glu), glutamine (Gln), and γ-aminobutyric acid (GABA) (Figure 1). NAA, the most prominent 1H-MRS signal, is widely considered a marker of neuronal integrity (18). NAAG appears to be involved in excitatory neurotransmission and in glutamate synthesis (19). Due to their overlapping peaks, NAA and NAAG are commonly expressed as a combined measure (total NAA or “tNAA”). Creatine and phosphocreatine are high-energy compounds reflecting cerebral bioenergetics (20). Choline-containing compounds, constituents of cellular membranes, may reflect abnormal membrane turnover (20). Myo-inositol has been used most commonly as a glial marker (20), but its exact significance remains unclear. Glutathione, an antioxidant, preserves hemoglobin in the ferrous state and supports amino acid transport (20). Lactate reflects mitochondrial function (20), but is still unexplored in OCD. Glu, Gln, and GABA are critical regulators of neuronal excitation and inhibition and are integral to neuronal and glial metabolism (21). The complex and overlapping multiplet structures of Glu and Gln (and, to some extent, GABA) have been difficult to separately and reliably quantify. Thus, until recently, Glu and Gln have been reported as a composite measure (termed “Glx”) comprised primarily of Glu + Gln with minor contributions from GABA and macromolecular resonances.
Below, we summarize available 1H-MRS studies measuring these neurochemicals in various brain regions in OCD, discuss methodological factors that may explain inconsistencies among studies, and suggest possible future 1H-MRS study strategies.
METHODS AND MATERIALS
Using the key words “magnetic resonance spectroscopy,” “obsessive-compulsive disorder,” and “OCD,” we searched PubMed (http://www.ncbi.nlm.nih.gov/pubmed) for studies in English utilizing 1H-MRS to compare levels of various neurochemicals in OCD patients versus healthy individuals or to examine changes following pharmacologic or psychosocial treatment. We accepted both pediatric and adult OCD studies regardless of sample size or brain regions studied, although we address these important factors below.
RESULTS
Twenty-eight studies met our criteria above; 20 compared neurochemical levels in OCD patients vs. healthy individuals (see Table S1 in Supplement 1) and eight examined changes in levels in patients following treatment with SSRIs or CBT (see Table S2 in Supplement 1). Five of these eight studies also included a healthy comparison group to assess baseline differences in levels.
Thirteen (46%) of the 28 studies examined pediatric OCD patients (see Tables S1 and S2). Samples were often small; only eight studies (29%) evaluated more than 20 patients. All studies excluded individuals with psychotic and substance-use disorders, but only nine (32%) excluded all comorbid axis I disorders. Of 25 studies comparing OCD patients and healthy individuals, 15 (60%) evaluated patients taking no psychiatric medications and 10 (40%) evaluated entirely medication-naïve patients.
Looking at 1H-MRS techniques, only four (14%) of 28 studies used field strengths ≥ 3 Tesla (3T). Twenty-six (93%) examined regions within the CSTC circuit (basal ganglia, ACC, OFC, thalamus). Twenty-one (75%) used single-voxel 1H-MRS with voxel sizes ≤ 8 cm3. All studies examined tNAA, tCr, and tCho, but only 16 (57%) examined glutamate-related neurochemicals (14 Glx only; 1 Glx and Glu; 1 Glu and Gln). Eleven (39%) used internal references to tCr or tCho as the main quantification method.
N-acetylaspartate + N-acetylaspartylglutamate (tNAA)
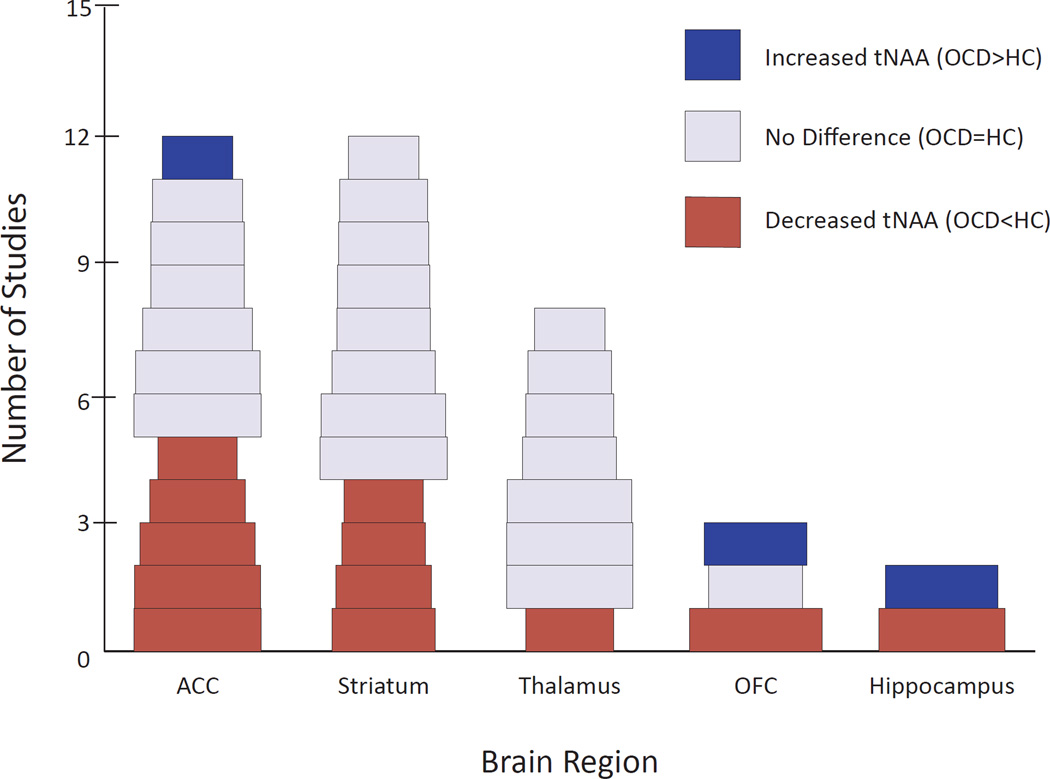
Among 25 studies using healthy comparison individuals, 10 (nine adult, one pediatric) reported significantly decreased tNAA within one or more brain regions in OCD patients (or patient subgroups) vs. comparison individuals (Figure 2) (22–30). Of studies specifically looking at ACC or caudate, 5/12 and 4/12, respectively, reported reduced tNAA in OCD. One additional study (31) reported decreased tNAA in ACC approaching statistical significance (p = .09). Five studies found increased tNAA levels in certain brain regions (27, 32–35), but only one of these (33) found increased tNAA in a CSTC region (left rostral ACC), which then decreased significantly following 12 weeks of CBT. In contrast, another study found significantly increased left caudate tNAA after CBT (36), and three studies found tNAA unchanged after treatment with the SSRI paroxetine (37, 38) or CBT (39).
Figure 2.
Proton Magnetic Resonance Studies of Patients with Obsessive-Compulsive Disorder Assessing Total N-Acetylaspartate by Brain Region. The width of each study block is proportional to the square root of the total sample size (number of OCD subjects plus number of healthy control subjects). ACC, anterior cingulate cortex; HC, healthy controls; OCD, obsessive-compulsive disorder; OFC, orbitofrontal cortex; tNAA, total N-acetylaspartate.
Glutamate-Related Neurochemicals (Glu, Gln, Glx)
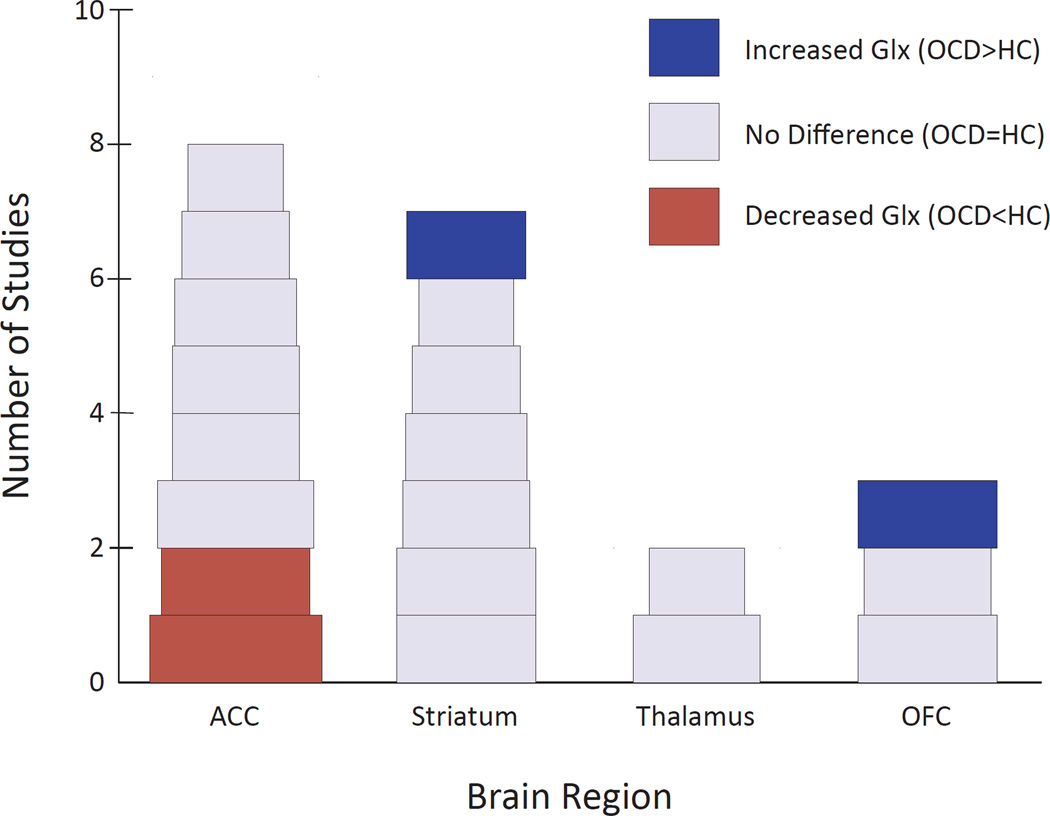
Among 14 studies comparing Glx in OCD vs. healthy individuals, 2/8 reported significantly decreased Glx in ACC (31, 40) and 1/3 reported increased Glx in OFC (35) (Figure 3). One additional study (41), which lacked a healthy comparison group and was therefore not included in our primary analysis, reported decreased ACC Glx in a genetically distinct OCD subgroup (discussed below). Among seven studies examining caudate, one investigation of 11 pediatric OCD patients (42) found increased Glx in the head of the left caudate, which decreased significantly following 12 weeks of paroxetine treatment, with Glx decreases significantly correlated with decreases in OCD symptomatology—replicating an earlier case report from the same laboratory (37). One of the 11 patients showed persistently decreased Glx and OCD symptoms on three-month follow-up after paroxetine discontinuation (38). Another laboratory found caudate Glu and Glx levels positively correlated with symptom severity within OCD patients (43), but no significant overall Glx or Glu differences between patients and healthy individuals in brain regions studied. Finally, four longitudinal studies found no Glx changes in various regions following 12 weeks of CBT (33, 36, 39) or six months of combined SSRI + CBT (44). The one remaining glutamate-related study (23) separately quantified Glu and Gln, and found no differences between OCD patients and healthy individuals in left corpus striatum.
Figure 3.
Proton Magnetic Resonance Studies of Patients with Obsessive-Compulsive Disorder Assessing Glx by Brain Region. The width of each study block is proportional to the square root of the total sample size (number of OCD subjects plus number of healthy control subjects). ACC, anterior cingulate cortex; HC, healthy controls; OCD, obsessive-compulsive disorder; OFC, orbitofrontal cortex.
Choline-Containing Compounds (tCho)
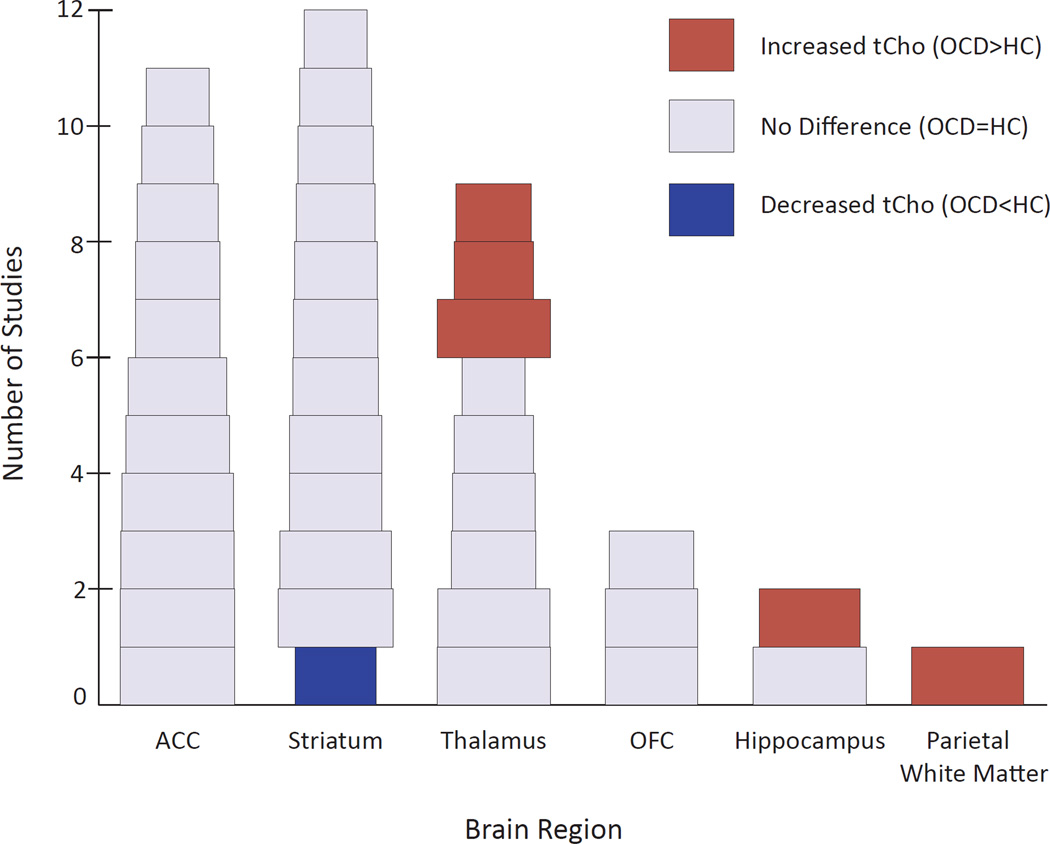
Five of 24 comparison studies found increased tCho in OCD vs. healthy individuals (three in thalamus (24, 45, 46), one in parietal white matter (47), and one in hippocampus (30); see Figure 4), but surprisingly, one (44) found decreased left striatal tCho in OCD and one (33) of eight treatment studies reported increased thalamic tCho in pediatric OCD patients following response to CBT.
Figure 4.
Proton Magnetic Resonance Studies of Patients with Obsessive-Compulsive Disorder Assessing Total Choline by Brain Region. The width of each study block is proportional to the square root of the total sample size (number of OCD subjects plus number of healthy control subjects). ACC, anterior cingulate cortex; HC, healthy controls; OCD, obsessive-compulsive disorder; OFC, orbitofrontal cortex; tCho, total choline.
Creatine + Phosphocreatine (tCr)
All 28 studies measured tCr levels, but since tCr frequently serves as an internal reference, only 16 compared absolute tCr in OCD patients vs. healthy individuals. Of these, two (27, 48) reported increased tCr in OCD (one in ACC (27) and one in thalamus (48)) and one (42) found a trend (p = .09) in the same direction. Conversely, one study found decreased tCr in right OFC in OCD (36). Seven studies analyzed change in tCr following treatment (33, 36–39, 42, 44), with only one (33) reporting a significant decrease in left rostral ACC after CBT.
Myo-inositol (mI)
Of 11 studies assessing mI in OCD patients vs. healthy individuals, one (31) found significantly increased levels in right rostral and dorsal ACC in OCD, while one (35) found significantly decreased levels in caudate. None of seven treatment studies found significant mI changes following SSRIs or CBT.
DISCUSSION
Many of the above-cited studies are compromised by sample heterogeneity, insufficient statistical power, and the lower “assay sensitivity” of older MRS technologies—problems all likely to inhibit detection of differences when differences actually exist. These issues cannot be resolved by meta-analysis, because disparities in study sample selection and technical methodology render combining information across studies inappropriate. Nevertheless, despite these factors mitigating against detection of differences, several findings are possibly converging: 1) reduced tNAA in ACC and caudate; 2) reduced Glx in ACC; 3) increased Glx in caudate; and 4) increased tCho in thalamus, parietal white matter, and hippocampus. Though it is premature to conceptualize these "points of convergence" as results clearly emerging from the literature, they nevertheless suggest preliminary hypotheses for future testing, which we discuss below.
Reduced tNAA in ACC and Caudate
Reduced tNAA may reflect neuronal loss or atrophy (49). Thus 1H-MRS findings of reduced ACC tNAA in OCD appear consistent with structural MRI findings of decreased ACC volume in OCD, as reported in three meta-analyses (50–52). However, 1H-MRS also suggests reduced tNAA in caudate—yet these same meta-analyses found caudate volume equal (51) or increased (50, 52) in OCD across studies collectively—though some individual studies reported reduced caudate volume in OCD (53, 54). Moreover, one meta-analysis demonstrated increased thalamic volumes in OCD (52), whereas all but one 1H-MRS study (29) found no differences in thalamic tNAA between OCD patients and controls. Also weighing against a hypothesis of neuronal loss, several above-cited studies found increased tNAA levels in certain brain regions in OCD (27, 32-35). These inconsistencies make it difficult to relate reduced tNAA to neuronal loss in OCD.
Alternatively, reduced tNAA levels in OCD might not reflect permanent neuronal loss, but rather potentially reversible abnormalities, as suggested by two studies reporting normalization of tNAA levels after treatment with citalopram (28) or CBT (36). NAA is likely involved in numerous biological processes, including neuronal mitochondrial metabolism, myelin lipid synthesis in oligodendrocytes, NAAG synthesis, and neuronal osmoregulation (18). Thus, reduced tNAA in OCD might reflect dysfunction in processes such as these, rather than outright neuronal atrophy. Further investigations using recent advanced 1H-MRS techniques, which can separately quantify the overlapping NAA and NAAG signals (55), may better elucidate the neurochemical pathology of the NAA-NAAG-Glu system.
Reduced Glx in ACC
Cortico-striatal neuronal pathways are primarily glutamatergic, and growing evidence implicates glutamatergic dysfunction in OCD (56). Given recent evidence that glutamatergic neurotransmitter activity is coupled with neuronal glucose metabolism (57), together with evidence of hypermetabolism in ACC from prior 18FDG-PET studies (6–8), one might predict increased ACC glutamatergic activity in OCD. However, the meaning of Glu and Gln levels in 1H-MRS is debated. Glutamate exists both intra- and extracellularly, influencing neuronal metabolism and neurotransmission, respectively. Therefore, Glu levels do not reflect one specific function (21). The combined measure, Glx, may be even less specific. As detailed below, newer advanced 1H-MRS techniques can now quantify Glu and Gln separately, which in turn may help to reconcile 1H-MRS observations with prior 18FDG-PET findings.
Although several studies (22, 25, 43, 58) found no ACC Glx abnormalities in adult OCD patients overall, the possibility remains that Glx is reduced only in certain subgroups defined, for example, by mutations in genes affecting glutamatergic neurotransmission (41), or perhaps simply by gender. Specifically, one study (31) found significantly reduced ACC Glx levels, correlated with OCD symptom severity, in women but not men with OCD.
Increased Glx in Caudate
Although OCD patients may show reduced Glx in ACC, they may show increased Glx in caudate (42), which may decline following response to paroxetine (37, 38, 42). These findings must be interpreted cautiously, however, given other studies failing to find such differences between OCD patients and comparison individuals (22, 33, 35, 36, 43, 44), or declines in caudate Glx following CBT (33, 39). To explain the possible inverse relationship between ACC and caudate Glx levels, Rosenberg and colleagues (40) propose that reduced tonic glutamatergic tone in the ACC might predispose to increased phasic stress-related glutamate release in the caudate.
Further evidence for striatal glutamatergic dysfunction in OCD comes from genetically engineered mice lacking the gene encoding for the anchoring/signaling complex protein SAP90/PSD-95-associated protein-3 (SAPAP3). These mice exhibit increased anxiety and compulsive self-grooming, which declines following chronic treatment with the SSRI fluoxetine (59). Notably, SAPAP3 plays a key role in glutamatergic synaptic signaling and is strongly expressed in the striatum (60). Although some studies have implicated the SAPAP3 gene in human OCD-related disorders, it has not been clearly linked to OCD per se (56).
Increased tCho in Thalamus, Parietal White Matter and Hippocampus
Choline-containing compounds are components of cell membranes (61), and increased tCho levels thus suggest increased membrane turnover from membrane breakdown or synthesis (62). Increased tCho levels have been identified in neurodegenerative disorders such as Alzheimer’s (63) and Huntington’s diseases (64)—perhaps reflecting membrane breakdown associated with neuronal loss. Also, patients with multiple sclerosis show increased tCho levels in and around active plaques (65), suggesting an association between increased tCho and demyelination. Thus the occasional findings of increased tCho in OCD (24, 30, 45–47) might indicate myelin breakdown. This interpretation is strengthened by findings of white matter abnormalities in OCD patients (66–68), and the potential association between OCD and genes involved in myelination (69). Conversely, tCho levels appear normal in other CSTC circuit regions in OCD (22, 23, 25–28, 31, 35, 40, 42, 58, 70), weighing against a demyelination hypothesis.
Limitations
Despite the areas of convergence discussed above, the existing 1H-MRS literature in OCD remains cloudy, likely due to small and heterogeneous study samples, together with widely varying imaging methodology (as reviewed elsewhere (71) in the case of mood disorders). Specifically, the median patient sample size in the above-cited studies was 13—which, assuming equal numbers of healthy controls, yields 90% power to detect only a very large effect size of approximately 1.3. Moreover, samples differed widely in age, illness duration, illness severity, comorbidity, and concomitant medications, which could all influence neurochemical levels. For instance, 45% of reviewed studies examined pediatric patients who, compared with adults, showed shorter illness duration, and likely higher proportions of early-onset OCD—a disorder possibly neurobiologically distinct from later-onset illness. Also, nearly 70% of studies included OCD patients with comorbid depressive or anxiety disorders—conditions themselves associated with neurochemical abnormalities in 1H-MRS studies (72, 73)—and over half of all studies examined patients taking concomitant psychiatric medications.
Looking next at limitations related to 1H-MRS methodology, most studies (86%) employed magnetic field-strengths below 3T, limiting spectral resolution and hence accurate quantification of metabolites, especially for overlapping resonances such as Glu and Gln. Second, 75% of studies used single-voxel 1H-MRS to examine metabolites in restricted brain regions, precluding simultaneous assessment of metabolites across the large neuronal networks implicated in OCD. Third, 39% of studies quantified metabolite concentrations as ratios using tCr or tCho as internal standards—an approach that assumes no inherent differences in tCr or tCho levels between OCD patients and healthy individuals. But tCho may be increased in OCD, as discussed above, and tCr, though still largely unexplored in OCD, appears altered in other psychiatric disorders (74, 75), rendering it also suspect as an internal standard. Fourth, differences in metabolite concentrations between white and gray matter require studies using absolute concentrations (rather than ratios) to account for differences in tissue content and cerebrospinal fluid within voxels. Of the 18 studies using absolute concentrations, only nine used tissue-segmentation techniques to control for voxel tissue composition. This is a concern, especially for metabolites such as tCr, tCho, and glutamate-related measures, which have been shown to differ between white and gray matter (76).
Future Directions
Addressing Clinical Heterogeneity
To address these limitations, one important step is to study more homogeneous patient samples. Sample homogeneity might be improved not only on demographic measures (e.g., age, sex, illness duration, and concomitant medications), but also by applying symptom dimensions to define OCD subgroups (77), as already successfully performed in studies using other neuroimaging modalities (78–81). Existing research findings might suggest further ways to improve homogeneity. For example, the SLC1A1 gene, which encodes the neuronal glutamate transporter EAAC1, appears associated with OCD, but only in males with early-onset illness (82–87)—thus encouraging future 1H-MRS studies examining glutamatergic dysfunction in this subpopulation. To offer another example, future 1H-MRS studies could examine neurochemical differences between OCD patients who exhibit CSTC hyperactivation during symptom provocation vs. those who do not. Indeed, 1H-MRS studies using all of these strategies might elucidate endophenotypes within OCD, ultimately leading to more individualized treatments.
Higher Field Strength/Advanced Spectral Editing Techniques
Glutamatergic abnormalities are probably important in OCD, but technically difficult to assess with 1H-MRS, because the resonance signatures of Glu and Gln overlap substantially with each other and with more dominant resonances, making it difficult to quantify these metabolites separately. However, with higher-field magnets (≥ 3T) and new spectral editing techniques such as two-dimensional J-resolved 1H-MRS (88), one can now accurately differentiate Glu and Gln levels. Still, interpretation of these levels remains complicated. In the so-called glutamate-glutamine (Glu-Gln) cycle, neuronal Glu is released into the synapse, taken up by glial cells and converted to Gln, which is then shuttled back to neurons and reconverted to Glu (21). Additionally, Glu is synthesized in mitochondria through the tricarboxylic acid cycle. Therefore, static Glu and Gln levels do not precisely measure glutamatergic neurotransmitter activity. An aggregate index, the Gln/Glu ratio, may better gauge glutamatergic neurotransmission (albeit still imperfectly) because it reflects Glu release and the reciprocity of Glu and Gln. Increased Gln/Glu ratios likely indicate intensified flux through the Glu-Gln cycle and increased overall glutamatergic neurotransmitter activity (89), making Gln/Glu perhaps the most useful measure for future 1H-MRS studies in OCD. These issues might be clarified by future animal studies coupling 1H-MRS with in vivo microdialysis to localize Glu signals to synapse, glia, or neuron—an approach already employed using nuclear magnetic resonance spectroscopy (90). Advanced editing techniques also permit measurement of other neurochemicals that are not easily quantified due to overlap with other resonances or low brain concentration, such as NAAG (55) and GSH (91). NAAG is both an N-methyl-D-aspartate (NMDA) receptor antagonist and an mGluR3 receptor agonist (19). Thus abnormal NAAG metabolism may cause glutamatergic dysfunction, which in turn may contribute to the pathophysiology of OCD (33). In the 1H-MRS spectrum, 15–25% of the NAA peak derives from NAAG (92), making it valuable to quantify these neurochemicals separately. GSH is a key antioxidant in the central nervous system, and oxidative stress may contribute to the pathophysiology of OCD (93–95). Also, as previously discussed, genetic findings in OCD involve the SLC1A1 gene (82–87), which modulates glutamate neurotransmission and GSH production (96) (e.g., mice lacking EAAC1 exhibit reduced GSH levels (97)).
Multiple-Voxel 1H-MRS Techniques
To date, most 1H-MRS studies in OCD have employed single-voxel 1H-MRS due to its simplicity and resultant high-quality spectra. However, with newer high field-strength magnets, multiple-voxel 1H-MRS techniques (also known as proton magnetic resonance spectroscopy imaging or 1H-MRSI) are increasingly feasible, including even two-dimensional 1H-MRSI using advanced editing techniques in time frames suitable for human subjects (98). This technique can partition individual brain slices into voxels and thus measure neurochemical levels across an entire network of brain regions. Also, 1H-MRSI can measure differences in neurochemical levels between gray and white matter within brain regions (98), thus assessing whether neurochemical abnormalities in OCD are specific to tissue type. For example, given preliminary evidence of both white matter abnormalities and abnormal tCho levels (a possible marker of demyelination) in OCD, this technology could assess white matter tCho levels in OCD patients relative to comparison groups. Additionally, 1H-MRSI could be used with and without symptom provocation to examine acute fluctuations in CSTC neurochemical markers relative to illness expression.
Multimodal Imaging
With increasingly efficient modern 1H-MRS protocols, one can add other neuroimaging techniques (e.g., functional MRI (fMRI), diffusion tensor imaging (DTI), or structural MRI) during the same scanning session to assess functional or structural abnormalities simultaneously with neurochemical dysfunction. The only study (25), to our knowledge, to use this approach in OCD combined 1H-MRS with fMRI and found 1) reduced tNAA in the dorsal ACC in OCD versus healthy individuals, and 2) a negative correlation between tNAA and blood oxygen-level dependent (BOLD) activation during an activation task. This demonstration of linked neurochemical and functional abnormalities supports the role of ACC neuronal dysfunction in OCD, and encourages further 1H-MRS/fMRI studies correlating neurochemical abnormalities and aberrant BOLD activation in other CSTC regions. Similarly, combined 1H-MRS/resting state fMRI might reveal the neurochemical substrates underlying connectivity abnormalities within the CSTC network, and combined 1H-MRS/DTI approaches could investigate the association of specific metabolites, such as tNAA or tCho, with white matter integrity in OCD—a topic of great recent interest (66–68).
Genetics/1H-MRS
With growing evidence regarding the genetic underpinnings of OCD, 1H-MRS can also assess the impact of specific genes on brain biochemistry. For example, one preliminary study (41) demonstrated an association between the G/G genotype of the rs1019385 polymorphism of the GRIN2B gene, which encodes the NR2B subunit of the NMDA receptor, and decreased Glx levels in the rostral ACC of pediatric OCD patients. Despite its limitations (small sample, lack of a comparison group, and use of a low field-strength magnet, thus precluding separate quantification of Glu and Gln), this study illustrates the value of this approach for understanding the contributions of genes to pathophysiology. One can easily envisage other opportunities for 1H-MRS to investigate associations between Gln/Glu ratios or tNAA levels and the growing list of genes potentially associated with OCD and/or encoding proteins involved in glutamatergic neurotransmission, such as the GluR6 subunit of the kainate receptor (GRIK2) (99), and the aforementioned GRIN2B (100), SLC1A1 (82–87), and SAPAP3 genes (60). Similarly, 1H-MRS could assess the impact of different polymorphisms of the OLIG2 gene (69), which encodes a regulator of oligodendrocyte development, on tNAA and tCho levels in OCD.
Longitudinal Studies
Naturalistic longitudinal studies might explore the early evolution of OCD, for example by assessing whether OCD patients and healthy participants exhibit developmental differences in neurochemical markers. In OCD patients receiving treatment, shorter-term longitudinal studies may also elucidate treatment-associated neurochemical changes. For example, 1H-MRS studies in pediatric OCD have hinted that SSRIs might act by modulating cortico-striatal glutamatergic activity (37, 38, 42). This hypothesis should be pursued using advanced spectral editing to investigate SSRI effects on Glu and Gln separately, as well as the Gln/Glu ratio. Using 1H-MRSI, these effects could be further assessed longitudinally in multiple brain regions across the CSTC network—an important topic, given that SSRIs may differentially affect glutamatergic activity in different regions (40). Second, longitudinal treatment studies may identify baseline metabolite abnormalities or early biochemical changes that could predict treatment response—a major potential advance. Third, longitudinal 1H-MRS studies could use pharmacological probes to assess the role of specific neurochemical systems in OCD, possibly leading to improved treatments working through novel mechanisms. For example, 1H-MRS measures of glutamate-related metabolites immediately following treatment with glutamate-modulating medications such as riluzole or memantine—treatments showing possible benefit in OCD (56)—may enhance understanding of glutamatergic dysfunction in OCD, and identify acute neurochemical changes that herald clinical improvement.
Conclusions
Although current findings remain tentative and somewhat inconsistent, 1H-MRS has opened a new window for understanding the pathophysiology of OCD and its treatment. Existing limitations of this research can likely be overcome with larger and more homogeneous subject samples and also, particularly, with recent improvements in 1H-MRS technology. Advances in this domain would be further enhanced by complementary basic neuroscience research designed to better understand the roles of these MRS-measurable neurochemicals at the molecular, cellular, systems, and behavioral levels.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by Grant 1K23-MH092397 from the National Institute of Mental Health (BPB) and the David Judah Fund at Massachusetts General Hospital (BPB).
BPB has received research grant support from Transcept Pharmaceuticals. HGP has received consulting fees from Alkermes and Shire Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
None of the other authors reported any biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenike MA. Clinical practice. Obsessive-compulsive disorder. N Engl J Med. 2004;350:259–265. doi: 10.1056/NEJMcp031002. [DOI] [PubMed] [Google Scholar]
- 3.Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B, et al. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand. 2004;110:4–13. doi: 10.1111/j.1600-0447.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Prado HS, Sada P, et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158:1899–1903. doi: 10.1176/appi.ajp.158.11.1899. [DOI] [PubMed] [Google Scholar]
- 5.Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000;23:563–586. doi: 10.1016/s0193-953x(05)70181-7. [DOI] [PubMed] [Google Scholar]
- 6.Baxter LR, Jr, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. A comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- 7.Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM. Cerebral glucose metabolic rates in obsessive compulsive disorder. Neuropsychopharmacology. 1989;2:23–28. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 8.Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- 9.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–324. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 10.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 12.Baxter LR, Jr, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- 13.Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry. 1990;47:840–848. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- 14.Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002;59:250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- 15.Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry. 1992;49:690–694. doi: 10.1001/archpsyc.1992.01820090018003. [DOI] [PubMed] [Google Scholar]
- 16.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Dolan RJ, Cath DC, Boellaard R, et al. Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res. 2004;132:225–237. doi: 10.1016/j.pscychresns.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, et al. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res. 2003;122:37–47. doi: 10.1016/s0925-4927(02)00104-x. [DOI] [PubMed] [Google Scholar]
- 18.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- 20.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebert D, Speck O, Konig A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Res. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- 23.Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed MA, Smith MA, Schlund MW, Nestadt G, Barker PB, Hoehn-Saric R. Proton magnetic resonance spectroscopy in obsessive-compulsive disorder: a pilot investigation comparing treatment responders and non-responders. Psychiatry Res. 2007;156:175–179. doi: 10.1016/j.pscychresns.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 26.Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Besiroglu L, Sozen M, Ozbebit O, Avcu S, Selvi Y, Bora A, et al. The involvement of distinct neural systems in patients with obsessive-compulsive disorder with autogenous and reactive obsessions. Acta Psychiatr Scand. 2011;124:141–151. doi: 10.1111/j.1600-0447.2011.01726.x. [DOI] [PubMed] [Google Scholar]
- 28.Jang JH, Kwon JS, Jang DP, Moon WJ, Lee JM, Ha TH, et al. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive-compulsive disorder. Am J Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald KD, Moore GJ, Paulson LA, Stewart CM, Rosenberg DR. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biol Psychiatry. 2000;47:174–182. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- 30.Atmaca M, Yildirim H, Ozdemir H, Koc M, Ozler S, Tezcan E. Neurochemistry of the hippocampus in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2009;63:486–490. doi: 10.1111/j.1440-1819.2009.01993.x. [DOI] [PubMed] [Google Scholar]
- 31.Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- 32.Fan Q, Tan L, You C, Wang J, Ross CA, Wang X, et al. Increased N-Acetylaspartate/ creatine ratio in the medial prefrontal cortex among unmedicated obsessive-compulsive disorder patients. Psychiatry Clin Neurosci. 2010;64:483–490. doi: 10.1111/j.1440-1819.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 33.O'Neill J, Piacentini JC, Chang S, Levitt JG, Rozenman M, Bergman L, et al. MRSI correlates of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:161–168. doi: 10.1016/j.pnpbp.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell A, Cortese B, Lorch E, Ivey J, Banerjee SP, Moore GJ, et al. Localized functional neurochemical marker abnormalities in dorsolateral prefrontal cortex in pediatric obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2003;13(Suppl 1):S31–S38. doi: 10.1089/104454603322126322. [DOI] [PubMed] [Google Scholar]
- 35.Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive-compulsive disorder and anxiety. Psychiatry Res. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside SP, Abramowitz JS, Port JD. The effect of behavior therapy on caudate N-acetyl-l-aspartic acid in adults with obsessive-compulsive disorder. Psychiatry Res. 2012 Jan 25; doi: 10.1016/j.pscychresns.2011.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Moore GJ, MacMaster FP, Stewart C, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine therapy for pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 1998;37:663–667. doi: 10.1097/00004583-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Bolton J, Moore GJ, MacMillan S, Stewart CM, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. J Am Acad Child Adolesc Psychiatry. 2001;40:903–906. doi: 10.1097/00004583-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Benazon NR, Moore GJ, Rosenberg DR. Neurochemical analyses in pediatric obsessive-compulsive disorder in patients treated with cognitive-behavioral therapy. J Am Acad Child Adolesc Psychiatry. 2003;42:1279–1285. doi: 10.1097/01.chi.0000087562.01900.de. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- 41.Arnold PD, Macmaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive-compulsive disorder. Psychiatry Res. 2009;172:136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Starck G, Ljungberg M, Nilsson M, Jonsson L, Lundberg S, Ivarsson T, et al. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- 44.Lázaro L, Bargallo N, Andres S, Falcon C, Morer A, Junque C, et al. Proton magnetic resonance spectroscopy in pediatric obsessive-compulsive disorder: Longitudinal study before and after treatment. Psychiatry Res. 2012 Jan 24; doi: 10.1016/j.pscychresns.2011.01.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg DR, Amponsah A, Sullivan A, MacMillan S, Moore GJ. Increased medial thalamic choline in pediatric obsessive-compulsive disorder as detected by quantitative in vivo spectroscopic imaging. J Child Neurol. 2001;16:636–641. doi: 10.1177/088307380101600902. [DOI] [PubMed] [Google Scholar]
- 46.Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, et al. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: a magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- 47.Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive-compulsive disorder: a magnetic resonance spectroscopy study at 3-Tesla. Acta Psychiatr Scand. 2006;114:101–108. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- 48.Mirza Y, O'Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J Child Neurol. 2006;21:106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- 49.Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 50.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 51.Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 53.Robinson D, Wu H, Munne RA, Ashtari M, Alvir JM, Lerner G, et al. Reduced caudate nucleus volume in obsessive-compulsive disorder. Arch Gen Psychiatry. 1995;52:393–398. doi: 10.1001/archpsyc.1995.03950170067009. [DOI] [PubMed] [Google Scholar]
- 54.Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB, et al. Frontostriatal measurement in treatment-naive children with obsessive-compulsive disorder. Arch Gen Psychiatry. 1997;54:824–830. doi: 10.1001/archpsyc.1997.01830210068007. [DOI] [PubMed] [Google Scholar]
- 55.Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 56.Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sibson NR, Shen J, Mason GF, Rothman DL, Behar KL, Shulman RG. Functional energy metabolism: in vivo 13C-NMR spectroscopy evidence for coupling of cerebral glucose consumption and glutamatergic neuronalactivity. Dev Neurosci. 1998;20:321–330. doi: 10.1159/000017327. [DOI] [PubMed] [Google Scholar]
- 58.Bedard MJ, Chantal S. Brain magnetic resonance spectroscopy in obsessive-compulsive disorder: the importance of considering subclinical symptoms of anxiety and depression. Psychiatry Res. 2011;192:45–54. doi: 10.1016/j.pscychresns.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ting JT, Feng G. Glutamatergic Synaptic Dysfunction and Obsessive-Compulsive Disorder. Curr Chem Genomics. 2008;2:62–75. doi: 10.2174/1875397300802010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci. 1996;58:1929–1935. doi: 10.1016/0024-3205(96)00182-8. [DOI] [PubMed] [Google Scholar]
- 62.Hattingen E, Magerkurth J, Pilatus U, Hubers A, Wahl M, Ziemann U. Combined (1)H and (31)P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis. NMR Biomed. 2011;24:536–546. doi: 10.1002/nbm.1621. [DOI] [PubMed] [Google Scholar]
- 63.Meyerhoff DJ, MacKay S, Constans JM, Norman D, Van Dyke C, Fein G, et al. Axonal injury and membrane alterations in Alzheimer's disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins BG, Koroshetz WJ, Beal MF, Rosen BR. Evidence for impairment of energy metabolism in vivo in Huntington's disease using localized 1H NMR spectroscopy. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- 65.Arnold DL, Matthews PM, Francis GS, O'Connor J, Antel JP. Proton magnetic resonance spectroscopic imaging for metabolic characterization of demyelinating plaques. Ann Neurol. 1992;31:235–241. doi: 10.1002/ana.410310302. [DOI] [PubMed] [Google Scholar]
- 66.Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, et al. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety. 2007;24:440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- 67.Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, et al. White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- 68.Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, et al. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand. 2007;116:211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 69.Stewart SE, Platko J, Fagerness J, Birns J, Jenike E, Smoller JW, et al. A genetic family-based association study of OLIG2 in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:209–214. doi: 10.1001/archpsyc.64.2.209. [DOI] [PubMed] [Google Scholar]
- 70.Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H, et al. Proton magnetic resonance spectroscopy of lenticular nuclei in obsessive-compulsive disorder. Psychiatry Res. 1999;92:83–91. doi: 10.1016/s0925-4927(99)00040-2. [DOI] [PubMed] [Google Scholar]
- 71.Capizzano AA, Jorge RE, Acion LC, Robinson RG. In vivo proton magnetic resonance spectroscopy in patients with mood disorders: a technically oriented review. J Magn Reson Imaging. 2007;26:1378–1389. doi: 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- 72.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Mathew SJ, Mao X, Coplan JD, Smith EL, Sackeim HA, Gorman JM, et al. Dorsolateral prefrontal cortical pathology in generalized anxiety disorder: a proton magnetic resonance spectroscopic imaging study. Am J Psychiatry. 2004;161:1119–1121. doi: 10.1176/appi.ajp.161.6.1119. [DOI] [PubMed] [Google Scholar]
- 74.Frye MA, Watzl J, Banakar S, O'Neill J, Mintz J, Davanzo P, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 75.Öngür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, et al. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mataix-Cols D, Rosario-Campos MC, Leckman JF. A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- 78.Koch K, Wagner G, Schachtzabel C, Christoph Schultz C, Straube T, Gullmar D, et al. White matter structure and symptom dimensions in obsessive-compulsive disorder. J Psychiatr Res. 2012;46:264–270. doi: 10.1016/j.jpsychires.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Gilbert AR, Mataix-Cols D, Almeida JR, Lawrence N, Nutche J, Diwadkar V, et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: a voxel-based morphometry study. J Affect Disord. 2008;109:117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 80.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 81.Gilbert AR, Akkal D, Almeida JR, Mataix-Cols D, Kalas C, Devlin B, et al. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2009;48:936–944. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 82.Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, et al. A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:886–892. doi: 10.1002/ajmg.b.30914. [DOI] [PubMed] [Google Scholar]
- 83.Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, et al. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1027–1033. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 84.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–776. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 85.Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, et al. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–785. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 86.Samuels J, Wang Y, Riddle MA, Greenberg BD, Fyer AJ, McCracken JT, et al. Comprehensive family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:472–477. doi: 10.1002/ajmg.b.31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wendland JR, Moya PR, Timpano KR, Anavitarte AP, Kruse MR, Wheaton MG, et al. A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:408–416. doi: 10.1001/archgenpsychiatry.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jensen JE, Licata SC, Öngür D, Friedman SD, Prescot AP, Henry ME, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- 89.Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanamori K, Ross BD, Kondrat RW. Glial uptake of neurotransmitter glutamate from the extracellular fluid studied in vivo by microdialysis and (13)C NMR. J Neurochem. 2002;83:682–695. doi: 10.1046/j.1471-4159.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- 91.Emir UE, Raatz S, McPherson S, Hodges JS, Torkelson C, Tawfik P, et al. Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR Biomed. 2011;24:888–894. doi: 10.1002/nbm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed. 1997;10:73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 93.Orhan N, Kucukali CI, Cakir U, Seker N, Aydin M. Genetic variants in nuclear-encoded mitochondrial proteins are associated with oxidative stress in obsessive compulsive disorders. J Psychiatr Res. 2012;46:212–218. doi: 10.1016/j.jpsychires.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 94.Guldenpfennig M, Wolmarans de W, du Preez JL, Stein DJ, Harvey BH. Cortico-striatal oxidative status, dopamine turnover and relation with stereotypy in the deer mouse. Physiol Behav. 2011;103:404–411. doi: 10.1016/j.physbeh.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Selek S, Herken H, Bulut M, Ceylan MF, Celik H, Savas HA, et al. Oxidative imbalance in obsessive compulsive disorder patients: a total evaluation of oxidant-antioxidant status. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:487–491. doi: 10.1016/j.pnpbp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- 97.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 98.Jensen JE, Frederick Bde B, Renshaw PF. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 99.Sampaio AS, Fagerness J, Crane J, Leboyer M, Delorme R, Pauls DL, et al. Association between polymorphisms in GRIK2 gene and obsessive-compulsive disorder: a family-based study. CNS Neurosci Ther. 2011;17:141–147. doi: 10.1111/j.1755-5949.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA. Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004;174:530–538. doi: 10.1007/s00213-004-1847-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.