Abstract
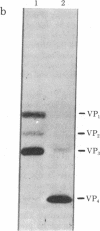
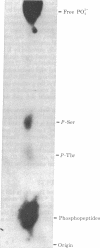
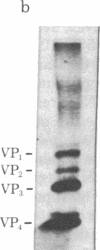
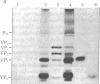
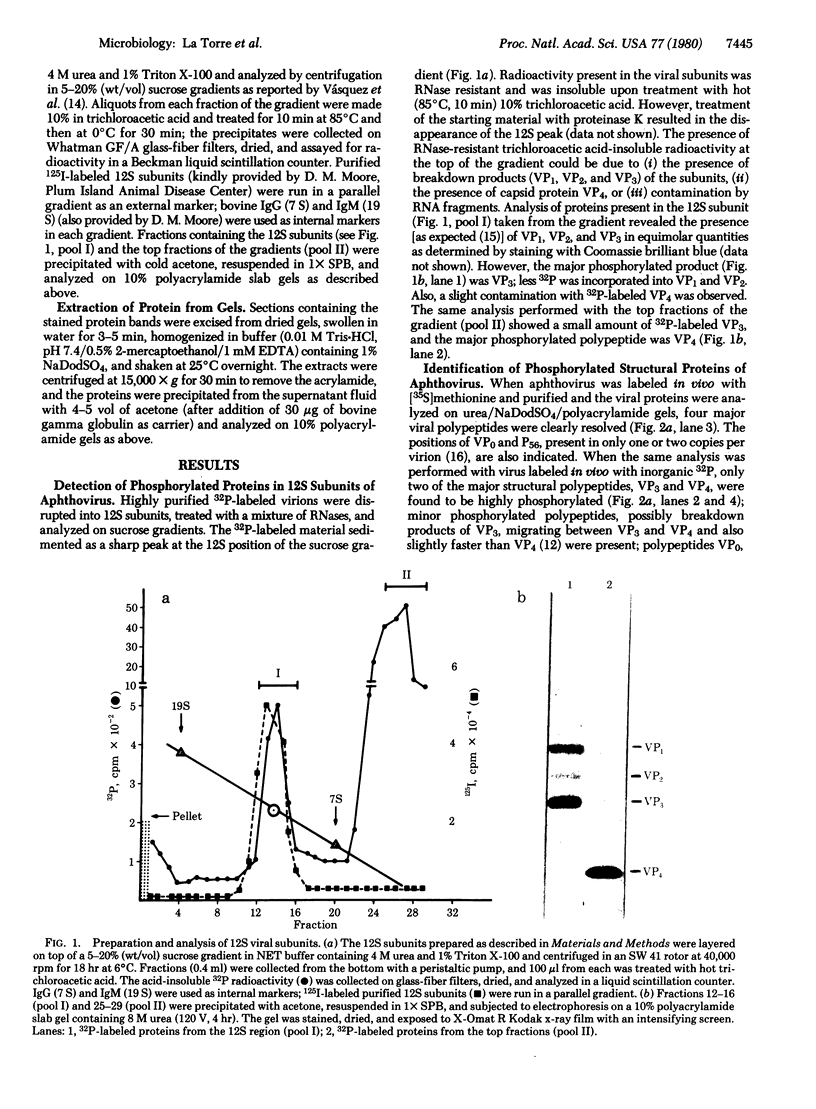
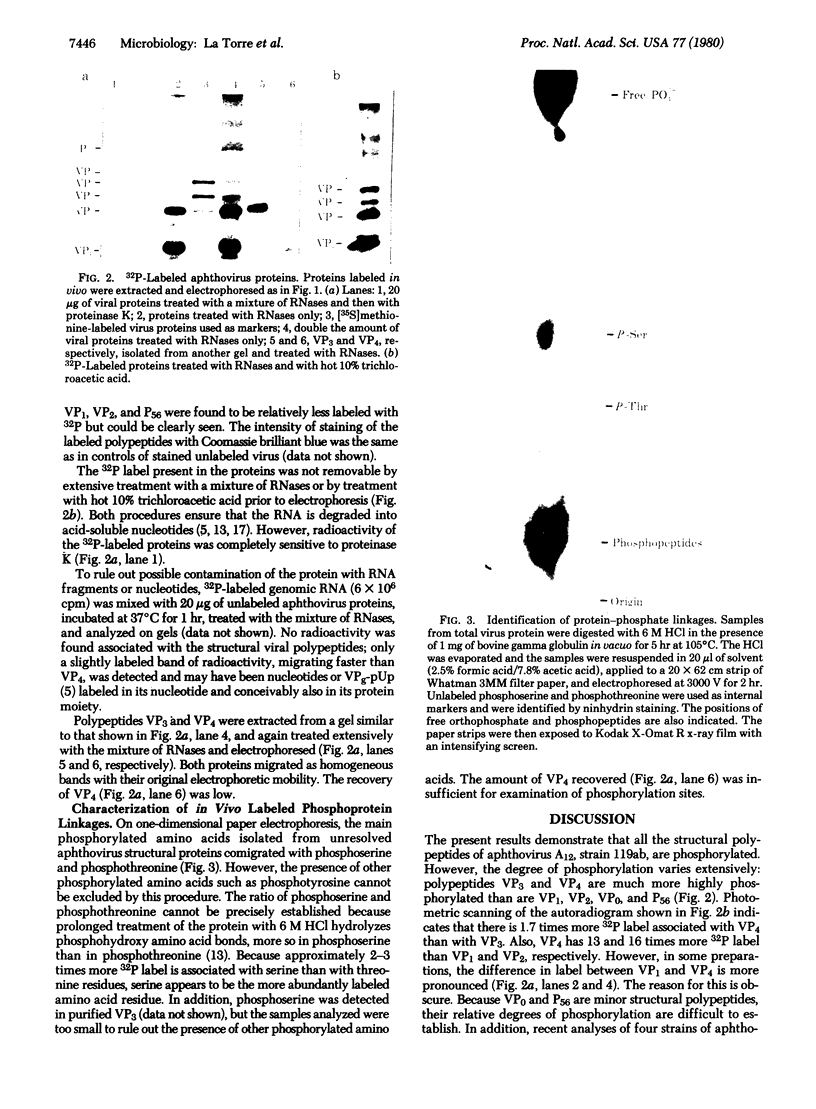
Analysis of aphthovirus A12, strain 119ab, grown in the presence of inorganic 32P revealed that two of the major viral polypeptides, VP4 and trypsin-sensitive protein VP3, were highly phosphorylated. The other major polypeptides, VP1 and VP2, were also phosphorylated but to a much lesser extent. Polypeptides VP0 and P56, of which there are approximately one of two copies per aphthovirion, were also labeled with 32P. Phosphoserine and phosphothreonine appeared to be the amino acids labeled with 32P.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Bachrach H. L., Moore D. M., McKercher P. D., Polatnick J. Immune and antibody responses to an isolated capsid protein of foot-and-mouth disease virus. J Immunol. 1975 Dec;115(6):1636–1641. [PubMed] [Google Scholar]
- Bachrach H. L., Morgan D. O., Moore D. M. Foot-and-mouth disease virus immunogenic capsid protein VPT: N-terminal sequences and immunogenic peptides obtained by CNBr and tryptic cleavages. Intervirology. 1979;12(2):65–72. doi: 10.1159/000149070. [DOI] [PubMed] [Google Scholar]
- Baxt B., Bachrach H. L. Early interactions of foot-and-mouth disease virus with cultured cells. Virology. 1980 Jul 15;104(1):42–55. doi: 10.1016/0042-6822(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Bitte L., Kabat D. Isotopic labeling and analysis of phosphoproteins from mammalian ribosomes. Methods Enzymol. 1974;30:563–590. doi: 10.1016/0076-6879(74)30056-0. [DOI] [PubMed] [Google Scholar]
- Denoya C. D., Scodeller E. A., Gimenez B. H., Vásquez C., La Torre J. L. Foot and mouth disease virus. I. Stability of its ribonucleic acid. Virology. 1978 Jan;84(1):230–235. doi: 10.1016/0042-6822(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Denoya C. D., Scodeller E. A., Vasquez C., La Torre J. L. Foot and mouth disease virus. II. Endoribonuclease activity within purified virions. Virology. 1978 Aug;89(1):67–74. doi: 10.1016/0042-6822(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Doel T. R., Sangar D. V., Rowlands D. J., Brown F. A re-appraisal of the biochemical map of foot-and-mouth disease virus RNA. J Gen Virol. 1978 Nov;41(2):395–404. doi: 10.1099/0022-1317-41-2-395. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Grubman M. J. The 5' end of foot-and-mouth disease virion RNA contains a protein covalently linked to the nucleotide pUp. Arch Virol. 1980;63(3-4):311–315. doi: 10.1007/BF01315038. [DOI] [PubMed] [Google Scholar]
- King A. M., Newman J. W. Temperature-sensitive mutants of foot-and-mouth disease virus with altered structural polypeptides. I. Identification by electrofocusing. J Virol. 1980 Apr;34(1):59–66. doi: 10.1128/jvi.34.1.59-66.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picornaviridae: second report. Intervirology. 1978;10(3):165–180. doi: 10.1159/000148981. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Cavanagh D., Brown F. Characterization of the minor polypeptides in the foot-and-mouth disease particle. J Gen Virol. 1976 Apr;31(1):35–46. doi: 10.1099/0022-1317-31-1-35. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Harris T. J., Brown F. Protein covalently linked to foot-and-mouth disease virus RNA. Nature. 1977 Aug 18;268(5621):648–650. doi: 10.1038/268648a0. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Talbot P., Brown F. A model for foot-and-mouth disease virus. J Gen Virol. 1972 May;15(2):163–169. doi: 10.1099/0022-1317-15-2-163. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Denoya C. D., La Torre J. L., Palma E. L. Structure of foot-and-mouth disease virus capsid. Virology. 1979 Aug;97(1):195–200. doi: 10.1016/0042-6822(79)90387-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]