Abstract
Objective
Characterization of the structural and functional progression of ocular von Hippel-Lindau (VHL) disease and analysis of patient factors influencing disease progression.
Design
Retrospective analysis of a case series from a longitudinal observational study.
Participants
Two hundred and forty-nine participants with clinically-defined systemic VHL disease and greater than two years of ophthalmic follow-up.
Methods
Standardized scoring of ocular phenotype and systemic characteristics was performed at each study visit and analyzed longitudinally to determine progression of ocular VHL disease.
Main Outcome Measures
Measures evaluated include: visual acuity, features of ocular VHL disease (presence, location, number, and extent of retinal capillary hemangioblastomas [RCHs]), germline mutation in the VHL gene, demographics (age, sex, age of onset of ocular disease), and patient characteristics (smoking status, body mass index).
Results
A majority of participants demonstrated relative anatomical and functional stability in ocular VHL disease status over a mean follow-up period of 8.2±4.0 years. About three-quarters (73%) of participants without ocular VHL disease at baseline remained disease-free at the end of follow-up. Among eyes with ocular VHL disease at baseline, 88% did not develop RCH in a new retinal location, 70% remained stable in RCH number, and 79% remained stable in the extent of RCH involvement. Mean visual acuity for all study eyes (n = 498) decreased by 5.1±0.6 letters across follow-up, with 16.1% decreasing by more than 10 letters. Among eyes affected at baseline, greater vision loss was associated with the presence of juxtapapillary RCHs, development of RCH in a new location, and increase in peripheral RCH number and extent. Younger baseline age, younger age at onset of ocular VHL disease, involvement of fellow eye with ocular VHL disease, and missense or protein-truncating germline mutations were significantly associated with increased anatomical involvement and functional deterioration.
Conclusions
Patients with ocular VHL disease maintain relative anatomical and functional stability, with only a minority demonstrating marked anatomical progression and marked vision loss. Systemic and ocular risk factors for anatomical progression and vision loss can help practitioners identify patients with a higher risk profile for counseling, closer follow-up, and proactive treatment.
Keywords: von Hippel-Lindau, VHL, hemangioblastoma, ocular tumor, retinal angioma
Von Hippel-Lindau (VHL) disease is an autosomal dominant, multi-systemic, progressive cancer syndrome arising from mutations in the VHL gene. While the incidence of VHL disease is low, affecting approximately 1 in 36,000 live births,1, 2 it is highly penetrant, as 95% of patients with characterized mutations in the VHL gene develop disease-related clinical manifestations by age 60 years.3 VHL disease manifestations include central nervous system (CNS) hemangioblastomas, renal tumors (renal cell carcinoma and clear cell carcinoma), pancreatic tumors, endolymphatic sac tumors, pheochromocytomas, and epididymal cystadenomas.4 Ocular manifestations of VHL disease consist of hamartomatous vascular lesions called retinal capillary hemangioblastomas (RCHs), which can disrupt retinal anatomy through structural and exudative effects, leading to vision loss and in severe cases, phthisis.5 For patients who carry a disease-associated mutation in the VHL gene, routine multi-systemic screening for disease manifestations is indicated, beginning early in life.4, 6
Genetic and molecular analysis of VHL function has revealed that the protein encoded by the VHL gene (pVHL) regulates the ubiquitination of hypoxia inducible factor (HIF), a transcription factor, leading to its breakdown.7–9 Intracellular levels of HIF are upregulated in hypoxia and result in increased vascular growth. Mutations in pVHL are thought to interfere with the regulated breakdown of HIF, resulting in excessive expression of HIF-regulated genes, which then produce dysregulated vascular growth in the retina and RCH formation.7 Recent reports have also characterized the role of pVHL in non-HIF-mediated mechanisms, including cell-cell adhesion and cell-extracellular matrix interactions, which can also contribute to RCH formation.10–13
We and others have previously characterized the phenotypes of patients with ocular VHL disease and described the associations between the genotype of the germline VHL mutation and the ocular VHL disease phenotype.14–19 While several studies have previously analyzed the longitudinal progression of ocular VHL disease,14, 15, 20 data arising from a large population of VHL patients that can be useful for prognosticating the course of ocular disease and counseling patients on the visual impact of VHL disease has not been previously available.
In the present study, we analyzed longitudinal data collected from 249 patients with documented mutations in the VHL gene to characterize the anatomical progression of ocular VHL disease. Anatomical disease progression was examined for (1) the development of ocular VHL disease de novo in previously unaffected eyes, and (2) increases in tumor burden in eyes with existing ocular VHL disease, either with new RCH or extent of the fundus involved. The long-term functional impact of ocular VHL disease in the study cohort was also analyzed and correlated with different types of anatomical progression of ocular VHL. Patient factors such as germline VHL mutation status, demographic features, and environmental features were also analyzed for their influence on ocular VHL disease progression.
METHODS
Patient Selection and Ascertainment
Patient data analyzed in the current study were obtained from participants of an institutional review board-approved study protocol at the National Cancer Institute (National Institutes of Health (NIH), Bethesda, Maryland) from 1988 to 2005 (NCT00001238, www.clinicaltrials.gov, accessed June 14, 2012). Potential study participants were screened with a multisystem evaluation that included history and physical examination, laboratory evaluation, radiographic (computed tomography or magnetic resonance imaging) studies of the abdomen, pelvis, brain, and spine, and a full ophthalmic evaluation, at the NIH Clinical Center. A clinical diagnosis of VHL based on family history, the presence of CNS hemangioblastomas, and related visceral tumors as previously defined21, 22 was required for study enrollment. Patients not meeting these clinical criteria, including asymptomatic family members with genetic mutations in the VHL gene, were not included in this study. Patients with ocular VHL disease received standard-of-care treatment during follow-up from a retinal specialist either at the NIH or elsewhere. The VHL medical team included urologic oncologists, neurosurgeons, surgical oncologists, genetic counselors, and ophthalmologists. This study was HIPAA-compliant and adhered to the tenets of the Declaration of Helsinki.
Genotypic analysis of the VHL gene
Analysis for VHL mutations was carried out on peripheral blood samples from at least one member of each participant pedigree. Germline mutations in the VHL gene were classified into one of the following categories: (1) missense mutations (resulting in a single amino acid substitution), (2) proteintruncating mutations (resulting from frameshift, nonsense, and partial deletion mutations), (3) complete deletion of the VHL gene, and (4) other mutation categories (including splice site mutations and single amino-acid deletions).
Assessment of ocular VHL phenotype
In the current longitudinal analysis, we identified participants who had been examined at the National Eye Institute (NEI) eye clinic (Bethesda, MD) with longitudinal standard ophthalmic examinations. Of 898 participants with clinically defined systemic VHL disease originally enrolled (with and without ocular VHL disease), 249 were identified as having received ophthalmic examinations on two or more occasions over a follow-up period of greater than two years. The initial standardized ophthalmic evaluation at the NEI eye clinic was designated the baseline visit, and subsequent visits were designated follow-up visits. The duration of ophthalmic follow-up was defined as the time period between baseline and final follow-up visits. Longitudinal analyses were performed primarily on overall follow-up data. Parallel analyses were also performed at the 3-year and 8-year time points (±1 year) following baseline on the subset of participants for which data at those time points were available.
At the baseline visit, data on the participant’s prior history of ocular VHL disease (including the date of ocular disease onset and history of previous ocular treatments) was collected. Best-corrected visual acuity at each study visit was measured in both eyes of each participant using the Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity chart. The presence or absence of ocular VHL disease was ascertained by a retinal specialist using slit-lamp and indirect funduscopic examination. Fundus imaging (film-based and digital color fundus photography and OPTOS wide angle photography) was obtained as part of clinical care. Information on the presence, location, number, and extent of VHL-associated retinal hemangioblastomas (RCHs) were recorded on a study scoring sheet as previously described.17 Briefly, in eyes with a clear visualization of the posterior pole, the location, number, and peripheral extent of RCHs were recorded. The location of tumors was scored as either (1) juxtapapillary (RCHs located on or within one-half disc diameter of the optic nerve), or (2) peripheral (RCHs located outside of the macular and juxtapapillary regions). The number of RCHs in each eye examined was recorded as the total number of peripheral RCHs; juxtapapillary RCHs were not included in the total. The extent of peripheral retinal involvement, defined as the fraction of the total peripheral retina associated with RCHs, was scored as either (1) less than or (2) greater than or equal to one quadrant of the peripheral retina. Eyes with extensive ocular VHL disease that had resulted in complications precluding funduscopic examinations (such as phthisis or enucleation), and could not be described using the phenotypic categories above, were designated as having “severe involvement.”
Assessment of patient factors related to VHL disease
Participants in this protocol received multidisciplinary care at the NIH Clinical Center. Retrospective chart review of electronic and paper medical records for each study participant was used to obtain patient characteristics at the baseline visit, including sex, race, genotypic category of germline VHL mutation (missense, protein-truncating, or deletion), smoking status (ever smoker or never smoker), presence of fellow eye involvement at baseline, presence of extraocular VHL disease at the baseline and 8 final follow-up visits, and history of surgery for extraocular VHL disease by the baseline and final follow-up visits, which were recorded and analyzed as categorical variables. Baseline age (defined as the participant’s age at the baseline visit), age of ocular disease onset (defined as the date of documented or self-reported age when ocular VHL disease was diagnosed), and body mass index (BMI) at the baseline visit, were analyzed as continuous variables.
Statistical analysis
We conducted analyses at the level of individual patients and at the level of individual eyes to assess the relationships between patient demographics, ocular phenotype, and visual function. Generalized linear model (GLM) analysis of variance (ANOVA) and unpaired t-tests were used to assess the relationship between clinical phenotypes and visual function. Where necessary, Welch’s correction for unequal variances between samples was applied. To evaluate the effect of baseline age, age of onset, sex, genotype, smoking status, BMI, presence of VHL disease affecting the contralateral fellow eye, number and extent of peripheral retinal involvement, and history of systemic VHL involvement or systemic VHL-related surgery on anatomical and functional disease progression, logistic regression using a generalized estimating equation (GEE) was used to account for the correlation between the fellow eyes of a given patient. Kaplan-Meier time-to-event analyses were performed to assess the relationship of genotype on ocular phenotype and visual function. These estimates were compared using the Wilcoxon test of equality over strata. All statistical analyses were conducted using the SAS software package (SAS Institute, Cary, NC). Data values are reported as mean±standard error of the mean unless otherwise noted.
RESULTS
Description of Study Population at Baseline
Participants in the current study were identified from a larger cohort of patients as having an ophthalmic follow-up duration of at least two years. Two hundred forty-nine patients were included in this longitudinal study, and their ophthalmic records were retrospectively analyzed. The distribution of follow-up durations is shown in Figure 1 (available at http://aaojournal.org). The mean±standard deviation (SD) follow-up time was 8.2±4.0 years, with a median of 7.7 years (range=2.0 to 19.8 years).
Baseline demographic and clinical data study participants are summarized in Table 1 (available at http://aaojournal.org). Approximately half (49%) of participants were male. The mean±SD baseline age was 33.3±13.5 years, and the majority of participants (90%) were Caucasian. At study baseline, 62% of participants had clinical evidence of ocular VHL in at least one eye, with involvement being bilateral in 33% and unilateral in 29% of participants. The mean±SD age of onset for ocular VHL disease was 25.2±12.0 years in participants with pre-existing ocular VHL disease at study baseline. Almost all study participants (96%) had a recorded germline mutation in the VHL gene; the remainder did not have available genetic testing information. Missense mutations were most prevalent (48%), followed by protein-truncating mutations (42%) and complete deletions (5%). Splice mutations and amino acid deletions comprised the remaining identifiable genotype mutations (5%).
Anatomical progression of ocular VHL disease
The progression of ocular VHL disease in study participants during follow-up was analyzed in two aspects: (1) the development of de novo ocular VHL disease (in participants without previous ocular involvement in either eye at study baseline) and (2) the change in RCH burden in participants with pre-existing ocular VHL disease at study baseline.
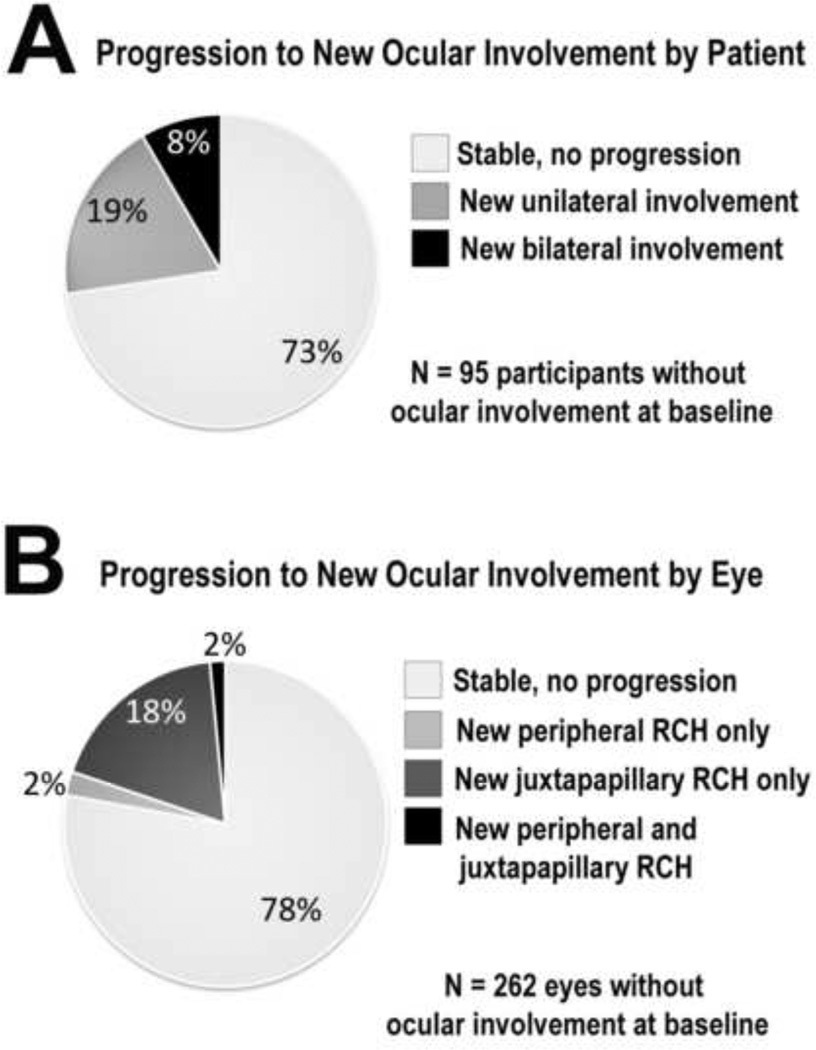
A total of 95 participants (38% of all 249 participants) had no evidence of ocular VHL disease in either eye at the baseline visit. By the final visit, a majority of these (73%, n=69 participants) remained without ocular VHL disease, while about a quarter (27%, n=26 participants) developed de novo ocular VHL disease in at least one eye. In this latter group, 18 participants developed new ocular VHL disease in only one eye, while eight participants developed disease in both eyes (Fig. 2A).
Figure 2. Progression to new onset ocular von Hippel-Lindau (VHL) disease during study followup.
(A) Of participants without ocular VHL disease in either eye at baseline, a majority (73%) remained free of ocular involvement at final follow-up. (B) Of study eyes without ocular VHL disease at baseline, the majority of eyes (78%) were disease-free at final follow-up. RCH = retinal capillary hemangioblastomas
On the level of individual eyes in the study, 498 eyes of 249 participants were examined at the baseline visit. About one half (53%, n=262 eyes) were without ocular VHL disease. At the final visit, a similar majority of these eyes (78%, n=204 eyes) remained without ocular involvement. About a quarter (22%, n=58 eyes) of eyes developed de novo ocular involvement, with 18% (n=48 eyes) developing new RCHs in a peripheral retinal location only, 2% (n=6 eyes) developing new RCHs in a juxtapapillary location only, and 2% (n=4 eyes) developing new RCHs in both juxtapapillary and peripheral locations (Fig. 2B).
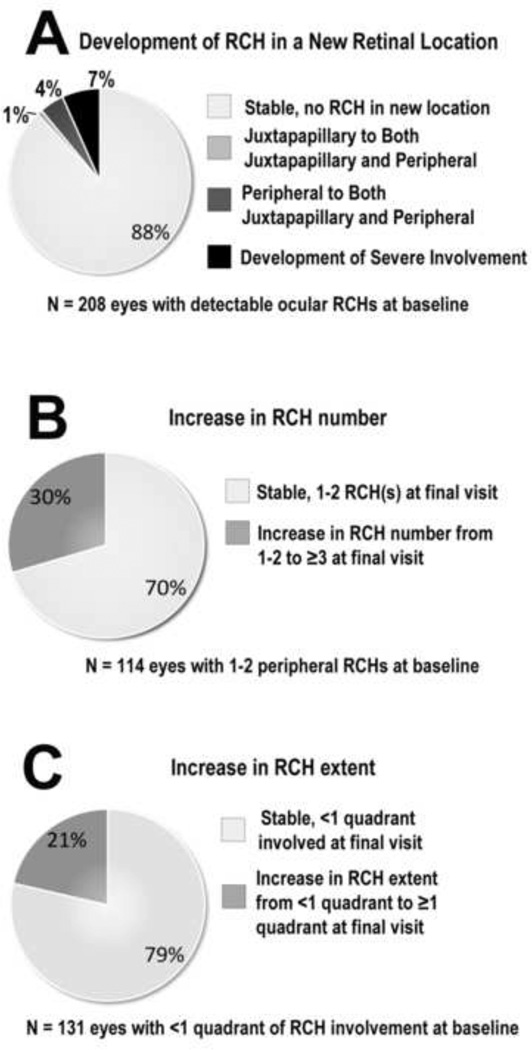
Eyes with pre-existing ocular VHL disease at study baseline were followed for changes in the burden of ocular disease during follow up. Increase in RCH burden was scored according to three separate parameters: (1) development of RCHs in a new retinal location (either peripheral or juxtapapillary) or progression to severe involvement (either phthisis or enucleation), (2) increase in peripheral RCH number (from 1–2 RCH(s) to ≥3 RCHs), and (3) increase in peripheral RCH extent (from <1 quadrant to ≥1 quadrant of retinal involvement). All 236 eyes with pre-existing ocular VHL disease at study baseline were scored according to these progression parameters. For each parameter, the majority of eyes that were susceptible to progression demonstrated stability in terms of all three progression parameters through the final study visit; 88% (n=183 of 208 susceptible eyes) of eyes did not demonstrate development of RCHs in a new location or progression to severe anatomical involvement (Fig. 3A), 70% (n=80 out of 114 susceptible eyes) of eyes remained stable in peripheral RCH number (Fig. 3B); and 79% (n=103 of 131 susceptible eyes) did not increase in the peripheral extent of RCH involvement (Fig. 3C). Of note, although a minority of eyes demonstrated anatomical progression, a small proportion (7%, n=14 eyes) demonstrated progression to the end-stage phenotype (severe involvement) involving phthisis or enucleation.
Figure 3. Anatomical progression of ocular von Hippel-Lindau (VHL) disease in eyes with preexisting ocular VHL disease at baseline.
(A) Of 208 eyes with clinically visualized retinal capillary hemangioblastomas (RCHs) at baseline, most (88%) demonstrated stability in terms of the retinal location of their lesions during study follow-up. Of note, 7% of eyes (10% of participants) progressed to severe involvement (involving phthisis or enucleation). (B) About 30% of eyes (25% of participants) with 1–2 peripheral RCH(s) increased to ≥3 peripheral RCHs during study follow-up. (C) About 21% of eyes (22% of participants) with <1 quadrant of retinal extent of peripheral RCH involvement increased in peripheral RCH extent to ≥1 quadrant during study follow-up.
We additionally performed parallel follow-up analyses at the 3-year and 8-year time points (±1 year) following study baseline examining the rates of anatomical progression in the subsets of participants for which data were available. The results for the same measures of progression are summarized in Table 2. These time point-specific analyses revealed similar long term stability in terms of the examined anatomical parameters, with the progression rates at the 8-year time point being comparable to those for the overall length of follow up (median = 7.7 years).
Table 2.
Anatomical progression of ocular VHL disease at 3 - and 8 - year time points following study baseline
| Baseline | 3 years | 8 years | Final Follow-up (median 7.7 years) |
|
|---|---|---|---|---|
| Patients with no ocular involvement in either eye at baseline | ||||
| Subset of patients evaluated at time point, n (%) | 95 | 40(42%) | 36(38%) | 95 |
| Patients without ocular involvement in either eye, n (%) | 31(78%) | 25(69%) | 69(73%) | |
| Eyes with no ocular involvement at baseline | ||||
| Subset of eyes evaluated at time point, n (%) | 262 | 117(45%) | 99(39%) | 262 |
| Eyes without ocular involvement, n (%) | 91(78%) | 74(75%) | 204(78%) | |
|
Eyes with ocular involvement at baseline at risk for progression in terms of spatial location |
||||
| Subset of eyes evaluated at time point, n (%) | 208 | 106(51%) | 75(37%) | 208 |
| Eyes remaining stable in terms of spatial location, n (%) | 96(91%) | 61(81%) | 183(88%) | |
|
Eyes with ocular involvement at baseline at risk for progression in terms of number of peripheral RCHs |
||||
| Subset of eyes evaluated at time point, n (%) | 114 | 60(53%) | 40(35%) | 114 |
| Eyes remaining stable in terms of number of peripheral RCHs, n (%) | 49(82%) | 28(70%) | 80(70%) | |
|
Eyes with ocular involvement at baseline at risk for progression in terms of extent of peripheral RCHs |
||||
| Subset of eyes evaluated at time point, n (%) | 131 | 66(50%) | 44(34%) | 131 |
| Eyes remaining stable in terms of extent of peripheral RCHs, n (%) | 62(94%) | 35(80%) | 103(79%) |
Key: VHL = von Hippel- Lindau, RCH = retinal capillary hemangioblastoma
Functional consequences of ocular VHL progression
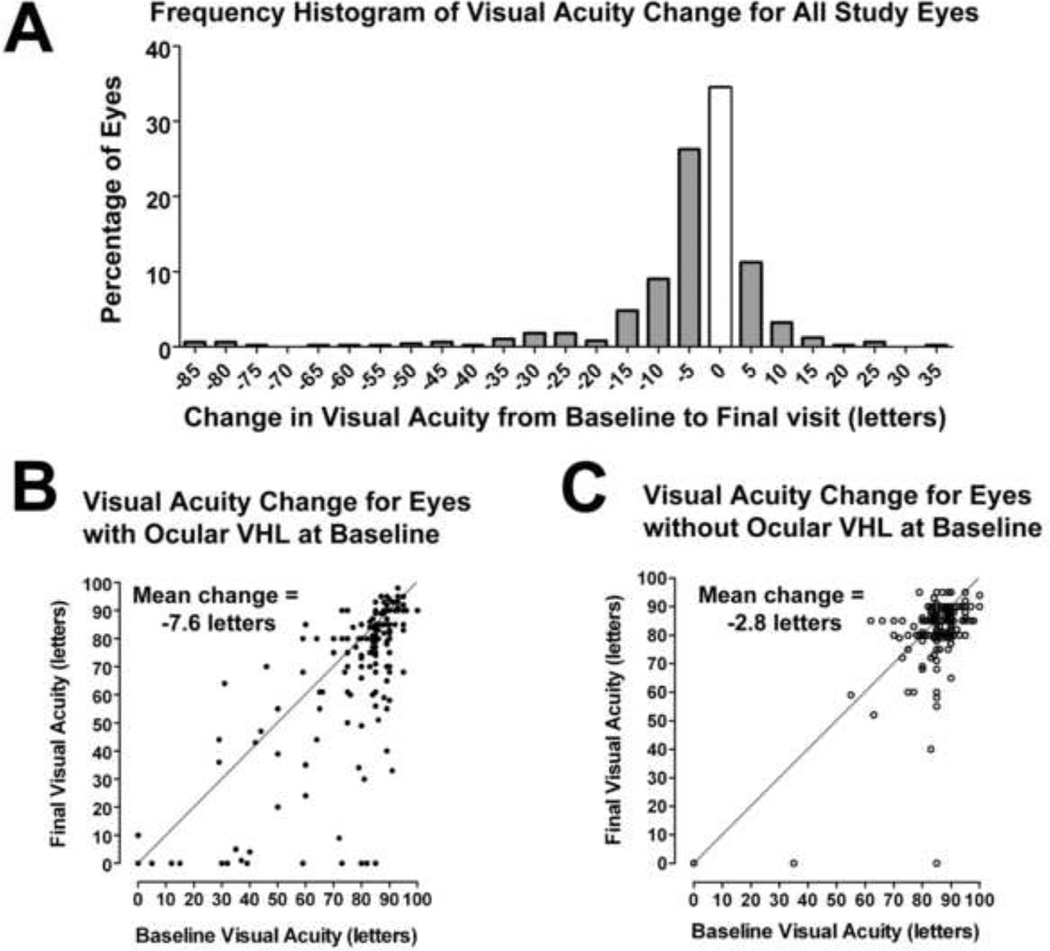
Changes in visual function in study eyes over follow up were analyzed and correlated with the presence or absence of ocular VHL disease at study baseline. Change in best-corrected visual acuity (BCVA) between study baseline and final visit was measured for each eye, and the overall distribution is shown in Figure 4A. In all study eyes (n=498), the mean change in BCVA was −5.1±0.6 letters, with 16.1% (n=80) of eyes demonstrating a decrease of >10 letters. Among eyes with pre-existing ocular VHL disease at baseline (n=236), the mean change in BCVA was −7.6±1.2 letters, with 22.5% (n=53) of eyes demonstrating a decrease of >10 letters during follow-up (Fig. 4B). Among eyes without pre-existing ocular VHL disease at baseline (n=262), mean BCVA change was −2.8±0.6 letters, with 9.9% (n=26) of eyes decreasing in visual acuity by >10 letters (Fig. 4C). Compared to eyes with pre-existing ocular VHL disease, eyes without pre-existing ocular VHL disease demonstrated a smaller mean BCVA change (p=0.0003, unpaired t-test), with a smaller proportion demonstrating a decrease in BCVA of more than 10 letters (Z-score=3.93, p<0.0001).
Figure 4. Change in best-corrected visual acuity (BCVA) in study eyes (n=498) from study baseline to final visit.
(A) Mean BCVA in study eyes decreased by about five ETDRS letters (or approximately one line) during study follow-up. (B and C) Scatter plots relating baseline BCVA to final BCVA in eyes with ocular von Hippel Lindau (VHL) disease at baseline (n=236) (B), and in eyes without ocular VHL disease at baseline (n=262) (C). Points falling below the diagonal line y=x correspond to individual eyes that decreased in BCVA during follow-up.
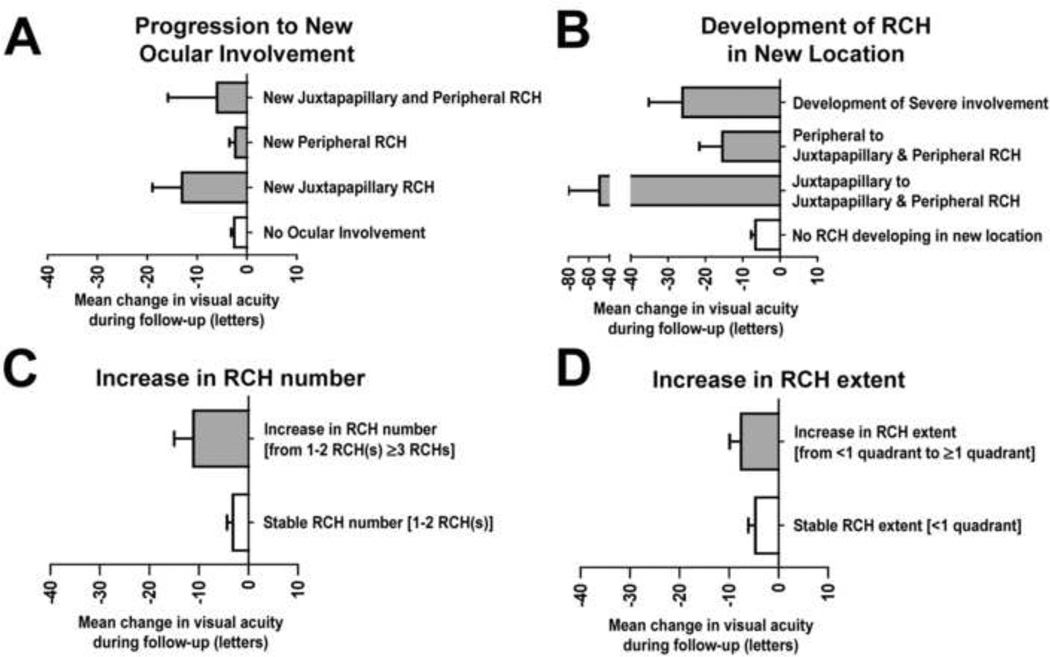
Changes in visual acuity were also correlated with aspects of anatomical progression in ocular VHL disease during follow up. The mean change in BCVA during follow-up was examined among eyes without ocular VHL disease at baseline. Eyes that remained free of ocular VHL involvement from study baseline to final visit demonstrated a small decrease in BCVA (−2.5±0.6 letters, n=204), while eyes developing ocular VHL disease de novo during follow-up (n=58) demonstrated a greater decrease in BCVA (−4.1±1.4 letters) (Fig. 5A). Changes in visual acuity varied significantly according to the presence and nature of new ocular progression (p=0.006, GLM ANOVA). Eyes that developed new peripheral involvement only (n=48) decreased in BCVA by −2.3±1.2 letters, which was similar to eyes that did not develop ocular VHL during follow-up (p=0.86, unpaired t-test). However, eyes that developed new juxtapapillary tumors (n=10) demonstrated a greater decrease in mean visual acuity (−12.7±5.2 letters) compared to either eyes without involvement or eyes developing peripheral RCH only.
Figure 5. Functional significance of anatomical progression of ocular von Hippel-Lindau (VHL) disease.
(A) The mean change in best corrected visual acuity (BCVA) of eyes remaining free of ocular VHL involvement during follow-up (n=204) was small in magnitude (−2.5±0.6 letters), similar that of eyes developing new peripheral RCHs (−2.3±1.2 letters). Eyes developing new juxtapapillary RCHs demonstrated a greater mean decrease in BCVA of −12.7±5.2 letters. (B) Among eyes with pre-existing VHL disease (n=236), the mean decrease in BCVA was greatest in eyes with juxtapapillary RCHs at baseline that developed new peripheral tumors and in eyes developing severe involvement. (C) Eyes that increased in number of peripheral RCHs from 1–2 RCH(s) to ≥3 RCHs demonstrated a mean decrease in BCVA of −11.1±3.9 letters, compared to eyes that maintained 1–2 RCH(s), which demonstrated a mean decrease in BCVA of −3.6±1.3 letters). (D) Eyes that increased from <1 quadrant to ≥1 quadrant of retinal extent of peripheral RCH involvement demonstrated a mean decrease in BCVA of −7.7±2.6 letters, compared to eyes that maintained <1 quadrant of involvement, which demonstrated a mean decrease in BCVA of −4.4±1.2 letters. RCH = retinal capillary hemangioblastomas
The impact of increasing tumor burden in eyes with pre-existing ocular VHL disease at study baseline was also investigated (Fig. 5B). Changes in visual acuity also varied significantly according to whether additional RCH developed and the location of new development (p= 0.04, GLM ANOVA). Eyes that did not develop RCHs in a new retinal location (either juxtapapillary or peripheral) (n=172 eyes) during follow-up decreased in BCVA by −6.6±1.2 letters. In comparison, eyes that developed RCHs in a new retinal location (either juxtapapillary or peripheral) (n=11 eyes), demonstrated a greater decrease in BCVA (−21.6±7.8 letters; p=0.08, unpaired t-test with Welch’s correction for unequal variances). Eyes with juxtapapillary RCHs at baseline which subsequently developed new peripheral involvement during follow-up (n=2) exhibited the greatest decrease in BCVA (−49.5±30.5 letters). Eyes progressing to severe involvement (for which the location of new RCHs could not be ascertained) (n=14) also demonstrated a significantly greater decrease in visual acuity (−26.1±9.0 letters; p=0.05, unpaired t-test with Welch’s correction for unequal variances) compared to eyes that did not progress to severe involvement (−7.4±1.2 letters, n=194).
The effect of increasing tumor burden in terms of increased number of peripheral RCHs and increased retinal extent of peripheral RCH involvement was also examined. Eyes with relatively stable peripheral RCH number [i.e. eyes with 1–2 peripheral RCH(s) at study baseline and also at final visit] showed a small decrease in visual acuity (−3.6±1.3 letters, n=80 eyes) during follow-up. Eyes that increased from 1–2 to ≥3 peripheral RCHs demonstrated a greater decrease in BCVA (−11.1±3.9 letters, n=34 eyes; p=0.07, unpaired t-test with Welch’s correction for unequal variances) (Fig. 5C). The mean change in visual acuity in eyes that were relatively stable in terms of retinal extent of peripheral RCH 13 involvement (n=103 eyes remaining with <1 quadrant of involvement throughout follow-up), relative to eyes that increased in retinal extent of peripheral RCH involvement (n=28 eyes increasing from <1 quadrant to ≥1 quadrant of involvement), was also lower in magnitude (−4.4±1.2 letters vs. −7.7±2.6 letters, respectively), but this difference did not reach statistical significance (p=0.21, unpaired t-test) (Fig. 5D).
Factors influencing progression of VHL disease
Multivariate logistic regression with GEE was performed to detect ocular factors that may be associated with ocular VHL disease progression. Table 3 (available at http://aaojournal.org) shows the p-values for the effect of patient factors on ocular VHL disease progression. Risk factors that were significantly associated with more than one type of anatomical progression included: (1) younger age at baseline visit, (2) younger age at onset of ocular VHL disease, and (3) involvement of fellow eye with ocular VHL disease. In addition, extraocular VHL disease at study baseline (present/absent), and surgery for extraocular VHL disease at study baseline and during follow-up (present/absent) were also analyzed but were not found to be significantly correlated with anatomical progression of ocular VHL disease (data not shown).
A similar analysis was also performed to detect ocular factors that may be associated with functional disease progression in terms of visual acuity loss. Table 4 (available at http://aaojournal.org) shows the p-values for the effect of the same factors on change in visual acuity during follow up. This was analyzed in terms of proportion of eyes (1) progressing to moderate (VA ≤ 35 ETDRS letters) or severe (VA ≤ 15 ETDRS letters) visual loss, and (2) demonstrating clinically significant decrease in visual acuity (decrease in VA ≥ 15 ETDRS letters). Increased risk for visual acuity decrease was also consistently associated with the following: (1) younger age at onset of ocular VHL disease, (2) involvement of fellow eye with ocular VHL disease, as well as (3) increased peripheral extent of involvement.
The genotype category of the germline VHL mutation, while not significantly associated with disease progression in univariate or multivariate analyses, was associated with ocular VHL progression using Kaplan-Meier time-to-event analyses on the basis of individual participants. These highlighted a significantly decreased estimated probability of developing RCH in participants with a complete deletion mutation of the VHL gene, relative to participants with a missense or protein-truncating mutation (p=0.01, Wilcoxon test of equality over strata, Fig. 6A). This genotype-phenotype correlation was also confirmed in a similar analysis carried out on the larger cross-sectional cohort of 868 patients with VHL disease (p=0.0006, Wilcoxon test of equality over strata, Fig. 6B). (Figure 6 is available at http://aaojournal.org.)
DISCUSSION
Using a large cohort of patients with VHL disease, the present study provides a comprehensive longitudinal characterization of the ocular phenotype, visual function, and patient factors affecting disease progression. Here, the diagnosis of VHL disease in study participants was rigorously ascertained using multi-systemic clinical examination in all cases and confirmed by genetic analysis in nearly all cases. After diagnosis, participants were followed longitudinally with multi-systemic evaluations that were performed in a single research medical center, and the phenotype of ocular VHL disease was systematically collected and scored using a standardized grading system. To our knowledge, this is the largest longitudinally-followed cohort of patients with VHL disease to be described in the ophthalmic literature. The study data may be limited by the comparatively short duration of follow-up (8 years) relative to the expected lifespan of participants, and a potential bias towards an over-representation of participants with ocular symptoms during follow-up, despite the institution of regular ophthalmic examinations for all participants. Also, the total length of follow-up in individual patients was variable in this retrospective study and therefore entailed disadvantages of analysis compared to a prospective cohort study with uniform follow-up time. To provide additional time-specific progression data, we performed parallel analyses utilizing visit data at the 3- and 8-year time points following baseline. The rates for anatomical progression in these subsets demonstrated a similar stability for the majority of participants examined, corroborating the analyzed results for overall study follow-up.
In a smaller longitudinal study of 57 patients with ocular VHL disease by Kreusel et al.,20 seven of 33 participants (21.2%) progressed from unilateral to bilateral ocular involvement of VHL disease over a mean of 7.3 years of follow-up. Similarly, in another study of 103 participants by Dollfus et al.,14 approximately 28% of patients with unilateral disease at initial visit progressed to bilateral involvement by final visit over a mean of 8.4 years of follow-up. In the current study of 249 patients, we found a somewhat higher but comparable 33% rate of progression over a mean follow-up of 8.2 years. To our knowledge, no previous longitudinal study has commented on the de novo development of ocular VHL disease in individual patients over follow-up.
Our results indicate that in general, eyes with ocular VHL disease and receiving regular treatment and follow-up are likely to remain phenotypically stable over the time scale of study follow-up. Despite this, anatomical progression in a small minority of eyes in this study was found to be marked, resulting in the overall disruption of the globe and vision loss. The data in the current study also provide a general profile for how visual function changes in a population of patients with VHL disease. A large proportion of the study population maintained its baseline level of visual acuity over study follow-up. While previous studies have also described decreases in visual acuity during follow-up in eyes with pre-existing ocular VHL disease,20 we had additionally compared this decrease relative to eyes without baseline ocular involvement, underscoring that despite general stability of ocular VHL disease, the impact of long-term ocular involvement is appreciable and likely cumulative. .
The data here revealed that the nature of anatomical progression during study follow-up can significantly influence visual function outcomes. The findings of this study indicate that eyes which either (1) develop new juxtapapillary RCHs, (2) develop RCHs in a new retinal location, or (3) increase in peripheral RCH number (from <3 to ≥3 peripheral RCHs) may be expected to be at greater risk of vision loss. This suggests that patients who undergo these patterns of anatomical progression may benefit from closer clinical follow-up and be counseled on the poorer visual prognosis associated with these anatomical progressions.
It is interesting that among the eyes progressing to new ocular involvement, those developing only new peripheral involvement maintained relatively stable mean visual acuity, and were comparable to eyes free of ocular VHL disease, in this regard. This likely indicates that peripheral RCHs which are newly emerging in the early course of disease, because of their distance from the macula, amenability to ablative treatment, and frequently non-exudative nature, may have a smaller impact on central vision compared to emerging juxtapapillary RCHs, which are more central and less amenable to treatment. Our results also indicate, however, that when RCH “load” increases in an affected eye, such as when peripheral RCH involvement increase in number or when peripheral RCHs emerge in combination with juxtapapillary tumors, affected eyes can progress more readily to vision loss.
The analysis was also able to highlight the contribution of patient factors (younger age of onset, younger baseline age, and presence of fellow eye involvement) to overall anatomical and functional progression. The age-dependent associations here indicate that pre-existing ocular VHL disease is more likely to progress during follow-up in a younger patient compared to an older one, resulting in a larger deterioration of visual acuity. Kreusel et al.,20 had previously reported that a younger age of ocular disease onset was associated with poor vision (defined as Snellen VA ≤ 20/1000 / ETDRS 0.05). Dollfus et al.,14 also reported that younger patients, aged 15–25 years, are in a critical age group for the development of hemangioblastomas. However, these associations were not apparent in a cross-sectional analysis performed by Webster et al.,23 in which the prevalence of ocular hemangioblastomatosis was not found to increase with age. Future investigations into age-dependent interactions with VHL gene function may further illuminate these observations, but no mechanisms are currently known.
Fellow-eye involvement as a risk factor for ocular VHL disease progression posits that particular systemic factors may play a role. In our analyses, factors such as smoking status, BMI, or sex did not significantly contribute. Involvement of VHL disease in other organ systems, which was not analyzed in this study, has been inconsistently associated with ocular disease severity; Allen et al., found a correlation between the presence of VHL renal involvement and the severity of CNS involvement,24 while Dollfus et al., were unable to detect a significant relation between ocular VHL involvement and the presence of other systemic VHL manifestations, including renal involvement.14 As previously described, the genotype of the germline VHL mutation to disease phenotype can be a contributory systemic factor.15, 16, 24–27 While logistic regression analyses in our dataset did not discover significant correlations between mutation genotype and progression rates in this study, Kaplan-Meier analyses, which estimate lifetime risk, associated deletion mutations in the VHL gene with a lower lifetime risk for ocular VHL disease. The mechanisms underlying these genotype-phenotype correlations remain an area of active investigation.28
In summary, these findings provide a longitudinal, comprehensive, and quantitative description of the natural history of ocular involvement of VHL disease. These findings provide data that can be helpful to practitioners to prognosticate the course of ocular VHL disease, to clinically manage affected patients, and to counsel patients and their families of the general functional impact of VHL disease. Associations between ocular and systemic factors and the risk of ocular VHL disease and progression may assist in segmenting patients into different risk categories for closer follow-up or aggressive management and may inform future investigations into the biology of ocular VHL disease.
Supplementary Material
Acknowledgments
Financial Support: This work has been supported by the National Eye Institute Intramural Research Program. BCT was supported by the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting; Fort Lauderdale, FL; April 2010).
Conflict of Interest: No conflicting relationship exists for any author.
Financial Disclosure: None of the authors has any financial interests to disclose.
REFERENCES
- 1.Maher ER, Iselius L, Yates JR, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28:443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neumann HP, Wiestler OD. Clustering of features and genetics of von Hippel-Lindau syndrome [letter] Lancet. 1991;338:258. doi: 10.1016/0140-6736(91)90401-a. [DOI] [PubMed] [Google Scholar]
- 3.Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 4.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 5.Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001;46:117–142. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 6.VHL Family Alliance. VHL Handbook. 4th ed. Boston, MA: VHL Family Alliance; 2012. [Accessed May 29, 2012]. pp. 46–51. Available at: http://www.vhl.org/handbook/handbook40.pdf. [Google Scholar]
- 7.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 8.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 9.Lei L, Mason S, Liu D, et al. Hypoxia-inducible factordependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol. 2008;28:3790–3803. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaelin WG., Jr New cancer targets emerging from studies of the Von Hippel-Lindau tumor suppressor protein. Ann N Y Acad Sci. 2010;1210:1–7. doi: 10.1111/j.1749-6632.2010.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thoma CR, Toso A, Meraldi P, Krek W. Double-trouble in mitosis caused by von Hippel-Lindau tumor-suppressor protein inactivation. Cell Cycle. 2009;8:3619–3620. doi: 10.4161/cc.8.22.9907. [DOI] [PubMed] [Google Scholar]
- 12.Turturro F. Beyond the Knudson's hypothesis in von Hippel– Lindau (VHL) disease-proposing vitronectin as a "gene modifier". J Mol Med (Berl) 2009;87:591–593. doi: 10.1007/s00109-009-0466-z. [DOI] [PubMed] [Google Scholar]
- 13.Wiesener MS, Maxwell PH, Eckardt KU. Novel insights into the role of the tumor suppressor von Hippel Lindau in cellular differentiation, ciliary biology, and cyst repression. J Mol Med (Berl) 2009;87:871–877. doi: 10.1007/s00109-009-0504-x. [DOI] [PubMed] [Google Scholar]
- 14.Dollfus H, Massin P, Taupin P, et al. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest Ophthalmol Vis Sci. 2002;43:3067–3074. [PubMed] [Google Scholar]
- 15.Webster AR, Maher ER, Moore AT. Clinical characteristics of ocular angiomatosis in von Hippel-Lindau disease and correlation with germline mutation. Arch Ophthalmol. 1999;117:371–378. doi: 10.1001/archopht.117.3.371. [DOI] [PubMed] [Google Scholar]
- 16.Wong WT, Agrón E, Coleman HR, et al. Genotype-phenotype correlation in von Hippel-Lindau disease with retinal angiomatosis. Arch Ophthalmol. 2007;125:239–245. doi: 10.1001/archopht.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong WT, Agrón E, Coleman HR, et al. Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel-Lindau disease. Ophthalmology. 2008;115:181–188. doi: 10.1016/j.ophtha.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong WT, Chew EY. Ocular von Hippel-Lindau disease: clinical update and emerging treatments. Curr Opin Ophthalmol. 2008;19:213–217. doi: 10.1097/ICU.0b013e3282fb7c04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong WT, Liang KJ, Hammel K, et al. Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology. 2008;115:1957–1964. doi: 10.1016/j.ophtha.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreusel KM, Bechrakis NE, Krause L, et al. Retinal angiomatosis in von Hippel-Lindau disease: a longitudinal ophthalmologic study. Ophthalmology. 2006;113:1418–1424. doi: 10.1016/j.ophtha.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Melmon KL, Rosen SW. Lindau's disease: review of the literature and study of a large kindred. Am J Med. 1964;36:595–617. doi: 10.1016/0002-9343(64)90107-x. [DOI] [PubMed] [Google Scholar]
- 22.Lamiell JM, Salazar FG, Hsia YE. von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore) 1989;68:1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Webster AR, Richards FM, MacRonald FE, et al. An analysis of phenotypic variation in the familial cancer syndrome von Hippel-Lindau disease: evidence for modifier effects. Am J Hum Genet. 1998;63:1025–1035. doi: 10.1086/302037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen RC, Webster AR, Sui R, et al. Molecular characterization and ophthalmic investigation of a large family with type 2A Von Hippel-Lindau Disease. Arch Ophthalmol. 2001;119:1659–1665. doi: 10.1001/archopht.119.11.1659. [DOI] [PubMed] [Google Scholar]
- 25.Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- 26.Maher ER, Webster AR, Richards FM, et al. Phenotypic expression in von Hippel-Lindau disease: correlations with germline VHL gene mutations. J Med Genet. 1996;33:328–332. doi: 10.1136/jmg.33.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mettu P, Agrón E, Samtani S, et al. Genotype-phenotype correlation in ocular von Hippel-Lindau (VHL) disease: the effect of missense mutation position on ocular VHL phenotype. Invest Ophthalmol Vis Sci. 2010;51:4464–4470. doi: 10.1167/iovs.10-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011;15:187–195. doi: 10.1111/j.1582-4934.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.