Background: IL-15 can either be transpresented by IL-15Rα or be secreted.
Results: New N- and C-terminal splice versions of human IL-15Rα determine whether IL-15 is secreted or stays bound to the cell membrane.
Conclusion: IL-15Rα isoforms determine the mode of action of IL-15.
Significance: IL-15Rα isoforms may modify immune response outcomes in humans.
Keywords: Glycosylation, Immunology, Interleukin, Intracellular Processing, Secretion
Abstract
Species-specific differences of post-translational modifications suggested the existence of human IL-15Rα isoforms. We identified eight new isoforms that are predicted to modify the intracellular C termini of IL-15Rα, and another N-terminal exon “Ex2A” that was consistently present in all but one of the C-terminal isoforms. Ex2A encodes a 49-amino acid domain that allowed the transfer of IL-15/IL-15Rα complex to the cell surface but prevented its cleavage from cell membranes and its secretion thus facilitating the transpresentation of IL-15 as part of the immunological synapse. The Ex2A domain also affected the O-glycosylation of IL-15Rα that explained the species-specific differences. The Ex2A domain appeared to be removed from major IL-15Rα species during protein maturation, but both Ex2A and IL-15Rα appeared on the surface of monocytic cells upon activation. The membrane-associated form of the only C-terminal isoform that lacked Ex2A (IC3) was retained inside the cell, but soluble IL-15/IL-15Rα complexes were readily released from cells that expressed IL-15/IL-15Rα-IC3 thus limiting this IL-15/IL-15Rα isoform to act as a secreted molecule. These data suggest that splice versions of IL-15Rα determine the range of IL-15 activities.
Introduction
IL-15 is a common gamma chain cytokine with a well-defined set of target cell populations, mainly NK cells and CD8 T cell subsets (1, 2). The cytokine is necessary for their survival and proliferation and contributes to their activation.
IL-15 and IL-15Rα appear to form a dimeric cytokine. IL-15 and IL-15Rα are strictly co-induced on monocytic cells (3–5). Strong inductions are achieved with a combination of an interferon and either TLR-ligands or CD40 ligand, and the transcriptions of both IL-15 and IL-15Rα depend on interferon-response factors and on NF-κB recognition sites (6–8). Adoptive transfer experiments in mice showed a necessity of both IL-15 and IL-15Rα expression in the same cells for the survival of target cells (3, 5). The expression of IL-15Rα on both CD11b+ and CD11c+ cells contribute to the survival of IL-15-dependent NK and CD8 cells (9).
IL-15/IL-15Rα complexes function via several distinct modes: In the well-documented “transpresentation” mode, the dimeric cytokine stays anchored on the surface of monocytic cells forcing target cells to accept co-stimulatory signals in addition to the cytokine stimulation (10). Alternatively, IL-15/IL-15Rα complexes can be cleaved from the surface of producing cells (11) to act as a soluble heterodimer on neighboring cells in a paracrine fashion. IL-15 also exerts effects on cells that produce the cytokine themselves in that it increases the survival of mature dendritic cells (DCs)2 and regulates protease activities in mastocytes (4, 12). These effects may be accomplished by autocrine activities of IL-15, and similar defects that we observed in DCs that lacked either the cytokine or part of the receptor appear to support this scenario (4). Alternatively, the binding of signaling molecules to the intracellular portion of IL-15Rα itself may also suggest reverse signaling events (13). The precise basis for the various modes of IL-15 activity is currently unknown.
Directing immune responses to specific targets such as cancerous cells by treatments with IL-15 may represent a valuable approach (14). We and others have shown an additive effect of soluble IL-15Rα on IL-15 activity both in vivo and in vitro (15–17). IL-15Rα had accessory activity on the amplitudes of CD8 responses as well as during the response against NK cell-sensitive tumors in vivo. The IL-15Rα effect may be partially caused by an increased half-life of IL-15 when bound to IL-15Rα, but direct activity-modulating effects also appear to be mediated by IL-15Rα. A potential clinical use of IL-15Rα accessory to IL-15 appears to necessitate knowledge about the exact nature of this protein under physiologic conditions.
Here we explore the molecular basis for the various modes of IL-15 action. We report that a number of previously unrecognized isoforms of human IL-15Rα exists that direct the cytokine to paracrine versus transpresentation activities by facilitating secreted or membrane-bound forms of the cytokine.
EXPERIMENTAL PROCEDURES
Mice and Human Cells
C57BL/6 wildtype mice were purchased from the Jackson Laboratory and were housed and treated within published guidelines of humane animal care, and all procedures were approved and performed according to National Cancer Institute Animal Care and Use Committee-approved protocols for animal research. The use of human cells was approved by The Institutional Review Board of the National Cancer Institute, NIH, and all samples were obtained with informed consent.
Cell Culture
293HEK and U2OS cells (ATCC) were propagated in DMEM supplemented with 10% FBS. For transfections, cells were trypsinized, washed, and seeded into 6-well plates. Transfections were done 18 h later using a total amount of 3 μg of plasmid DNA per well containing various amounts of specific plasmid DNA and empty pcDNA3.1 (Invitrogen) and 4 μl of Lipofectamine 2000 (Invitrogen), resulting in greater than 90% transfection efficiency for 293 cells. The culture medium was exchanged 8 h later with RPMI containing 8% human AB serum (Cellgro) to generate supernatants for human NK cell proliferation, or otherwise with DMEM-10% FBS. Supernatants were collected 48 h later; otherwise cells were used 24 h after transfection. Where indicated, the O-glycosylation inhibitor Benzyl-GalNAc was added into the culture medium to 4 mm 1 h after transfections. Stable U2OS clones were generated by the presence of 400 μg/ml G418 (Invitrogen) and limited dilution.
To generate membrane fractions containing human CD40 ligand, 293HEK cells that had been transfected with a CD40 ligand-encoding plasmid 24 h prior were washed, resuspended in 210 mm d-mannitol, 70 mm sucrose, 1 mm EDTA, 10 mm Hepes, pH 7.2, mechanically disrupted by 30 passages through 27-gauge needles and centrifuged at 14,000 × g. The resulting pellet from ∼5 × 106 transfected cells was used to mature ∼5 × 106 human DCs. As a control we used membrane fractions prepared from mock-transfected 293HEK cells that did not affect DC maturation or their expression of IL-15 or IL-15Rα (not shown).
Human DCs were derived from elutriated monocytes (Blood Bank, NIH, Bethesda) and cultured for 5 days in RPMI supplemented with 8% human serum and 50 ng/ml recombinant human GM-CSF and IL-4 (Peprotech). Maturations were done by overnight exposures to combinations of 20 ng/ml human IFN-γ (Peprotech) and 100 ng/ml LPS (Escherichia coli 055:B5, Sigma), the membrane fraction of CD40 ligand-transfected 293HEK cells, 10 μg/ml poly I:C (Sigma), exponentially growing listeria monocytogenes bacteria at a multiplicity of infection of 0.5 or vaccinia virus (Western Reserve, multiplicity of infection of 10). Maturations were verified by FACS analyses of CD80, CD86, and MHC class II expression (not shown). Murine bone marrow-derived DCs were prepared by growing C57BL/6 bone marrows in RPMI supplemented with 10% FBS and 40 ng/ml recombinant murine GM-CSF (Peprotech) for 5 days. To generate murine monocyte-derived DCs, murine PBMCs were isolated via Ficoll-centrifugation of blood samples and allowed to adhere to tissue culture plates for 2 h. Plates were rinsed repeatedly, and the remaining adhering cells were incubated for 5 days in RPMI containing 10% FBS, 40 ng/ml murine GM-CSF, and 50 ng/ml murine IL-4 (Peprotech). DCs were matured overnight in RPMI containing 10% FBS, 50 ng/ml LPS (E. coli 055:B5, Sigma), and 20 ng/ml murine IFN-γ (Peprotech).
To derive human NK cells, blood samples from healthy donors were depleted of erythrocytes via Ficoll-centrifugation and sorted with the negative NK cell isolation kit (Miltenyi). NK cells were expanded in culture in RPMI containing 8% human AB serum and 1 nm human IL-2 (Peprotech) for 7 days prior to use in proliferation assays. PBMCs were also used directly after labeling with CFSE (500 nm, 10 min at 37 °C, Invitrogen).
Generation of Antibodies
The entire Ex2A domain was expressed as a GST fusion protein (pGEX-2T, Pharmacia) in E. coli, isolated and used to immunize rabbits and mice. Rabbit antiserum was produced by Prosci, Poway, CA. Mouse mAbs were generated using standard procedures (18). The screening of mAbs was performed by ELISA using supernatants from 293HEK cells that had been transfected with plasmids encoding IL-15 and a fusion construct of the extracellular portion of human IL-15Rα containing Ex2A and the two C-terminal constant regions of human IgG1. Ascites were produced from positive clones by Harlan Laboratories, Indianapolis, IN. For use in cytometry, mAbs were biotinylated using the EZ-Link Sulfo-NHS-LC-Biotinylation Kit (Pierce). Point mutation analyses showed binding of αEx2A1/2/3 mAbs to be affected by amino acids C27, C36, and H41 of the mature Ex2A domain in cytometry analyses, while binding of αEx2A4 in immunoblots involved amino acids E15, F17, H19, and E20 (not shown).
Cytometry
Blood cells were analyzed after removing erythrocytes via Ficoll-centrifugation. Cells were blocked with a mixture of mouse IgG1, IgG2a, IgG2b, IgG3, and hamster IgG for 15 min at room temperature that was followed by a 30-min incubation on ice with the specific antibody. For biotinylated antibodies, an additional 15-min incubation on ice was done with streptavidin-PerCP-CY5.5 (Ebioscience). Antibodies against the following proteins were used: CD3, clone SK7; CD14, M5E2; CD20, L27; MHC class II, TU39 (all BD biosciences); CD56, MEM188; CD8, RPA-T8 (Ebioscience); IL-15, M111 (ATCC), IL-15Rα, 7A4 (10) and anti-Ex2A antibodies as described above.
Proliferation Assay
Human NK cells that had been cultured for 7 days in 1 nm IL-2 were washed three times and plated into 96-well plates at 5 × 104 cells per well. Cells were incubated for 48 h with various amounts of supernatants that had been collected from transfected 293HEK cells. [3H]Thymidine (1 mCi, Perkin-Elmer) was present during the final 12 h of the assay.
RT-PCR and Sequencing
Total RNA was prepared with Trizol (Invitrogen) according to the manufacturer's instructions. 1 μg of RNA was subsequently transcribed into cDNA using an oligo-dT primer and 200 units of Superscript III reverse transcriptase (Invitrogen). Primers used for PCR are given in the supplemental Table S1. Parameters for all PCR amplifications were 3 min at 94 °C, followed by 35 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 90 s, with a final extension of 72 °C for 5 min. To amplify the entire coding region of human IL-15Rα, a nested step was necessary in which 1 μl of the first PCR was used as template for the nested step. PCR reactions were size-fractionated, bands were isolated with GenElute Spin Columns (Sigma), and sequenced directly. The new IL-15Rα sequences that are described in this report have been scanned against the database, and no sequence with significant relatedness to the new sequences other than genomic sequence has been identified. The nucleotide sequences reported in this paper have been submitted to GenBankTM with accession numbers HQ401283, JX987304, JX987305, JX987306, JX987307, JX987308, JX987309, JX987310, JX987311.
Plasmid Constructs
Coding sequences for human IL-15, IL-15Rα, CD25, and CD40 ligand were amplified by RT-PCR, cloned into pCR2.1-TOPO (Invitrogen), verified by sequencing and recloned into the mammalian expression vector pcDNA3.1. The generation of chimeric expression constructs containing the extracellular portion of IL-15Rα and the two C-terminal constant regions of human IgG1 has been described previously (15). Oligonucleotides that were used to generate other chimeric, deletion, and tagged constructs are given in the supplemental Table S1, and cloning steps should be self-explanatory. Point mutations were introduced using the QuikChange II XL Kit (Agilent).
Immunoprecipitation and Western Blot
All antibodies used for immunoprecipitation were covalently bound to protein G-agarose (Pierce). As controls for goat anti-human and goat anti-mouse IL-15Rα antibodies (AF247 and AF551 from R&D Systems) we used goat IgG (Sigma), as control for mouse anti-human IL-15Rα (7A4), mouse anti-human IL-15 (M111) and anti-Ex2A we used mouse IgG (Pierce). Cell lysates were used from ∼5 × 106 DCs for the detection of endogenous proteins, and from 106 transfected 293HEK cells for the detection of overexpressed proteins per sample. Lysis was done in 150 mm NaCl, 1% Triton-X-100, 25 mm Hepes, pH 7.3, 10% glycerol, supplemented with protease inhibitors (Roche). Cell lysates were cleared by centrifugation and incubated with agarose-bound immunoprecipitating antibodies for 2 h at 4 °C with constant rotation, washed four times with lysis buffer and subjected to SDS-PAGE (Tris-Glycine Gels, Invitrogen) that was followed by immunoblotting with antibodies against human IL-15Rα (SC12378 from Santa Cruz Biotechnology and SAB1100259 and 1100260 from Sigma), murine IL-15Rα (AF551, R&D Systems), CD25 (SC665, Santa Cruz Biotechnology), human IL-15 (SC7889 from Santa Cruz Biotechnology), the Flag tag (M2 from Sigma), or the Ex2A domain (described above).
Pulse-Chase and Glycosidase Treatments
Proteins were expressed in 293 cells and immunoprecipitated with anti-Flag M2 antibodies (Sigma) as described above. For pulse-chase experiments, cells were metabolically labeled with 35S-protein labeling mix (100 μCi/ml, MP Biomedicals, 15 min), and chased for the times indicated with complete DMEM/10% FBS. Proteins were eluted either in 0.1% (w/v) SDS/0.1 M 2-mercaptoethanol and boiling for 10 min or in 1% (w/v) SDS/0.1 m 2-mercaptoethanol and boiling that was followed by extensive dialysis into 150 mm NaCl. The resulting preparations were digested with endoglycosidase H (Roche) in 100 mm sodium citrate, pH 5.5, O-sialoglycoprotease (Cedarlane) in 50 mm Hepes, pH 7.4, 1% Nonidet P-40, neuraminidase, and O-glycosidase (NEB, using the provided buffers), Chondroitinase ABC (Sigma, 40 mm Tris acetate, pH 8.0) and Heparinase I/II/III mixtures (Sigma, 40 mm Tris acetate, pH 7.0) at 37 °C overnight. Digests were subjected to SDS-PAGE, and unlabeled proteins were analyzed by Western blotting.
ELISA
Nunc Maxisorp plates were coated with antibody (5 μg/ml anti-IL15 M111 or goat anti-human IgG from Pierce in PBS at 4 °C overnight) and blocked with 1% w/v bovine serum albumin at 37 °C for 1 h. Samples were added and incubated at 37 °C for 1 h that was followed by the second antibody (anti-human IL-15Rα AF247, R&D Systems, anti-IL-15 M111, or anti-Ex2A1, all biotinylated and at 1 μg/ml), alkaline phosphatase-conjugated streptavidin (Pierce, 1 μg/ml, 37 °C for 1 h) and substrate.
Microscopy
For immunofluorescence microscopy, U2OS clones were grown on coverslips, fixed with methanol (−20 °C for 5 min), blocked with 1% BSA and 5% goat serum (37 °C for 1 h), incubated with primary antibodies (rabbit anti-GM130 or anti-PD1, Abcam, 37 °C for 1 h), incubated with secondary antibody (goat anti-rabbit-Cy3, Invitrogen, 37 °C for 1 h), incubated with DAPI (100 nm, room temperature, 20 min) and mounted. Confocal fluorescence images were acquired using a Zeiss LSM510 META laser-scanning microscope equipped with a 63× Plan-aprochromat (N.A. 1.4) oil immersion objective lens, with an optical slice thickness of 0.9 μm, a x-y pixel sampling of 0.07 μm, and a z-step size of 0.35 μm. The resultant z-stack image series were background subtracted and an intensity threshold mask applied representative of the respective cell labels used to calculate colocalization coefficients through the volume of the sample using the colocalization analysis module of Imaris software (v 7.0).
RESULTS
Cloning of IL-15Rα Isoforms
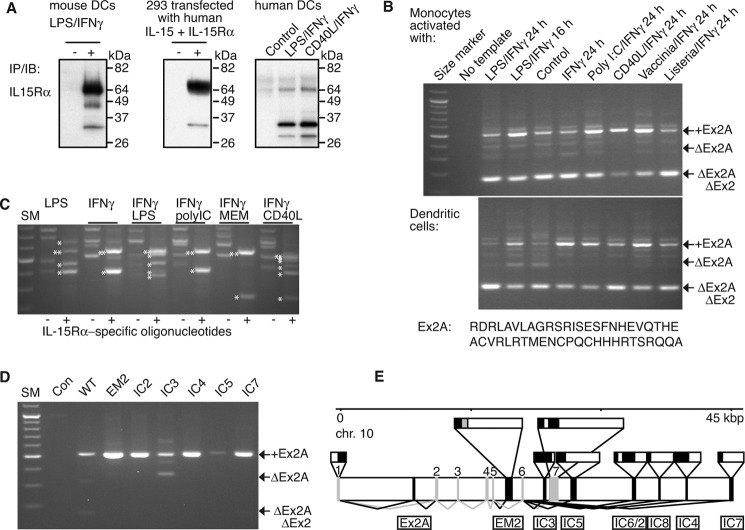
Activated monocytes and DCs are major sources of IL-15. As previously reported (4, 19, 20) and as shown in Fig. 1A, endogenous IL-15Rα from activated human DCs differs from endogenous murine IL-15Rα and from human IL-15Rα generated by overexpression in that the majority migrates at a lower molecular weight. We had previously shown that O- and N-glycosylation events modify IL-15Rα resulting in migration at a higher molecular weight (19). The glycosylation discrepancies as shown in Fig. 1A could have been caused by at least two different mechanisms: (A) human DCs could possess a specific glycosylation-inhibiting mechanism, or (B) sequence of human IL-15Rα may not be fully known, and additional domains may affect glycosylation patterns. We investigated the latter possibility. We generated cDNAs from human DCs that had been matured with combinations of IFN-γ and TLR ligands or CD40 ligand and applied several PCR-based cloning strategies (supplemental Fig. S1). To search for additional internal exons of fully spliced message, a first PCR step used oligonucleotides that annealed inside the first and last exon of IL-15Rα mRNA that was followed by a nested step to amplify a sequence that comprised one or two exon borders. We observed that all IL-15Rα mRNAs from variously treated monocytes and DCs contained an additional 153-bp sequence inserted between the previously dedicated exons 1 and 2 that we will refer to as “exon 2A” or “Ex2A” (Fig. 1B, the predicted amino acid sequence is shown below the panels). It is important to note that amplification of Ex2A largely depended on the first PCR step that targeted full-length and fully spliced IL-15Rα mRNA in that most PCR products lacked Ex2A when the exon 1/2 border was amplified from cDNA directly. In contrast to human IL-15Rα, GenBankTM searches failed to reveal any sequence predicted to encode a homologous domain in the murine intron 1. In addition, we were unable to detect a corresponding exon by PCR in cDNAs that had been derived from mature murine bone marrow- or monocyte-derived DCs. These data suggest that human DC-derived IL-15Rα mRNA contains an additional exon not found in mice.
FIGURE 1.
Cloning of IL-15Rα isoforms. A shows differences in the migratory patterns of IL-15Rα on SDS-PAGE. Its expression was induced in murine and in human DCs by exposure to LPS/IFN-γ or CD40L/IFN-γ and in 293 cells by over-expression of human IL-15/IL-15Rα. Analyses were done by immunoprecipitations/immunoblots. While most murine IL-15Rα from DCs and human IL-15Rα from transfected 293 cells migrated at ∼65 kDa with minor species at 35 kDa, the majority of IL-15Rα from human DCs was detected at 35 kDa with a minor species at 65 kDa. Human IL-15Rα from 293 cells and DCs were detected with the same antibody combinations, and two alternative antibodies that were used for both immunoprecipitations and immunoblots gave similar results. B, cDNAs were derived from variably activated human monocytes and DCs and subjected to RT-PCR. A first step with oligonucleotides annealing in the first and last exons of IL-15Rα was followed by a nested step that used oligonucleotides in exons 1 and 3. All resulting PCR products that contained the sushi domain-encoding and IL-15-binding exon 2 also contained an additional 153-bp sequence between exons 1 and 2 that we termed “exon 2A”. The predicted amino acid sequence is shown below the panels. C, further analyses revealed the existence of alternative IL-15Rα splice products at its 3′-end in cDNAs that had been derived from activated DCs. Specific PCR products that contained IL-15Rα sequences are indicated by asterisks left of the bands, double- asterisks denote wild-type IL-15Rα. D, targeting the various C-terminal isoforms in the first PCR step revealed IC3 to be the only isoform that may lack the Ex2A sequence. All PCR products were verified by direct sequencing. E, genomic organization of newly identified exons of human IL-15Rα on chromosome 10. New exons are shown in black and are named below the horizontal bar. Previously described exons are gray and are numbered above the horizontal bar. Lines below the bar depict splicing (black for new and gray for previously known). Exon structures are depicted above the bar with coding regions in black and noncoding regions in white. EM2 encodes an alternative membrane domain that is shown in gray.
A second cloning series targeted alternative C-terminal isoforms of IL-15Rα. Using 3′-RACE on cDNA samples as above we identified seven new splice versions that modified the 3′-end (Fig. 1C) in addition to the previously known IL-15Rα message that we refer to as “wildtype” in this manuscript. Alignments with genomic sequences revealed that all exons were located within ∼45 kbp of exon 1 (Fig. 1E). One isoform (EM2) is predicted to encode an additional extracellular domain and an alternative membrane domain. All other isoforms retained the wildtype membrane domain but altered the intracellular amino acid sequence. The two isoforms IC2 and IC6 resulted from alternative splice locations of the same C-terminal exon similar to what has been described for wildtype IL-15Rα (21).
We also investigated whether the inclusion of Ex2A is linked to C-terminal isoforms. We performed initial PCR steps with oligonucleotides annealing in the first and last exons of the respective isoforms that was followed by a nested step to amplify the exon 1/2 border. Fig. 1D shows the consistent presence of Ex2A in all C-terminal isoforms with the exception of IC3 for which species with or without Ex2A were detected. Together these data show the existence of additional coding exons in the human IL-15Rα gene.
IL-15/IL-15Rα Complex with the Ex2A Domain Is Functional
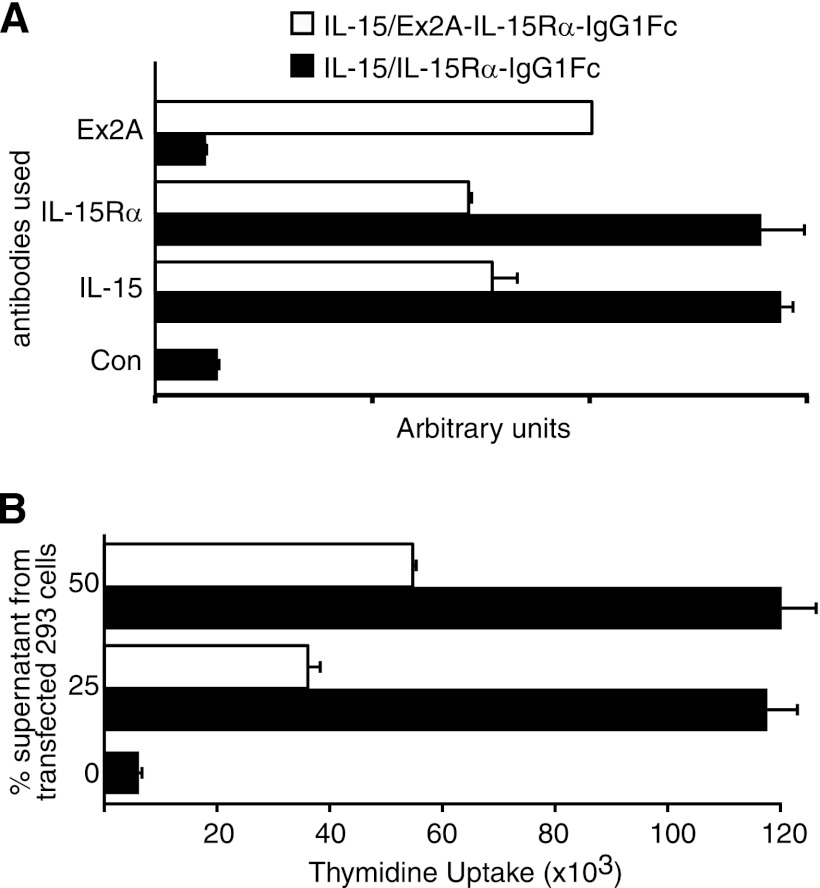
Ex2A is predicted to encode an amino acid domain immediately N-terminal of the IL-15-binding sushi domain. We investigated whether this constellation would render the resulting IL-15/IL-15Rα complex inactive. We generated chimeric constructs in which the membrane and intracellular domains of IL-15Rα were replaced by immunoglobulin constant domains of human IgG1 thus inducing their secretion (15). We analyzed supernatants from transfected 293 cells by ELISA and observed that antibodies to both IL-15 and IL-15Rα recognized the IL-15/IL-15Rα-IgFc complex that had been captured by anti-Fc antibodies (Fig. 2A) indicating that binding of IL-15 to IL-15Rα remained unaffected by Ex2A although its presence appeared to decrease the levels of secretion. The same supernatants also induced the proliferation of native human NK cells (Fig. 2B) with differences that corresponded to the levels of secretion. Therefore, there appeared to be no interference by Ex2A with the binding or activity of IL-15.
FIGURE 2.
IL-15Rα containing Ex2A is functional. A, chimeric proteins comprising human IL-15Rα with or without the Ex2A domain and an Fc portion of human IgG1 were co-expressed with IL-15 in 293 cells, and the resulting supernatants were analyzed by ELISA with plate-bound anti-Fc antibodies and soluble antibodies recognizing Ex2A, IL-15Rα, or IL-15. Although the presence of Ex2A appeared to decrease secretion levels, heterodimers were detected by antibodies against both IL-15Rα and IL-15, suggesting that the binding of IL-15 to IL-15Rα was not affected by Ex2A. B, exposure of human NK cells from healthy donors to the same supernatants caused proliferation rates that were equivalent to the amounts detected by ELISA. This suggests that a presence of the Ex2A domain did not inhibit the proliferation-inducing activity of the IL-15/IL-15Rα complex.
Ex2A Affects the Glycosylation of IL-15Rα
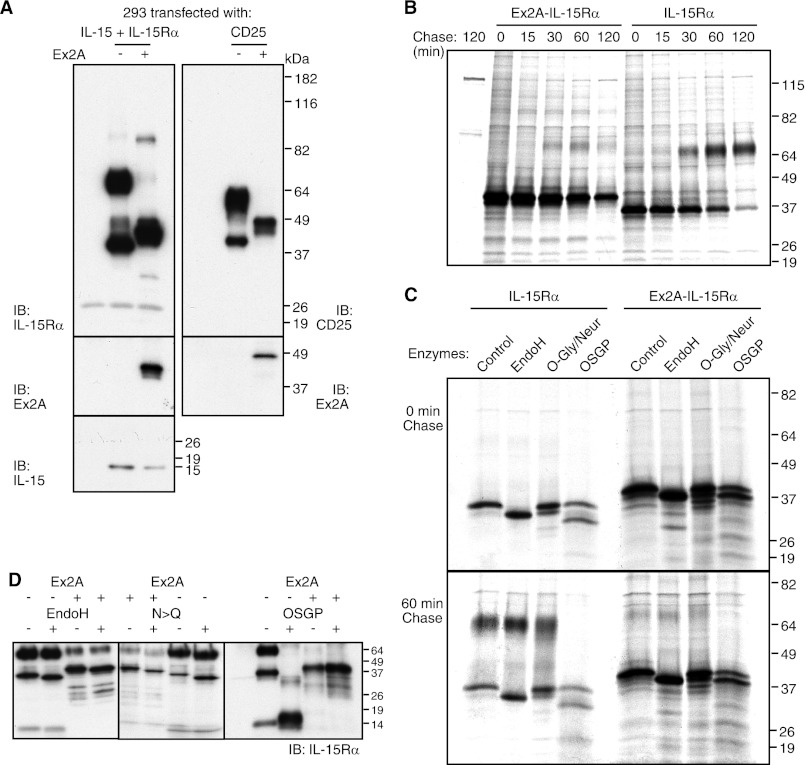
We addressed the initial question of the causes of glycosylation differences. When Ex2A domain was present, IL-15Rα indeed migrated at a lower molecular weight on SDS-PAGE (Fig. 3A, left panels). To investigate causality we expressed the Ex2A domain at the N-terminal end of mature CD25 and observed similar mobility changes (Fig. 3A, right panels). Pulse-chase experiments (Fig. 3B) showed that for IL-15Rα lacking Ex2A, the higher molecular weight species started to appear after a 30-min chase and remained stable while unmodified IL-15Rα disappeared. The presence of Ex2A appeared to prevent such a modification to result in unstable protein only. These data suggest that the observed protein modification confers stability. Ex2A appeared to be acting in cis only since co-expressions of Ex2A-containing IL-15Rα or CD25 failed to affect migration patterns of IL-15Rα or CD25 lacking Ex2A (supplemental Fig. S2). We failed to identify amino acid motifs within the Ex2A domain that caused the changes in the migratory patterns (supplemental Fig. S3) suggesting that Ex2A changes the conformation of IL-15Rα. Together these data show that the Ex2A domain affects glycosylation of IL-15Rα in cis.
FIGURE 3.
Ex2A affects migratory patterns of IL-15Rα. A, IL-15 and IL-15Rα with or without Ex2A were transfected into 293 cells and analyzed by Western blotting (left panels, the first lane represents a control of untransfected cells). The presence of Ex2A induced the major species of IL-15Rα to migrate at a lower molecular weight (∼42 instead of 65 kDa). Generating a chimeric Ex2A-CD25 protein (upper right panel) similarly caused migratory shifts of CD25. The expression of Ex2A is shown in the two middle panels. IL-15 migrated independently of IL-15Rα at ∼14 kDa (lower left panel). B, pulse-chase experiments revealed that lower molecular weight versions of IL-15Rα are initially generated while an IL-15Rα of higher molecular weight appeared after 30 min of chase only if Ex2A was absent. C, digestions of 35S-labeled proteins showed that a removal of N-linked carbohydrate chains with EndoH caused small shifts of IL-15Rα regardless of Ex2A presence. The high molecular weight version of IL-15Rα that was present after 60 min of chase in the absence of Ex2A was digested by O-sialoglycoprotease (OSGP) but not by O-glycosidase/neuraminidase suggesting an unusual type of modification by O-linked carbohydrate chains. D, similar results were obtained when non-labeled cells were analyzed by Western blotting: The Ex2A domain did not appear to affect N-glycosylation of IL-15Rα since small shifts were induced by EndoH digestions of immunoprecipitated IL-15Rα regardless of Ex2A presence (left), and replacements of the asparagines that are targeted by N-glycosylation also caused IL-15Rα shifts regardless of Ex2A (middle). OSGP cleaved most of IL-15Rα that lacked Ex2A, but the presence of Ex2A prevented this cleavage. The first lane represents an enzyme-only control. Since the activity of this enzyme requires tight clusters of sialylated O-linked oligosaccharides (22), its inability to cleave Ex2A-IL-15Rα suggests that the Ex2A domain prevents O-glycosylation.
IL-15Rα undergoes multiple glycosylation events (19). We studied the nature of the protein modifications that were associated with the presence of Ex2A. The early N-glycosylation appeared to be unaffected since both digestions with EndoH as well as amino acid substitutions of the targeted asparagines shifted IL-15Rα regardless of the presence of Ex2A (Fig. 3, C and D). In contrast, O-sialoglycoprotease digestion was prevented by the presence of Ex2A (Fig. 3, C and D). This suggests that Ex2A affected O-glycosylation since O-sialoglycoprotease requires this protein modification for its activity (22). O-Glycosylation modifications of IL-15Rα may be atypical since digestions with neuraminidase/O-glycosidase failed to affect the high molecular weight-species of IL-15Rα (Fig. 3C). The existence of such atypical modifications may further be supported by the inability of the O-glycosylation inhibitor Benzyl-GalNAc to affect mobility (see below). Digestions with chondroitinase, heparinases, or combinations thereof also failed to affect IL-15Rα mobility (not shown). In conclusion, the presence of the Ex2A domain appears to inhibit the O-glycosylation of IL-15Rα in cis.
The Ex2A Domain Prevents Release of IL-15/IL-15Rα into the Soluble Phase
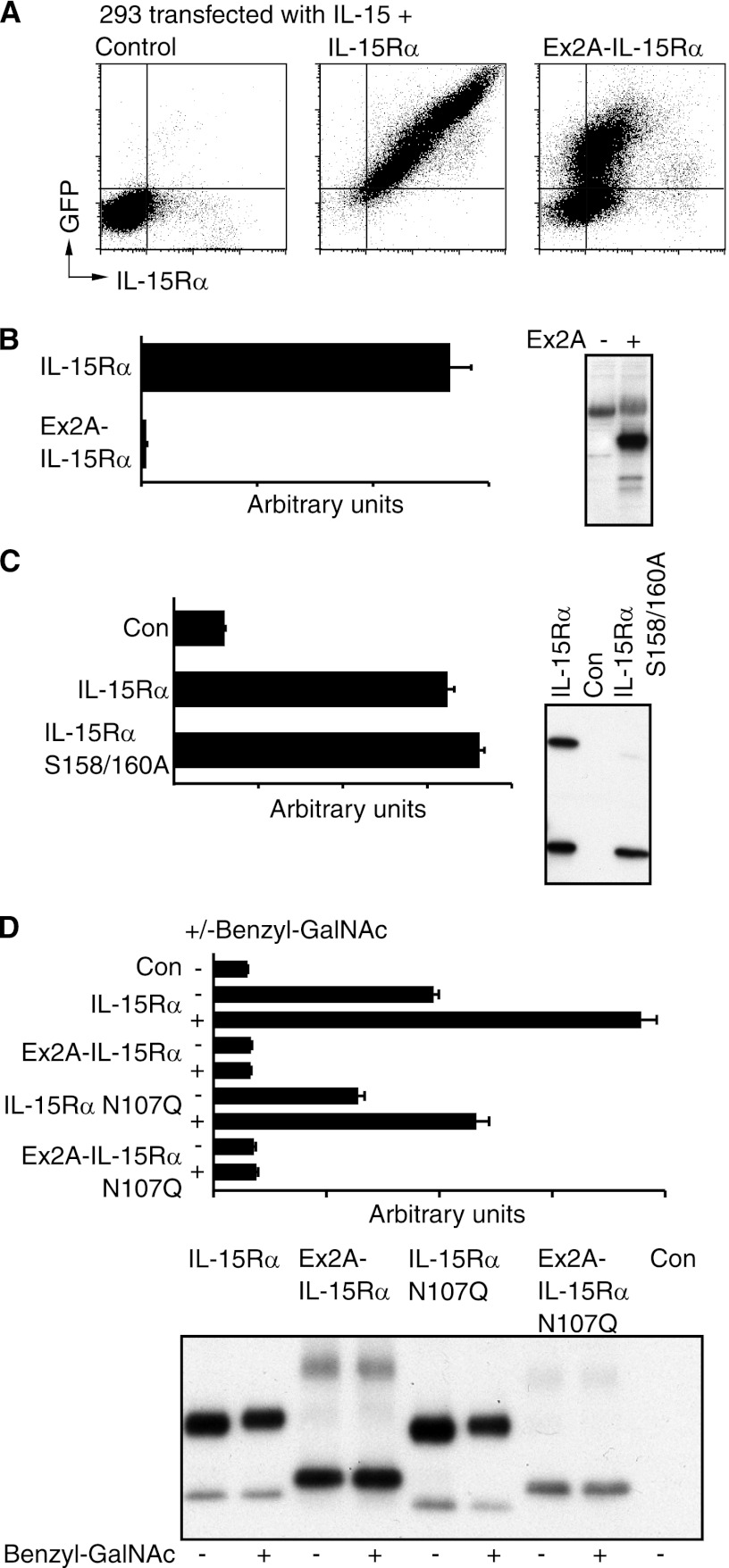
We investigated the functional consequences of Ex2A presence. As mentioned above, the IL-15/IL-15Rα complex acts as either a membrane-bound cytokine or after its release into the soluble phase. To study the former we generated IL-15Rα ± Ex2A with C-terminal GFP tags that were expressed together with IL-15 in 293 cells and analyzed by cytometry. Fig. 4A shows that for IL-15Rα containing Ex2A, increases of intracellular GFP detection were accompanied by smaller increases of surface IL-15Rα suggesting that Ex2A inhibited but did not prevent the surface export of IL-15Rα. Ex2A also slightly inhibited the transport of IL-15Rα from the endoplasmatic reticulum to the Golgi apparatus (supplemental Fig. S4). When concentrations of IL-15/IL-15Rα heterodimer in supernatants were determined by ELISA using plate-bound anti-IL-15 and soluble anti-IL-15Rα antibodies as a measure of cytokine release into the soluble phase (Fig. 4B), Ex2A completely abolished the ability of 293 cells to release the IL-15/IL-15Rα complex into the soluble phase. It therefore appears that Ex2A inhibits surface export and prevents release of the soluble IL-15/IL-15Rα heterodimer.
FIGURE 4.
Ex2A inhibits surface expression and prevents secretion of IL-15/IL-15Rα heterodimer. A, IL-15Rα with C-terminal GFP tags and IL-15 were co-expressed in 293 cells and stained for intracellular GFP and extracellular IL-15Rα. Increased GFP expression was accompanied by a smaller increase in the expression of extracellular IL-15Rα when Ex2A was present suggesting that Ex2A inhibited the surface export of IL-15/IL-15Rα heterodimer. B, no IL-15/IL-15Rα heterodimer was detected by ELISA in the supernatant when Ex2A was present. The panel on the right shows the levels of cell-associated IL-15Rα for this experiment. C, wildtype IL-15Rα without Ex2A as well as a construct with two amino acid substitutions (S158/160A) that lacked IL-15Rα modified by O-glycosylation similar to when Ex2A is present were coexpressed with IL-15 and analyzed by ELISA (graph) and Western blotting (panel). Secreted IL-15/IL-15Rα heterodimers were detected for both conditions suggesting that Ex2A-induced changes in the O-glycosylation of IL-15Rα and its secretion are not linked. D, both O- and N-glycosylation affect IL-15/IL-15Rα heterodimer secretion. The presence of the O-glycosylation inhibitor Benzyl-GalNAc increased levels of IL-15/IL-15Rα heterodimer secretion (graph) despite a lack of discernible effects on IL-15Rα migration on Western blots suggesting that an O-glycosylation of alternative proteins is involved in determining secretion levels. Expressions of an asparagine mutant (N107Q) that prevented N-glycosylation decreased IL-15/IL-15Rα heterodimer secretion suggesting a positive effect of IL-15Rα N-glycosylation on its secretion.
As described above, Ex2A affected O-glycosylation and release of IL-15Rα. We studied whether both processes are linked. For unrelated reasons we had generated a number of IL-15Rα mutants where serines had been replaced by alanines and that did not include Ex2A. The analysis of one of these clones (S158/160A) by Western blot revealed a lack of O-glycosylation (Fig. 4C). Contrary to the presence of Ex2A however, these amino acid replacements did not decrease the IL-15/IL-15Rα heterodimer concentration in supernatants suggesting that the effects of Ex2A on O-glycosylation and heterodimer release are not linked.
We also studied the contributions of O- and N-glycosylation to IL-15/IL-15Rα heterodimer release. Fig. 4D (graph) shows that the presence of the O-glycosylation inhibitor Benzyl-GalNAc increased the release of the IL-15/IL-15Rα complex that lacked Ex2A without an obvious effect on O-glycosylation of IL-15Rα itself as analyzed by the migration on Western blots (Fig. 4D, panel) suggesting that O-glycosylation of alternative proteins affected the IL-15/IL-15Rα complex release. In contrast, N-glycosylation of IL-15Rα itself appeared to support the secretion since substituting the target asparagine (N107Q) in IL-15Rα decreased IL-15/IL-15Rα complex concentration in the supernatant (Fig. 4D). Together these data reveal a complex picture in which independent effects of N- and O-glycosylation as well as the presence of the Ex2A domain determine IL-15/IL-15Rα heterodimer release.
Mature IL-15Rα Lacks the Ex2A Domain
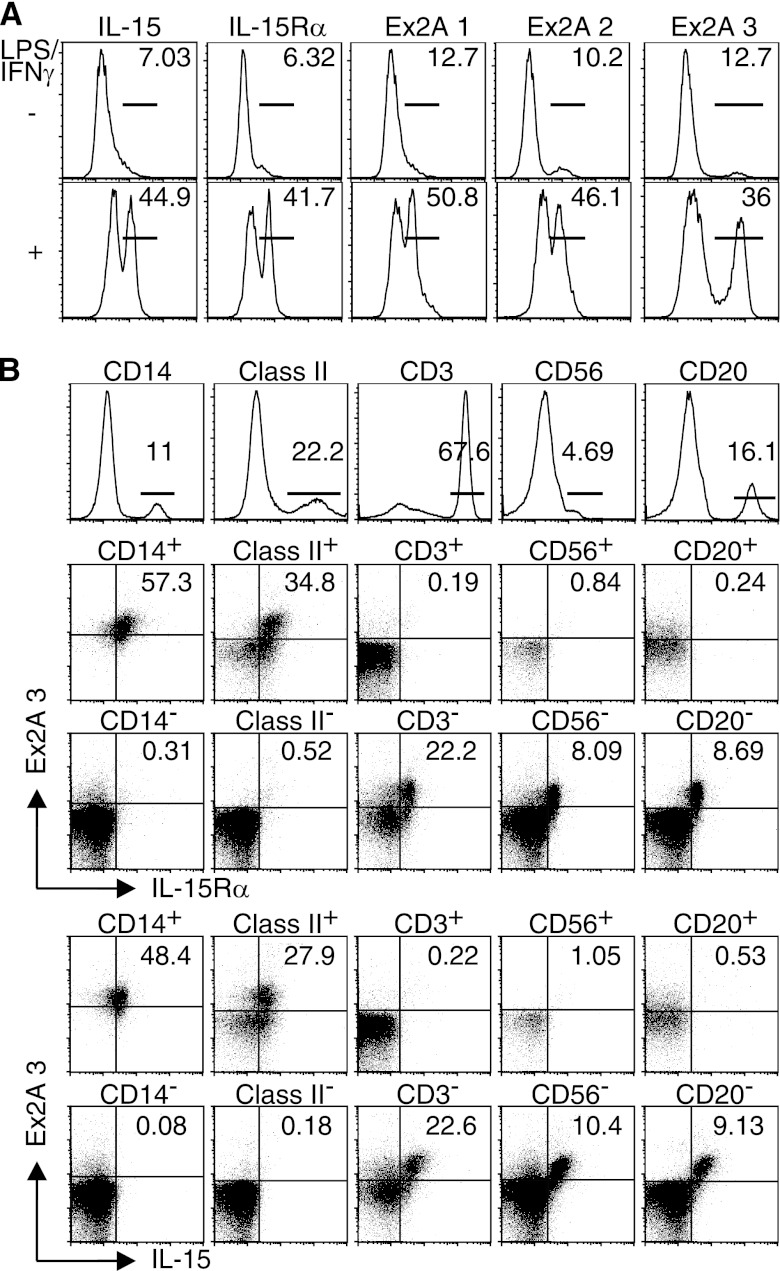
To detect and study the Ex2A-encoded domain, we generated several mAbs in mice that could be used for cytometry and immunoblotting (supplemental Fig. S5 and not shown). FACS analyses revealed the induction of Ex2A on the surface of DCs upon activation with LPS/IFN-γ (Fig. 5A). Ex2A was co-expressed together with IL-15 and IL-15Rα on monocytic (CD14+, MHC class II+) but not on lymphocytic (CD3+, CD56+, CD20+) cells among PBMCs from healthy donors (Fig. 5B). Despite co-expression, immunoprecipitation/immunoblot analyses revealed that the major species of IL-15Rα in activated DCs did not comprise the Ex2A domain (Fig. 6). Analyses of monocytes and DCs that had been activated under various conditions also revealed that Ex2A was consistently expressed on cells that were positive for IL-15 (supplemental Fig. S6) or IL-15Rα (not shown). These data suggest that the Ex2A domain is removed from the major IL-15Rα species during protein maturation and that both proteins appear on the surface separately.
FIGURE 5.
Ex2A is expressed on the surface of monocytes and DCs. A, three newly developed mouse mAbs were used to detect the Ex2A domain that was induced on human DCs after activations with LPS/IFN-γ. B, cytometry analyses of human PBMCs revealed that Ex2A was co-expressed with IL-15Rα and with IL-15 on cells that also expressed CD14 or MHC class II, but not on T cells (CD3+), NK cells (CD56+) or B cells (CD20+).
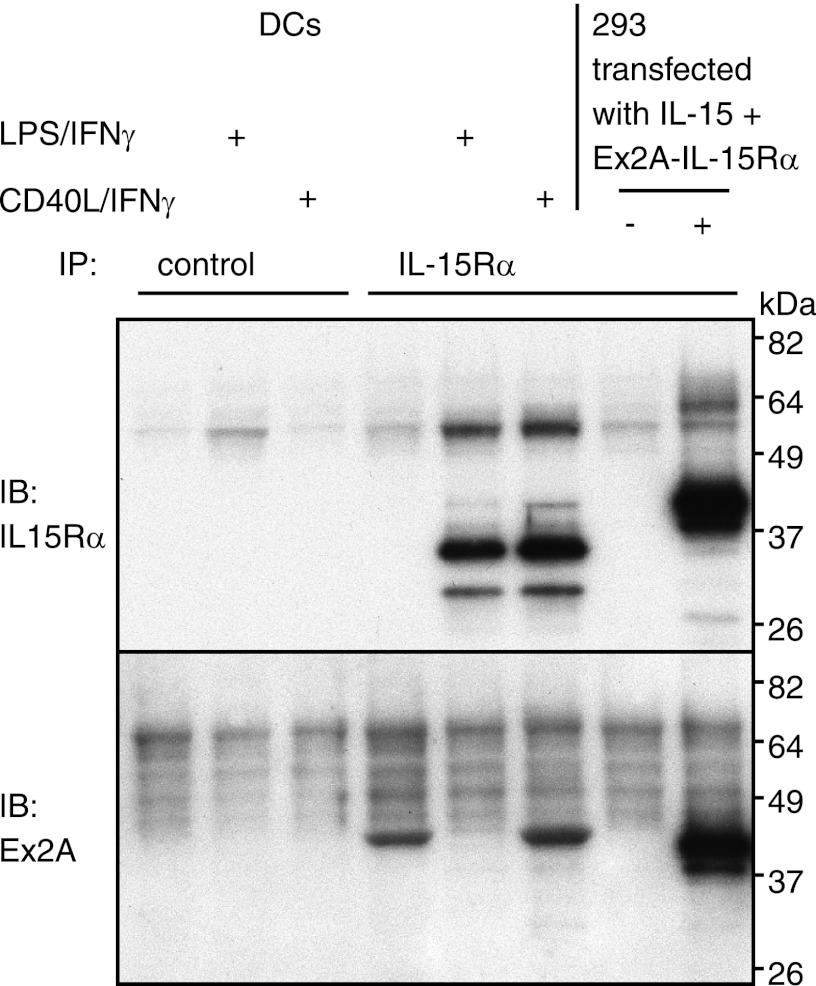
FIGURE 6.
Ex2A is absent from the major species of mature IL-15Rα in DCs. Human DCs with or without LPS/IFN-γ- or CD40L/IFN-γ-induced maturations as well as Ex2A-IL-15Rα-transfected 293 cells were subjected to immunoprecipitation with antibodies directed against extracellular IL-15Rα that was followed by immunoblotting with antibodies against the intracellular portion of wildtype IL-15Rα or against Ex2A. The major 35-kDa IL-15Rα species that was induced by DC maturation was detected with immunoblotting antibodies against IL-15Rα (upper) but not against Ex2A (lower) suggesting that both epitopes were not part of the same protein. A 42-kDa protein that was present in immature DCs and after CD40L/IFN-γ treatment appeared to contain both Ex2A and extracellular but not intracellular IL-15Rα sequence (lower panel) since an antibody against extracellular IL-15Rα was used to precipitate and anti-Ex2A was used to blot. The exact nature of this protein remains unclear.
Alternative C-terminal Isoforms of IL-15Rα
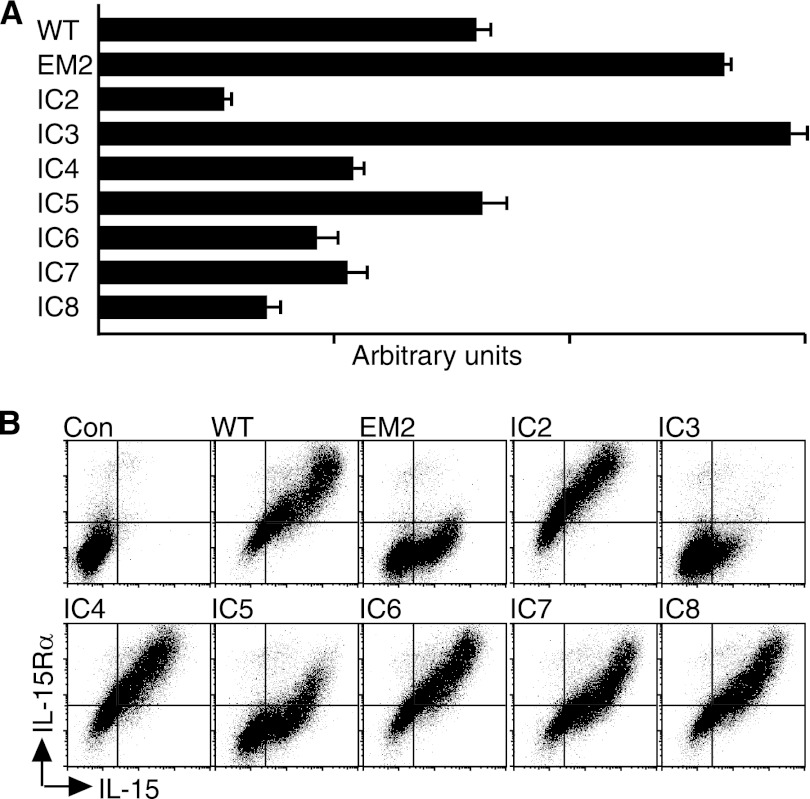
We investigated whether the newly identified C-terminal isoforms of human IL-15Rα similarly affected secretion and/or surface expression. Fig. 7A shows that the expression of two alternative isoforms was associated with an increased secretion of IL-15/IL-15Rα heterodimer into the supernatants when compared with wildtype. When surface expressions were analyzed (Fig. 7B), most isoforms showed behaviors similar to wildtype IL-15Rα in that the surface expressions of IL-15 and IL-15Rα increased proportionally. However, the C-terminal isoforms with the highest secretion levels differed: Levels of IL-15Rα increased less when compared with IL-15 for the isoforms EM2 and IC5 that we interpret as conformational changes that were induced by the alternative C termini and that affected the detection by the anti-IL-15Rα antibody. In addition, the isoform with the highest secretion level (IC3) was nearly absent from the cell surface. These data suggest that C-terminal isoforms of IL-15Rα determine secretion and surface expression.
FIGURE 7.
C-terminal isoforms of IL-15Rα affect the secretion and surface expression of IL-15/IL-15Rα heterodimer. IL-15 and wildtype or 8 newly identified C-terminal isoforms of IL-15Rα were expressed in 293 cells. The resulting supernatants were analyzed by ELISA (A), and surface expressions were measured by cytometry (B). The surface expression of isoforms with the highest secretion levels differed in that non-proportional IL-15 versus IL-15Rα increases were observed for EM2 and IC5 by cytometry, and IC3 showed only minimal surface expression.
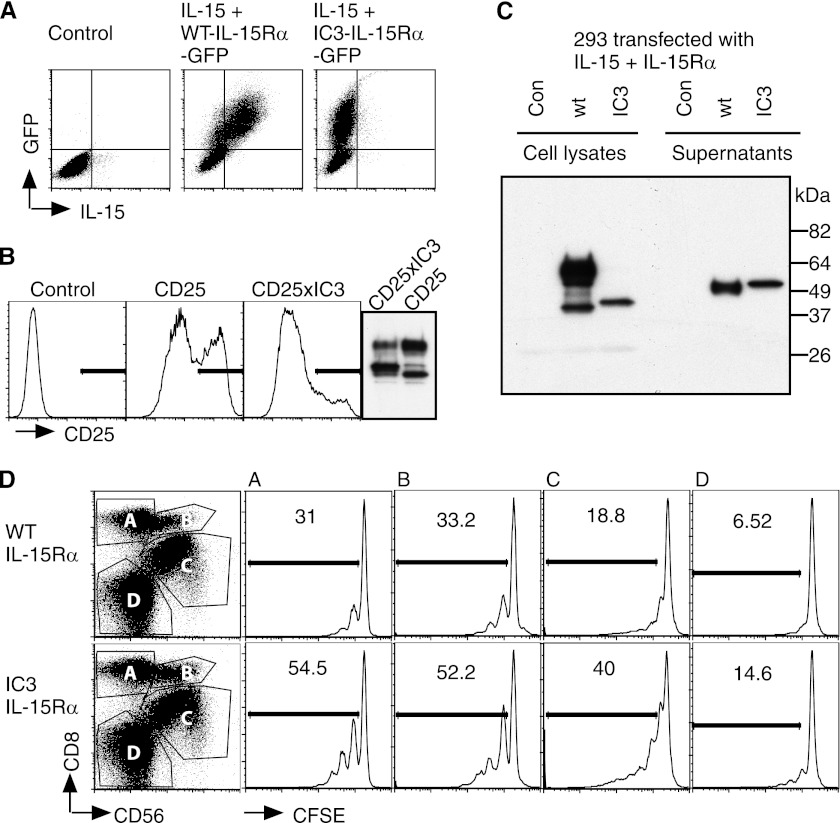
We further investigated IC3. We generated and analyzed three deletion mutants (supplemental Fig. S7). Deleting parts of the N-terminal or C-terminal ends of the intracellular portion of IC3 was accompanied by both higher surface expression levels and by lower secretion levels suggesting that secretion and intracellular retention were linked. Membrane-bound IC3-IL-15Rα appeared to be prevented from entering the Golgi apparatus as most protein co-localized with the endoplasmatic reticulum in microscopic analyses (supplemental Fig. S8). IC3-IL-15Rα retained IL-15 inside the cell (Fig. 8A) since increased levels of the C-terminal and therefore intracellular GFP tag were accompanied by surface appearance of IL-15 for wildtype but not for IC3-IL-15Rα. Replacing the intracellular portion of CD25 with IC3 also caused a reduced surface appearance of CD25 suggesting causality between IC3 and intracellular retention (Fig. 8B). We also analyzed the cell-associated and secreted forms of wildtype and IC3-IL-15Rα by immunoprecipitation/immunoblot and made several observations (Fig. 8C): 1) The intracellular presence of IC3 prevented O-glycosylation similar to the presence of the Ex2A domain (see above). 2) The molecular weight of both isoforms differed for both surface and secreted proteins. As expected, the intracellular portion of IL-15Rα was present only in cell-associated but not in secreted protein (not shown). The molecular weight difference of the secreted protein suggests alternative cleavage sites for wildtype and IC3-IL-15Rα. 3) The molecular weight of secreted IC3-IL-15Rα showed an unexpected increase when compared with cell-associated protein. 4) Cleavage of both IL-15Rα isoforms appeared to occur inside or close to the membrane domain since the secreted proteins were recognized by antibodies directed against the 15 amino acids immediately N-terminal of the membrane domain. A cleavage inside the membrane domain is further supported by the finding that IL-15/IL-15Rα heterodimer secretions were detected for all natural IL-15Rα variants that lacked various parts of the extracellular domain encoded by exons three through five (supplemental Fig. S9). We also determined the proliferation-inducing capacity of secreted IL-15/IC3-IL-15Rα complex. Equimolar amounts of IC3-IL-15Rα/IL-15 complex as determined by ELISA appeared to be superior to wildtype IL-15Rα/IL-15 in inducing the proliferation of CD8 and NK cells among PBMCs (Fig. 8D). Together these data suggest that the intracellular isoform IC3 prevented surface expression of the IL-15Rα/IL-15 complex but caused high levels of secretion to induce the proliferation of target cells in a paracrine fashion.
FIGURE 8.
IL-15/IC3-IL-15Rα heterodimer is secreted but not transpresented. A, IL-15 and wildtype or IC3-IL-15Rα with C-terminal GFP tags were coexpressed in 293 cells. Analyses of intracellular GFP and extracellular IL-15 revealed that IC3 retained IL-15 inside the cell. B, replacing the intracellular domain of CD25 with IC3 similarly caused a decreased surface expression of CD25. The right panel shows total CD25 expressions for this experiment. C, cell lysates and supernatants of 293 cells that coexpressed IL-15 and wildtype or IC3-IL-15Rα were immunoprecipitated with an antibody against IL-15 and immunoblotted with an antibody against a domain of IL-15Rα immediately N-terminal of the membrane domain. Both cell-associated and secreted wildtype and IC3-IL-15Rα differed in size even though the intracellular domain was absent from the secreted complexes (not shown). D, PBMCs from a healthy donor were CFSE-labeled and grown in equimolar amounts of wildtype or IC3-IL-15Rα/IL-15 complex for 6 days. Cytokine complex comprising IC3-IL-15Rα proved efficient in stimulating the proliferation of the cell populations shown on the left.
DISCUSSION
The pro-inflammatory cytokine IL-15 has several modes of action. Paracrine activities involving secreted IL-15/IL-15Rα complex assure the survival of responding NK and CD8 T cells without the requirement of cell-to-cell interactions. Cell surface IL-15 transpresentation by IL-15Rα gives the potential for additional signaling from the IL-15-expressing to the IL-15-dependent cells. It has been shown that IL-15 transpresentation rather than soluble IL-15/IL-15Rα heterodimer serves as a co-activation signal for NK cells (23, 24), and similar mechanisms probably also apply to CD8 T cells where IL-15 signaling has distinct effects during various immune response phases (25, 26). Here we describe a number of previously unrecognized isoforms of human IL-15Rα that determine the cytokine mode of action. We observed that wildtype IL-15Rα appears to act exclusively via transpresentation due to the new N-terminal Ex2A domain that prevented its cleavage and secretion into the soluble phase while another isoform, IC3 caused the appearance of soluble but not cell membrane-anchored IL-15/IL-15Rα complex due to the retention of membrane-bound IL-15Rα in the endoplasmatic reticulum.
An interesting finding is the apparent absence of similar IL-15Rα-regulating mechanisms in non-primates. GenBankTM searches identified sequence homologous to Ex2A in chimpanzees only but neither murine, rat, nor bovine genomes appear to contain such an exon. In addition, cDNAs from various mouse monocyte and DC preparations lacked any additional sequence between the first two exons when analyzed by RT-PCR (not shown). This isoform-dependent IL-15/IL-15Rα regulation may therefore be a recent event in phylogenesis, and organisms such as mice may employ alternative mechanisms to achieve similar outcomes.
Our data also include the identification of a number of C-terminal isoforms in addition to IC3 that change the intracellular amino acid sequence of IL-15Rα. Most of these isoforms functioned in a fashion similar to wildtype IL-15Rα in that their cleavage from cell membranes was prevented by the presence of the Ex2A domain, but the surface export of membrane-anchored IL-15/IL-15Rα complex remained intact. This existence of alternative intracellular domains suggests reverse signaling in which various intracellular domains of IL-15Rα affect signaling events inside the IL-15/IL-15Rα-producing cells. Effects of IL-15 absence on IL-15-producing cells have been reported in the mouse for DCs and mast cells (4, 12). In addition, the binding of signaling molecules to IL-15Rα has been reported (13). Therefore, it appears possible that the expressions of various IL-15Rα isoforms affect cell differentiation states of IL-15-producing cells.
The new C-terminal isoforms were detected by RT-PCR at levels similar to the wildtype isoform of IL-15Rα (Fig. 1C). Most of these new isoforms encode short intracellular amino acid motifs. We attempted to generate rabbit antisera directed against these intracellular motifs. We were successful to generate one such antiserum that detected IC4 in immature DCs and after activation with CD40L/IFN-γ (supplemental Fig. S10). We observed another isoform in Fig. 6 that was immunoprecipitated with antibody against extracellular IL-15Rα and was recognized by an immunoblotting antibody against Ex2A but not against the intracellular portion of wildtype IL-15Rα. This band may represent the version of IC3 that contains Ex2A (Fig. 1D), and the prevention of membrane-anchored IC3 from exiting the endoplasmatic reticulum may also prevent the cleavage of Ex2A from IL-15Rα. The generation of additional antibodies is necessary to study various IL-15Rα isoforms on the protein level.
Our data appear to show that IL-15Rα and possibly CD25 proteins are modified by an unusual O-glycosylation. The proteolytic activity of O-sialoglycoprotease indicates the presence of O-linked oligosaccharides whose attachment appeared to be prevented by both Ex2A and IC3 (Fig. 3, C and D). IL-15Rα contains a typical mucin-type domain (27) encoded by exons three and four that are often modified by O-glycosylation. Expressions of IL-15Rα in 293 cells usually generate two species that do or do not contain the protein modification. The distances between these bands in analyses of natural splicing products that lack parts of exons three and four (supplemental Fig. S9) support the notion of multiple attachment sites in that region that is also typical for O-glycosylation. On the other hand, digestions with O-glycosidase/neuraminidase did not remove sugar moieties from IL-15Rα (Fig. 3C). O-Glycosidase is specific for cleaving only one O-linked disaccharide, Galβ1–3GalNAcα-Ser/Thr (22). In addition, the presence of an inhibitor of mucin-like O-glycosylation failed to affect the mobility of IL-15Rα while affecting its secretion (Fig. 4). The most likely explanation for these data is that IL-15Rα is modified by an atypical O-glycosylation that uses sugars other than GalNAc for its core attachment. Such atypical O-glycosylation events have been described for other proteins (28–30).
Glycosylation patterns in the immune system are highly regulated (31–35). Such patterns are altered during development, trafficking, activation, and apoptosis, and changes of glycosylation are linked to disease mechanisms such as auto-immunity. The function of a wide variety of receptors is affected by glycosylation (31–35). Changes of glycosylation may but do not have to affect receptor stability, surface expression, ligand binding, host-pathogen interactions, lectin binding, allosteric conformation, and intracellular signaling. Glycosylation steps also appear to affect IL-15 pathways. Our data show that N-glycosylation of IL-15Rα supports its secretion while O-glycosylations of other proteins are inhibitory.
In summary, our data reveal the existence of multiple IL-15Rα isoforms in humans that appear to determine the mode of action of the pro-inflammatory cytokine IL-15. The isoforms containing the Ex2A domain prevent the release of IL-15/IL-15Rα heterodimers from cell membranes thereby facilitating its transpresentation function. The IC3 isoform that does not contain the Ex2A domain functions as a soluble secreted cytokine.
Supplementary Material
This work was supported by the intramural research program of the NCI, National Institutes of Health.

This article contains supplemental Table S1 and Figs. S1–10.
- DC
- dendritic cell
- OSGP
- O-sialoglycoprotease.
REFERENCES
- 1. Kennedy M. K., Glaccum M., Brown S. N., Butz E. A., Viney J. L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C. R., Brasel K., Morrissey P. J., Stocking K., Schuh J. C., Joyce S., Peschon J. J. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lodolce J. P., Boone D. L., Chai S., Swain R. E., Dassopoulos T., Trettin S., Ma A. (1998) IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 3. Burkett P. R., Koka R., Chien M., Chai S., Boone D. L., Ma A. (2004) Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 200, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dubois S. P., Waldmann T. A., Müller J. R. (2005) Survival adjustment of mature dendritic cells by IL-15. Proc. Natl. Acad. Sci. U.S.A. 102, 8662–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandau M. M., Schluns K. S., Lefrancois L., Jameson S. C. (2004) Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Rα by the same cells. J. Immunol. 173, 6537–6541 [DOI] [PubMed] [Google Scholar]
- 6. Mariner J. M., Lantz V., Waldmann T. A., Azimi N. (2001) Human T cell lymphotropic virus type I Tax activates IL-15Rα gene expression through an NF-κB site. J. Immunol. 166, 2602–2609 [DOI] [PubMed] [Google Scholar]
- 7. Mariner J. M., Mamane Y., Hiscott J., Waldmann T. A., Azimi N. (2002) IFN regulatory factor 4 participates in the human T cell lymphotropic virus type I-mediated activation of the IL-15 receptor α promoter. J. Immunol. 168, 5667–5674 [DOI] [PubMed] [Google Scholar]
- 8. Waldmann T. A., Tagaya Y. (1999) The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 [DOI] [PubMed] [Google Scholar]
- 9. Mortier E., Advincula R., Kim L., Chmura S., Barrera J., Reizis B., Malynn B. A., Ma A. (2009) Macrophage- and dendritic-cell-derived interleukin-15 receptor α supports homeostasis of distinct CD8+ T cell subsets. Immunity 31, 811–822 [DOI] [PubMed] [Google Scholar]
- 10. Dubois S., Mariner J., Waldmann T. A., Tagaya Y. (2002) IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 [DOI] [PubMed] [Google Scholar]
- 11. Mortier E., Bernard J., Plet A., Jacques Y. (2004) Natural, proteolytic release of a soluble form of human IL-15 receptor α-chain that behaves as a specific, high affinity IL-15 antagonist. J. Immunol. 173, 1681–1688 [DOI] [PubMed] [Google Scholar]
- 12. Orinska Z., Maurer M., Mirghomizadeh F., Bulanova E., Metz M., Nashkevich N., Schiemann F., Schulmistrat J., Budagian V., Giron-Michel J., Brandt E., Paus R., Bulfone-Paus S. (2007) IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 13, 927–934 [DOI] [PubMed] [Google Scholar]
- 13. Horng T., Bezbradica J. S., Medzhitov R. (2007) NKG2D signaling is coupled to the interleukin 15 receptor signaling pathway. Nat. Immunol. 8, 1345–1352 [DOI] [PubMed] [Google Scholar]
- 14. Waldmann T. A. (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 [DOI] [PubMed] [Google Scholar]
- 15. Dubois S., Patel H. J., Zhang M., Waldmann T. A., Müller J. R. (2008) Preassociation of IL-15 with IL-15R α-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 180, 2099–2106 [DOI] [PubMed] [Google Scholar]
- 16. Mortier E., Quéméner A., Vusio P., Lorenzen I., Boublik Y., Grötzinger J., Plet A., Jacques Y. (2006) Soluble interleukin-15 receptor α (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rβ/γ. Hyperagonist IL-15 × IL-15Rα fusion proteins. J. Biol. Chem. 281, 1612–1619 [DOI] [PubMed] [Google Scholar]
- 17. Rubinstein M.P., Kovar M., Purton J. F., Cho J. H., Boyman O., Surh C. D., Sprent J. (2006) Converting IL-15 to a superagonist by binding to soluble IL-15R{α}. Proc. Natl. Acad. Sci. 103, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yokoyama W. M., Christensen M., Santos G. D., Miller D. (2006) Production of monoclonal antibodies. Curr Protoc Immunol Chapter 2: Unit 2 5 [DOI] [PubMed] [Google Scholar]
- 19. Dubois S., Magrangeas F., Lehours P., Raher S., Bernard J., Boisteau O., Leroy S., Minvielle S., Godard A., Jacques Y. (1999) Natural splicing of exon 2 of human interleukin-15 receptor α-chain mRNA results in a shortened form with a distinct pattern of expression. J. Biol. Chem. 274, 26978–26984 [DOI] [PubMed] [Google Scholar]
- 20. Koka R., Burkett P., Chien M., Chai S., Boone D. L., Ma A. (2004) Cutting edge: murine dendritic cells require IL-15Rα to prime NK cells. J. Immunol. 173, 3594–3598 [DOI] [PubMed] [Google Scholar]
- 21. Anderson D. M., Kumaki S., Ahdieh M., Bertles J., Tometsko M., Loomis A., Giri J., Copeland N. G., Gilbert D. J., Jenkins N. A. (1995) Functional characterization of the human interleukin-15 receptor α chain and close linkage of IL15RA and IL2RA genes. J. Biol. Chem. 270, 29862–29869 [DOI] [PubMed] [Google Scholar]
- 22. Freeze H. H. (2001) Use of glycosidases to study protein trafficking. Current Protocols in Cell Biology (Bonifacino J. S., eds) Chapter 15, Unit 15 12 [DOI] [PubMed] [Google Scholar]
- 23. Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. (2007) Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26, 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortier E., Woo T., Advincula R., Gozalo S., Ma A. (2008) IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J. Exp. Med. 205, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burkett P. R., Koka R., Chien M., Chai S., Chan F., Ma A., Boone D. L. (2003) IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. U.S.A. 100, 4724–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schluns K. S., Klonowski K. D., Lefrançois L. (2004) Transregulation of memory CD8 T-cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood 103, 988–994 [DOI] [PubMed] [Google Scholar]
- 27. Julenius K., Mølgaard A., Gupta R., Brunak S. (2005) Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology 15, 153–164 [DOI] [PubMed] [Google Scholar]
- 28. Lommel M., Strahl S. (2009) Protein O-mannosylation: conserved from bacteria to humans. Glycobiology 19, 816–828 [DOI] [PubMed] [Google Scholar]
- 29. Sakaidani Y., Nomura T., Matsuura A., Ito M., Suzuki E., Murakami K., Nadano D., Matsuda T., Furukawa K., Okajima T. (2011) O-linked-N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nature Communication 2, 583. [DOI] [PubMed] [Google Scholar]
- 30. Stanley P. (2007) Regulation of Notch signaling by glycosylation. Curr. Opin. Struct. Biol. 17, 530–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baum L. G. (2002) Developing a taste for sweets. Immunity 16, 5–8 [DOI] [PubMed] [Google Scholar]
- 32. Lowe J. B. (2001) Glycosylation, immunity, and autoimmunity. Cell 104, 809–812 [DOI] [PubMed] [Google Scholar]
- 33. Marth J. D., Grewal P. K. (2008) Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rudd P. M., Elliott T., Cresswell P., Wilson I. A., Dwek R. A. (2001) Glycosylation and the immune system. Science 291, 2370–2376 [DOI] [PubMed] [Google Scholar]
- 35. van Kooyk Y., Rabinovich G. A. (2008) Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.