Abstract
Acute liver failure (ALF) due to hepatitis A virus (HAV) infection is an uncommon but potentially lethal illness. The aim of this study was to identify readily available laboratory and clinical features associated with a poor prognosis among ALF patients with HAV infection. The presenting features of 29 adults with anti-HAV IgM positive ALF enrolled in the ALFSG between 1998 and 2005 were reviewed. The HAV patients listed for transplantation by UNOS were also reviewed. Acute HAV accounted for 3.1% of patients enrolled in the ALFSG. At 3 weeks follow-up, 16 had spontaneously recovered (55%), 9 underwent transplantation (31%), and 4 had died (14%). A prognostic model incorporating 4 presenting features (serum ALT <2,600 IU/L, creatinine >2.0 mg/dL, intubation, pressors) had an AUROC for transplant/death of 0.899 which was significantly better than the King’s College criteria (0.623, P=.018) and MELD scores (0.707, P=.0503). Between 1988 and 2005, the frequency of patients requiring liver transplantation for HAV in the UNOS database significantly decreased from 0.7 % to 0.1% (P < .001). In addition, the proportion of HAV cases enrolled in the ALFSG significantly decreased from 5% to 0.8% (P = .007). In conclusion, the frequency of HAV patients enrolling in the ALFSG and being listed for liver transplantation in the United States has declined in parallel. A prognostic index consisting of 4 clinical and laboratory features predicted the likelihood of transplant/death significantly better than other published models suggesting that disease specific prognostic models may be of value in non-acetaminophen ALF.
Over the past 20 years, the Centers for Disease Control and Prevention (CDC) have reported a significant decline in the incidence of acute hepatitis A virus (HAV) infection in the United States.1,2 However, sporadic outbreaks of HAV infection continue to be reported largely due to enteral spread of contaminated food or direct person-to-person contact.3,4 Although the majority of immunocompetent adults exposed to HAV experience an acute self-limited illness, the likelihood of developing symptomatic hepatitis with jaundice appears to be greater in adults compared to children and the rates of hospitalization and complications appear to be highest in the elderly.5-7 Since recent studies have demonstrated a decreasing incidence of protective immunity to HAV among adults in western countries, the potential for severe morbidity and mortality during sporadic outbreaks is substantial.2,8 Treatment for acute HAV infection is largely supportive but as many as 50% of HAV patients with acute liver failure (ALF) defined by the presence of coagulopathy and encephalopathy may die or require emergency liver transplantation.9-11 Therefore, rapid identification of HAV patients with a poor prognosis for recovery is important but has been difficult to establish due to the low incidence of ALF in the general population.11,12
The Acute Liver Failure Study Group (ALFSG) is a multi-center consortium of 24 sites formed in 1998 to identify and track the etiologies and outcomes of adults with ALF in the United States.13 Over 925 adults with ALF have been enrolled into the prospective observational study through late 2005 including 29 confirmed HAV cases. The United Network of Organ Sharing (UNOS) prospectively collects demographic, clinical, and diagnostic information on all patients listed for liver transplantation in the United States. Although the majority of patients awaiting liver transplantation have cirrhosis and chronic liver failure, ALF due to varying etiologies accounts for ~3% of annual liver transplants.14 The primary aim of the current study was to identify the presenting features associated with a poor prognosis (i.e., need for liver transplantion, or death) in HAV patients prospectively enrolled in the ALFSG observational study. A secondary aim was to develop an HAV specific prognostic model from the ALFSG database and compare its performance to other published models including the King’s College criteria and laboratory MELD scores.15,16 Finally, we compared the baseline clinical features and outcomes of HAV patients listed for a liver transplant in the ALFSG to those reported by UNOS during the same time period to determine if ALFSG patients are representative of the general U.S. ALF population.
Patients and Methods
Acute Liver Failure Study Group Patient Population
The ALFSG is a multi-center consortium funded by the National Institute of Diabetes and Digestive and Kidney Diseases to determine the etiology, clinical features, and outcomes of adult patients with ALF.13 Since its inception in 1998, 24 participating centers have enrolled more than 925 patients into the prospective observational study. Inclusion criteria include the presence of coagulopathy (i.e., prothrombin time >15 seconds or international normalized ratio [INR] ≥1.5) and hepatic encephalopathy within 26 weeks of symptom onset in the absence of pre-existing liver disease. Detailed demographic, clinical, and laboratory data are collected at study entry. In addition, clinical complications as well as serial laboratory and diagnostic studies are collected through 3 weeks after enrollment until final patient disposition of liver transplantation, death, or spontaneous survival. Candidacy for liver transplantation is determined at the individual centers according to UNOS guidelines. A written informed consent document approved by the local Institutional Review Board was signed by the patient’s next of kin at entry into the ALFSG observational study since the patients by definition had an impaired mental status. All data forms are sent to the data coordinating center at the University of Texas Southwestern Medical Center at Dallas for review and subsequently entered into a relational database. Annual site visits were conducted by the data coordinating center to verify source documents. A Certificate of Confidentiality was obtained from the National Institutes of Mental Health for the entire study.
The final etiology of ALF was assigned by the site investigator using standard diagnostic criteria and reviewed by the data coordinating center. Acute HAV infection was defined by the presence of detectable anti-HAV IgM and exclusion of competing causes of ALF including acute hepatitis B virus infection, acetaminophen overdose, ischemia, autoimmune hepatitis, and Budd-Chiari among others.13 The eligible ALF population reviewed for this analysis included 925 adult ALF patients enrolled between 1/1/1998 and 9/15/2005.
Prognostic Models of Survival
The 29 HAV patients included in this analysis were divided into 2 groups based on their status at 3 weeks following enrollment: survivors with spontaneous recovery or transplant/death who had died or undergone liver transplantation. The initial baseline and subsequent clinical factors associated with death/transplantation on univariate analysis were combined in a multivariate prognostic model (see Table 1). Variables selected for the ALFSG index were chosen based upon their relationship to poor prognosis and wide-spread availability. The performance of the ALFSG prognostic model was compared to the King’s College criteria for non-acetaminophen related ALF at study admission of (A) prothrombin time >100 seconds (INR >6.5) or (B) if any three of the following were present: (1) Age <10 or >40; (2) Cause: Non-A, Non-B hepatitis/idiosyncratic drug reaction; (3) Jaundice to encephalopathy >7 days; (4) Prothrombin time >50 seconds (INR >3.5); or (5) Serum bilirubin >17.5 mg/dL.15 In addition, the utility of the ALFSG index was compared to the day 1 laboratory MELD scores as recently described by Kremers et al.16
Table 1.
Day 1 Clinical Features of Patients With Hepatitis A Virus Enrolled in the Acute Liver Failure Study Group
| Total (n = 29) | Survivors (n = 16) | Transplant/Death (n = 13) | P * | |
|---|---|---|---|---|
| Age (years) | 48 ± 14 | 44 ± 16 | 54 ± 10 | .44 |
| % Female | 48% | 69% | 23% | .008 |
| Symptom onset to study enrollment (days) | 15.3 ± 12.7 | 10.8 ± 3.7 | 20.9 ± 17.2 | .17 |
| % with >21 days from symptom onset to enrollment | 17% | 0% | 38% | .07 |
| Hospital admission to study enrollment (days) | 3.0 ± 3.3 | 2.4 ± 2.0 | 3.8 ± 4.4 | .95 |
| Mean follow-up (days) | 5.6 ± 5.9 | 6.4 ± 5.7 | 4.5 ± 6.2 | .38 |
| Median follow-up (range) | 4 (1-22) | 5 (1-22) | 2 (1-21) | |
| Race/ethnicity | ||||
| Caucasian | 83% | 74% | 92% | .40 |
| Hispanic/Latino | 7% | 13% | - | - |
| Asian | 7% | 13% | - | - |
| African American | 3% | - | 8% | - |
| Study day 1 labs | ||||
| Lab MELD | 31 ± 6.5 | 29 ± 6.1 | 34 ± 6.1 | .77 |
| INR | 2.8 ± 1.2 | 3.1 ± 1.4 | 2.5 ± 0.6 | .73 |
| Serum ALT (IU/L) | 2606 ± 1626 | 3362 ± 1665 | 1675 ± 1000 | .03 |
| Serum ALT < 2600 (IU/L) | 48% | 25% | 77% | .03 |
| Serum AST (IU/L) | 1417 ± 1336 | 1679 ± 1240 | 1095 ± 1428 | .07 |
| Alkaline phoshatase (IU/L) | 151 ± 72 | 179 ± 82 | 118 ± 37 | .02 |
| Total bilirubin (mg/dL) | 14.5 ± 8.4 | 13.6 ± 9.7 | 15.6 ± 6.6 | .59 |
| WBC (103/mm3) | 9.5 ± 4.6 | 8.7 ± 5.2 | 10.4 ± 3.7 | .23 |
| Hemoglobin (g/dL) | 12.8 ± 2.1 | 13.2 ± 2.2 | 12.3 ± 1.9 | .65 |
| Platelet count (103/mm3) | 224 ± 83 | 242 ± 82 | 202 ± 83 | .46 |
| Creatinine (mg/dL) | 2.1 ± 1.9 | 1.2 ± 0.9 | 3.1 ± 2.2 | .04 |
| Creatinine >2.0 (mg/dL) | 31% | 13% | 54% | .20 |
| Phosphate (mg/dL) (n = 21) | 3.1 ± 1.8 | 2.3 ± 0.8 | 3.8 ± 2.2 | .25 |
| pH (n = 23) | 7.48 ± 0.06 | 7.49 ± 0.06 | 7.48 ± 0.07 | .98 |
| Study day 1 complications | ||||
| Grade 3-4 encephalopathy | 52% | 38% | 69% | .33 |
| On pressors | 21% | 0% | 46% | .0004 |
| On hemodialysis | 17% | 6% | 31% | .08 |
| Intubated | 52% | 25% | 85% | .01 |
NOTE. All results reported as % or mean +/− standard deviation with median (range) if non-normally distributed.
Abbreviations: WBC, white blood cell count; AST, aspartate aminotransferase.
Comparing spontaneous survivors at 3 weeks versus transplant/death.
United Network of Organ Sharing Patient Population
The United Network of Organ Sharing (UNOS) prospectively collects demographic, clinical, and laboratory data on all solid organ transplant candidates and recipients in the United States. Deidentified UNOS data were obtained from a publicly available Organ Procurement and Transplantation Network dataset which included 134,342 liver listing registrations from 1/1/87 through 9/15/05. The cases of acute HAV infection coded by UNOS as Acute Hepatic Necrosis: Type A were extracted and reviewed. Of the 79,250 subjects registered for liver transplantation between 1/1/98 and 9/15/05 (the period of our study), 92 (0.12%) were coded as acute HAV infection. From this cohort, 20 were subsequently excluded from this analysis due to age <15 years and 3 due to pretransplant waiting times that exceeded 6 months and presumably reflected diagnostic coding/data entry errors. Therefore, 69 adult subjects listed for liver transplantation within UNOS due to acute HAV infection were included in this analysis.
Statistical Analysis
Results are expressed as percentages or mean ± standard deviation (medians with ranges) unless otherwise stated. Univariate analysis was performed by dichotomizing continuous variables utilizing the median, then performing log-rank testing to identify predictors of transplant/death. Multivariate analysis was performed with forward stepwise (Wald) Cox regression modeling. Comparison of demographics between the ALFSG and UNOS populations was done using Student t test for continuous data and Fisher’s Exact Test for categorical data. Receiver operating characteristic (ROC) curves were compared using the method of Hanley et al.17 A P value less than .05 was considered significant. All statistical analyses and graph formation was completed using SPSS Version 13.0 (SPSS Inc., Chicago, IL). Analysis of the UNOS dataset was completed with SAS Version 9.1 (SAS Institute Inc., Cary, NC).
Results
ALFSG Patients With Hepatitis A
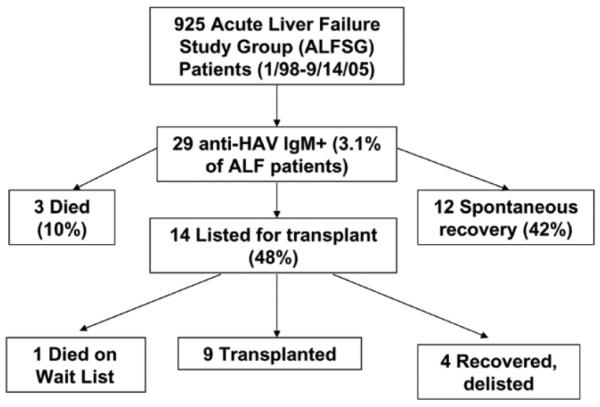
The day 1 laboratory and clinical features of the 29 HAV patients enrolled in the ALFSG are summarized in Table 1. There were an equal proportion of male and female patients and the mean subject age was 48 years (range: 21 to 72). Patients had been ill for a mean of 15 days prior to study entry but the mean duration of hospitalization prior to study enrollment was only 3 days (range: 1 to 16). The 29 HAV patients represented 3.1% of the 925 ALF patients enrolled in the ALFSG prospective observational study between 1/1/98 and 9/15/05. Within 3 weeks of enrollment, 3 HAV patients died, 12 spontaneously recovered and 14 were listed for liver transplantation. Subsequently, 9 of the listed patients underwent transplantation, 1 died and the remaining 4 patients recovered and were removed from the transplant waiting list (Fig. 1). Therefore, there were 16 HAV patients (55%) who spontaneously recovered and 13 (45%) who either died or required emergency liver transplantation.
Fig. 1.
Outcomes of patients with hepatitis A virus enrolled in the Acute Liver Failure Study Group. All patients were enrolled between 1/98 and 9/15/05 and followed for 3 weeks after enrollment.
The 16 spontaneous survivors were not significantly younger but they were significantly more likely to be female compared to the transplant/death group (69% vs. 23%, P = .008). On study day 1, survivors had significantly higher serum alanine aminotransferase (ALT) and alkaline phosphatase levels than the transplant/death group and significantly lower serum creatinine levels (Table 1). However, initial serum bilirubin, INR, pH levels, and MELD scores were similar in the two patient groups. Regarding clinical complications, none of the survivors were on pressors on study day 1 compared to 46% of the transplant/death group (P = .0004). In addition, survivors were significantly less likely to be intubated (25% vs. 85%, P = .01). Survivors also had a lower frequency of advanced encephalopathy (grade 3-4) and need for hemodialysis on study day 1 compared to transplant/death group but neither of these trends were statistically significant.
During their ALF hospitalization, survivors had significantly lower mean peak creatinine levels (1.4 vs. 4.3 mg/ dL, P = .004) but the mean peak serum ALT, INR, and bilirubin levels were not significantly different from the transplant/death group (Table 2). Survivors were also significantly less likely to require pressors, hemodialysis, or mechanical ventilation compared to the transplant/death group.
Table 2.
Peak Laboratory Values and Clinical Complications in Hepatitis A Patients Enrolled in the Acute Liver Failure Study Group Through Week 3
| Total (n = 29) | Survivors (n = 16) | Transplant/Death (n = 13) | P * | |
|---|---|---|---|---|
| WBC (103/mm3) | 14.5 ± 8.6 | 10.8 ± 4.7 | 19.0 ± 10.2 | .001 |
| INR | 3.9 ± 3.0 | 3.4 ± 1.4 | 4.5 ± 4.2 | .33 |
| Serum ALT (IU/ml) | 3560 ± 2191 | 3799 ± 1811 | 3266 ± 2634 | .37 |
| Serum AST (IU/mL) | 2523 ± 2226 | 2433 ± 2220 | 2633 ± 2320 | .75 |
| Total bilirubin (mg/dL) | 17.8 ± 8.8 | 17.1 ± 9.7 | 18.8 ± 7.8 | .55 |
| Creatinine (mg/dL) | 2.7 ± 2.4 | 1.4 ± 1.3 | 4.3 ± 2.7 | .004 |
| pH (nadir) (n = 23) | 7.41 ± 0.09 | 7.45 ± 0.07 | 7.39 ± 0.10 | .31 |
| Grade 3-4 encephalopathy | 69% | 50% | 92% | .14 |
| On pressors | 28% | 6% | 54% | .004 |
| On hemodialysis | 24% | 6% | 46% | .09 |
| Intubated | 66% | 38% | 100% | .01 |
NOTE. All results reported as % or mean +/− standard deviation with median (range) if non-normally distributed.
Abbreviation: WBC, white blood cell count.
Comparing spontaneous survivors at 3 weeks versus transplant/death.
All of the 29 subjects were fulminant HAV infection due to the presence of detectable anti-HAV IgM and exclusion of competing causes. One of the HAV subjects had preexisting liver disease due to chronic HBV and HCV infection acquired via intravenous drug use. This subject was thought to have fulminant HAV infection due to the recent exposure to a sick contact with known HAV and detection of anti-HAV IgM. In regards to acetaminophen use, only 4 subjects had serum acetaminophen levels above the lower limit of detection of 10 mg/dL (range: 12 to 28 mg/dL). However, the serum bilirubin levels at presentation were >12 mg/dL in all of these subjects and prior studies have demonstrated that increased serum bilirubin levels can lead to false positive serum acetaminophen assay results.18 In addition, the acetaminophen dose ingested in these 4 cases was not felt to be hepatotoxic or the primary cause of ALF by the local investigator and all of these subjects spontaneously recovered.
Survival Models in ALFSG Patients With Hepatitis A
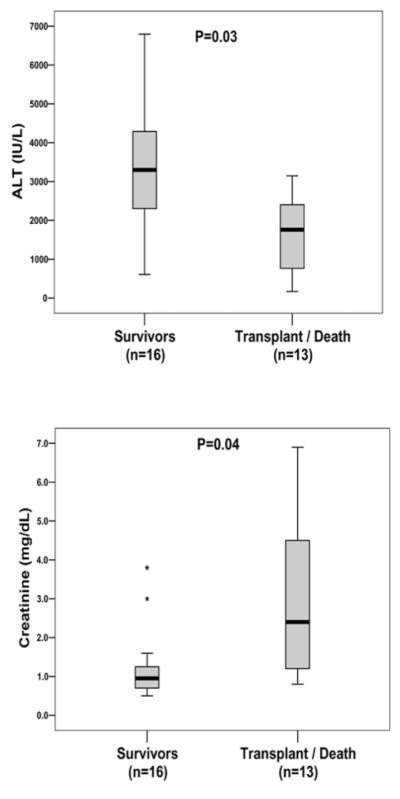
A day 1 serum creatinine level of > 2.0 mg/dl provided a sensitivity of 54% and specificity of 88% for predicting non-survival (Fig. 2). Similarly, a day 1 serum ALT <2,600 IU/mL identified the transplant/death group with a sensitivity of 77% and specificity of 75%. A prognostic index consisting of 4 clinical variables from day 1 that were significant predictors of transplant/death on univariate analysis was then constructed (i.e., creatinine ≥2.0 mg/dL, ALT ≤2600 IU/mL, intubation status, and pressors). The presence of 2 or more of these factors provided a sensitivity of 92%, specificity of 88%, and positive predictive value of 86% in identifying transplant/death (Table 3). In comparison, use of the King’s College Criteria to predict transplant/death on study day 1 provided a 31% sensitivity, 94% specificity, and positive predictive value of 80% (Table 3). Similarly, a day 1 laboratory MELD score of ≥35 provided a sensitivity of 54%, specificity 88%, and positive predictive value of 78% for transplant/death. The c-statistic from the area under the receiver operator curve (AUROC) for the ALFSG index was 0.899 which was significantly better than that obtained with the King’s College criteria (0.623, P=.018) and laboratory MELD score (0.707, P=.053).
Fig. 2.
Day 1 serum ALT and creatinine levels of patients with hepatitis A virus enrolled in the Acute Liver Failure Study Group. The mean serum ALT levels on study day 1 of survivors were significantly higher than the transplant/death group (3362 vs. 1675 IU/mL, P = .03) while the mean serum creatinine levels of survivors were significantly lower than the transplant/death group (1.2 vs. 3.1 mg/dL, P = .04). *Outliers were defined as patients with laboratory values that exceeded 1.5 times the interquartile range.
Table 3.
Models to Predict Transplant/Death in 29 Patients With Hepatitis A Enrolled in the Acute Liver Failure Study Group
| Model | n | Sensitivity | Specificity | PPV | NPV | AUROC |
|---|---|---|---|---|---|---|
| ALFSG index * | ||||||
| ≥ 1 Factor | 20 | 100% | 56% | 65% | 100% | .781 |
| ≥ 2 Factors | 14 | 92% | 88% | 86% | 93% | .899 |
| ≥ 3 Factors | 9 | 62% | 94% | 89% | 75% | .766 |
| 4 Factors | 1 | 8% | 100% | 100% | 57% | .538 |
| Other models | ||||||
| MELD ≥ 35 | 9 | 54% | 88% | 78% | 70% | .707 |
| King’s College | 5 | 31% | 94% | 80% | 62% | .623 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
ALFSG index includes 4 factors on day 1: creatinine ≥ 2.0 mg/dL, ALT ≤ 2600 IU/L, intubation status, and need for pressors.
UNOS Patients With Hepatitis A
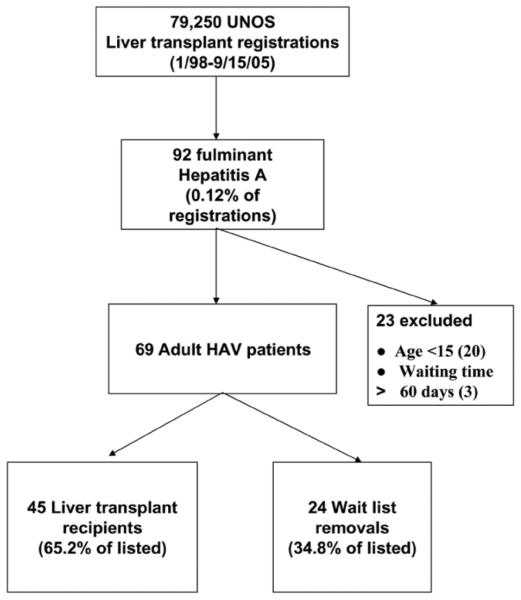
Of the 69 adult patients listed for liver transplantation with fulminant HAV between 1/98 and 9/05, 45 (65.2%) underwent transplantation (Fig. 3). The remaining 24 (34.8%) patients were removed from the liver transplant waiting list due to spontaneous improvement in 8, pretransplant death/too ill for transplant in 9, and unspecified reasons in 7. Therefore, the pretransplant mortality among the 69 UNOS HAV transplant candidates was at least 13% (9/ 69) and similar to that observed in the ALFSG HAV waitlisted patients of 7% (1/14) (Table 4).
Fig. 3.
Outcomes of patients with hepatitis A virus listed for liver transplantation in the UNOS database. All patients were listed between 1/98 and 9/15/05 and the diagnosis of fulminant HAV was made at the transplant center.
Table 4.
Patients With Hepatitis A Listed for Liver Transplantation in the Acute Liver Failure Study Group and in the UNOS Database (1998 to 2005)
| ALFSG-Enrolled Patients |
ALFSG-Listed Patients |
UNOS-Listed Patients |
P * | |
|---|---|---|---|---|
| Eligible study population reviewed | 925 | 925 | 79,250 | – |
| Adult HAV patients ** | 29 (3.1%) | 14 (1.5%) | 69 (0.09%) | – |
| Mean Age (years) | 48.3 ± 14.1 | 49.8 ± 12.4 | 44.4 ± 14.9 | .167 |
| % Female | 48% | 36% | 49% | .394 |
| Ethnicity | .446 | |||
| Asian | 1 | 3 | ||
| African American | 1 | 11 | ||
| Hispanic | - | 1 | ||
| Caucasian | 12 | 44 | ||
| Unknown | - | 10 | ||
| Reason for removal | .589 | |||
| Transplant | – | 9 (64.2%) | 45 (65.2%) | |
| Died | – | 1 (7.1%) | 7 (10.1%) | |
| Improved | – | 4 (28.9%) | 8 (11.5%) | |
| Too sick to undergo transplant | – | – | 2 (2.9%) | |
| Multilisting | – | – | 1 (1.4%) | |
| Not reported | – | – | 6 (8.7%) |
| ALFSG HAV Transplant Recipients (n = 9) |
UNOS HAV Transplant Recipients (n = 45) |
P *** | |
|---|---|---|---|
| Recipient age (years) | 51.7 ± 10.7 | 46.2 ± 13.6 | .201 |
| % Female | 22% | 51% | .153 |
| Ethnicity | .535 | ||
| Asian | – | 2 | |
| African American | 1 | 10 | |
| Caucasian | 8 | 27 | |
| Unknown | – | 6 | |
| Mean waiting time (days) | 3.3 ± 5.6 | 6.5 ± 13.0 | .251 |
| Median wait time (range) (days) | 1 (0-18) | 1 (0-53) |
Comparison between ALFSG-listed patients to UNOS-listed patients.
Adult defined by age >15.
Comparison between ALFSG transplant recipients and UNOS transplant recipients.
The mean age, gender, and ethnicity of the 69 UNOS HAV waitlisted patients was similar to that of the 14 ALFSG HAV patients listed for transplant (Table 4). In addition, the mean age, gender, and ethnicity of the 45 UNOS HAV transplant recipients was similar to that of the 9 ALFSG transplant recipients. Although the range of waiting times from listing to transplantation was greater in the UNOS population, the median waiting times of the ALFSG and UNOS patients were not significantly different (P = .251) (Table 4).
Incidence of Hepatitis A Related ALF
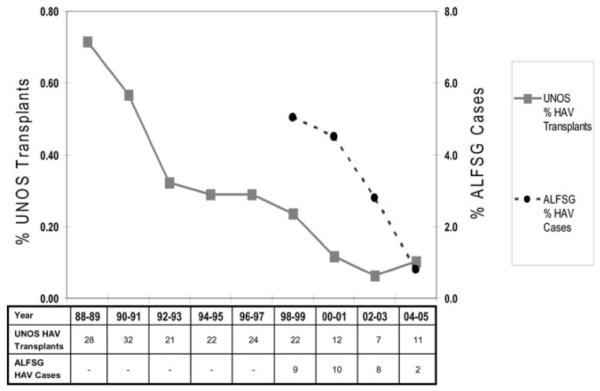
The frequency of patients undergoing liver transplantation for fulminant HAV was calculated in 2 year increments between 1988 and 2005 from the UNOS database. As seen in Fig. 4, the annual percentage of liver transplants for HAV related ALF declined from 0.7% in 1988-89 to 0.3% in 1996-97 and 0.1% through 2004-05. In addition, the proportion of liver transplants in UNOS for fulminant HAV compared to other etiologies of ALF also showed a similar decrease from 8.1% in 1988-89 to 1.3% in 2004-05 (Graph not shown). A parallel decline in the annual incidence of HAV patients enrolled in the ALFSG was also noted dropping from 5% in 1998-99 to 0.8% in 2004-05.
Fig. 4.
Trends in the incidence of hepatitis A virus related acute liver failure in the United States. The incidence of patients undergoing liver transplantation for HAV related ALF in the UNOS database significantly declined between 1988 and 2005 (P < .001). Similarly, the frequency of HAV patients enrolled in the ALFSG significantly declined between 1998 and 2005 (P = .007).
Discussion
Acute liver failure is a rare but potentially lethal illness that can affect previously healthy individuals of all ages. Although there are specific treatments for some etiologies of ALF (e.g., N-acetylcysteine for acetaminophen overdose), the risk of developing life-threatening cerebral edema or infectious complications is substantial and many patients require life-saving emergency liver transplantation.10,13 Previous studies have attempted to identify clinical and laboratory features at presentation in ALF patients associated with a low likelihood of spontaneous recovery.13,15,16 However, since the prognosis for spontaneous survival varies substantially by etiology (e.g., 0% in fulminant Wilson’s disease to 60% with acetaminophen overdose), disease-specific prognostic models may prove more useful if adequate numbers of patients can be studied.13,19 Since the annual incidence of ALF in the United States is low with an estimated 2,000 to 3,000 total cases per year, tracking trends in the outcomes from individual diseases like HAV is difficult and requires the development of a multicenter, surveillance network.20,21
In the current study, two large national registries, the ALFSG and UNOS databases, were used to identify trends in the incidence of fulminant HAV in the United States as well as predictors of poor outcomes. Overall, the proportion of ALFSG cases due to HAV was low at 3.1% and only 0.12% of the patients listed for liver transplantation during the same time period were for acute HAV. Interestingly, a decreasing incidence of subjects with fulminant HAV was observed over time in both the ALFSG and UNOS populations (Fig. 4). Epidemiologic data from the CDC support this finding with a decrease in the incidence of acute HAV from 24,238 reported cases in 1993 to 7,653 cases in 2003, although the number of acute HAV cases reported to the CDC is likely underreported by up to 10 fold.2 Possible explanations for the decreasing incidence of acute and fulminant HAV in the United States include the introduction of the hepatitis A vaccine in 1995 and successful vaccination of high risk groups.22-24 In addition, other public health measures such as improved sanitation and food preparation techniques as well as educational initiatives may have contributed to the decreasing incidence.25 Nonetheless, since less than a third of the adult US population has serological evidence of protective immunity to HAV, additional measures are needed to avoid future cases of sporadic HAV.2,22 Along those lines, the Advisory Committee of Immunization Practices has recently recommended that all children greater than 12 months of age be considered for HAV vaccination.26
Prior studies have suggested that women, subjects with underlying liver disease, and older individuals may be at increased risk of developing life-threatening ALF with acute HAV infection but the number of patients reported has been limited.5,11,12 For example, in a cohort of 76 subjects with acute hepatitis A, the 19 subjects with ALF were significantly older, had higher bilirubin levels, and were more likely to be female.11 In addition, HAV RNA was more likely to be undetectable in the fulminant patients compared to the non-ALF patients (9/19 vs. 5/31, P < .02). This suggests that an aberrant host immune response may be responsible for the severe liver injury encountered as has been postulated in patients with acute HBV developing ALF.27 In the current study, there was only 1 individual with pre-existing chronic hepatitis B and C infection who developed fulminant HAV infection but this patient spontaneously recovered.
Our data identified higher day 1 creatinine levels and lower serum ALT and alkaline phosphatase levels as being associated with transplant/death. The lower serum ALT values in the transplant/death group may reflect more extensive necrosis at presentation leading to “burnout” of the liver but it should be noted that the peak serum ALT levels were similar in the two patient groups.28 The finding of significantly lower serum alkaline phosphatase values among the transplant/death group is more difficult to explain but may also reflect less vigorous liver regeneration. The absence of a significant relationship between subject age with transplant/death may, in part, be due to selection/referral bias since the majority of patients enrolled in the ALFSG were transferred for liver transplant evaluation and elderly individuals may not have been referred.10 The association of the need for pressors and intubation at presentation with transplant/death is consistent with prior studies demonstrating that ALF patients with greater illness severity and evidence of multiorgan failure have a lower likelihood of recovery.13,15,29 The association between higher serum creatinine values and the need for hemodialysis with transplant/death is also consistent with prior studies demonstrating the poor prognosis associated with renal failure in ALF patients.30,31 Although recent studies from France have suggested that concomitant acetaminophen use may increase the risk of fulminant HAV infection, we did not find a significant relationship between the presence of detectable serum acetaminophen levels or a history of acetaminophen ingestion and clinical outcomes.11 However, the prognostic significance of serum acetaminophen-protein adducts in ALF patients with viral hepatitis is under investigation.31
The prognostic index we developed to predict transplant/death incorporated 4 variables that are easy to obtain and readily available to practicing clinicians. The presence of at least 2 of these component features predicted transplant/death significantly better than the King’s College Criteria as well as laboratory MELD scores (Table 3). The lower sensitivity and NPV of the King’s criteria may relate to the fact that the King’s criteria groups multiple etiologies together under the umbrella of “non-acetaminophen” ALF. In addition, previous studies have shown that the King’s College Criteria are less useful in predicting those who are likely to recover compared to those who are likely to die or require transplantation and this would further impact accuracy in a subgroup such as HAV ALF that has better overall outcomes.32 Nonetheless, the King’s criteria remain the most widely used prognostic criteria in ALF worldwide.15,18 The poorer performance of the laboratory MELD score is not surprising since ALF patients with rapid onset disease such as fulminant HAV infection and acetaminophen overdose frequently do not have high bilirubin or creatinine levels at presentation. However, it must be emphasized that the newly identified ALFSG prognostic index was derived using a small dataset and requires further validation in other patient populations.
While the current study design provided a unique opportunity to investigate fulminant HAV infection, there are several important limitations. Specifically, the total number of subjects in both the ALFSG (29) and UNOS cohorts (69) with fulminant HAV are small. In addition, there is potential for referral bias as all ALFSG study sites are also local liver referral centers and all but one site offers liver transplantation. However, the clinical and demographic features of the waitlisted and transplanted ALFSG and UNOS HAV patients were remarkably similar (Table 4). This suggests that that the findings from the ALFSG may be generalizable to liver transplant candidates and recipients with ALF throughout the country. However, it is possible that patients with fulminant HAV in the community were not referred to one of the study sites due to milder disease, advanced age, or other contraindications to transplantation. Therefore, only a population based study would be able to identify the prognostic features of all United States patients with fulminant HAV but such a study will likely be difficult to conduct due to the declining incidence of acute HAV and the low frequency of fulminant HAV.2,22
In conclusion, fulminant HAV is an uncommon disease in the United States that accounts for a minority of patients enrolling in the ALFSG observational study and being listed for liver transplantation. In parallel with a declining incidence of acute HAV infection in the general population, the incidence of fulminant HAV in both the ALFSG and UNOS databases has significantly decreased over the past 7 years. Among the ALFSG HAV patients, 4 readily available laboratory and clinical features at presentation may help identify patients in need of urgent liver transplantation but further validation is required. The HAV patients enrolled in the ALFSG appear to be representative of HAV patients listed for liver transplantation throughout the United States and provides external validity for the ALFSG. Since severe acute HAV infection is one of the few preventable etiologies of ALF, greater vaccination of the general population and high-risk sub-groups may lead to further reductions in the associated morbidity and mortality.
Acknowledgment
The authors would like to thank the investigators and study coordinators of the participating sites of the ALFSG which includes: William M. Lee (Principal Investigator), University of Texas Southwestern Medical Center, Dallas, Texas; Anne Larson, University of Washington, Seattle, Washington; Jeffery S. Crippin, Washington University, St. Louis, Missouri; Timothy J. Davern and Nathan Bass, University of California at San Francisco, San Francisco, California; Sukru Emre, Mt. Sinai Medical Center, New York, New York; Timothy M. McCashland, University of Nebraska, Omaha, Nebraska; J. Eileen Hay, Mayo Clinic, Rochester, Minnesota; Natalie Murray, Baylor University Medical Center, Dallas, Texas; A. Obaid Shakil, University of Pittsburgh, Pittsburgh, Pennsylvania; Andres T. Blei, Northwestern University, Chicago, Illinois; Atif Zaman, Oregon Health Sciences University, Portland, Oregon; Steven H.B. Han, University of California, Los Angeles, Los Angeles, California; Robert J. Fontana, University of Michigan, Ann Arbor, Michigan; Brendan McGuire, University of Alabama at Birmingham, Birmingham, Alabama; Raymond Chung, Massachusetts General Hospital, Boston, Massachusetts; Steven Lobritto, Robert Brown and Michael Schilsky, Columbia-Presbyterian Medical Center, New York, New York; M. Edwyn Harrison, Mayo Clinic Scottsdale, Phoenix, Arizona; Adrian Reuben, Medical University of South Carolina, Charleston, South Carolina; Santiago Munoz, Albert Einstein Medical Center, Philadelphia, Pennsylvania; Rajender Reddy, University of Pennsylvania, Philadelphia, Pennsylvania; R. Todd Stravitz, Virginia Commonwealth University, Richmond, Virginia; Lorenzo Rossaro, University of California, Davis Medical Center, Sacramento, California; Raj Santayanarana, Mayo Clinic, Jacksonville, Florida.
Supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK58369 to WML), the Jean Roberts and the Rollin and Mary Ella King Funds at the Southwestern Medical Foundation, Dallas. Also supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- ALF
acute liver failure
- ALFSG
Acute Liver Failure Study Group
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under the receiver operator curve
- CDC
Centers for Disease Control and Prevention
- HAV
hepatitis A virus
- INR
international normalized ratio
- MELD
model for end stage liver disease
- UNOS
United Network for Organ Sharing
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA. 2005;294:194–201. doi: 10.1001/jama.294.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Hepatitis Surveillance Report No. 60. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2005. [Google Scholar]
- 3.Wheeler C, Vogt TM, Armstrong GL, Vaughan G, Weltman A, Nainan OV, et al. An outbreak of hepatitis A associated with green onions. N Engl J Med. 2005;353:890–897. doi: 10.1056/NEJMoa050855. [DOI] [PubMed] [Google Scholar]
- 4.Hutin YJ, Pool V, Cramer EH, Nainan OV, Weth J, Williams IT, Gold-stein ST, et al. A multistate, foodborne outbreak of hepatitis A. National Hepatitis A Investigation Team. N Engl J Med. 1999;340:595–602. doi: 10.1056/NEJM199902253400802. [DOI] [PubMed] [Google Scholar]
- 5.Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious Hepatitis A: An analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998;128:111–114. doi: 10.7326/0003-4819-128-2-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Brown GR, Persley K. Hepatitis A epidemic in the elderly. South Med J. 2002;95:826–833. [PubMed] [Google Scholar]
- 7.Chau TN, Lai ST, Tse C, Ng TK, Leung VK, Lim W, et al. Epidemiology and clinical features of sporadic hepatitis E as compared with hepatitis A. Am J Gastroenterol. 2006;101:292–296. doi: 10.1111/j.1572-0241.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Gandolfo GM, Ferri GM, Conti L, Antenucci A, Marrone R, Frasca AM, et al. Prevalence of infections by hepatitis A, B, C, and E viruses in two different socioeconomic groups of children from Santa Cruz, Bolivia. Med Clin (Barc) 2003;120:725–727. doi: 10.1016/s0025-7753(03)73826-3. [DOI] [PubMed] [Google Scholar]
- 9.Schiodt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:448–453. doi: 10.1111/j.1572-0241.2003.t01-1-07223.x. [DOI] [PubMed] [Google Scholar]
- 10.Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29–34. doi: 10.1002/lt.500050102. [DOI] [PubMed] [Google Scholar]
- 11.Rezende G, Roque-Afonso AM, Samuel D, Gigou M, Nicand E, Ferre V, et al. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology. 2003;38:613–618. doi: 10.1053/jhep.2003.50366. [DOI] [PubMed] [Google Scholar]
- 12.Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 13.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 14.Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, et al. Liver and Intestine transplantation in the United States, 1995-2004. Am J Transplant. 2006;6:1170–1187. doi: 10.1111/j.1600-6143.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 16.Kremers WK, van IJperen M, Kim WR, Freeman RB, Harper AM, Kamath PS, et al. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39:764–769. doi: 10.1002/hep.20083. [DOI] [PubMed] [Google Scholar]
- 17.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 18.Polson J, Orsulak PJ, Wians F, Murray N, Balko J, Fuller D, Rossaro L, et al. Elevated bilirubin may cause false positive acetaminophen levels in hepatitis patients [Abstract] Hepatology. 2004;40(Suppl 1):496A. [Google Scholar]
- 19.Lee WM. Acute Liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle JH, Carithers RL, Jr, Shapiro C, Ascher N. Fulminant hepatic failure: Summary of a workshop. Hepatology. 1995;21:240–252. [PubMed] [Google Scholar]
- 21.Kim WR, Brown RS, Terrault NA, El-Serag H. Burden of liver disease in the United States: Summary of a workshop. Hepatology. 2002;36:227–242. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 22.Bell BP, Kruszon-Moran D, Shapiro CN, Lambert SB, McQuillan GM, Margolis HS. Hepatitis A virus infection in the United States: serologic results from the Third National Health and Nutrition Examination Survey. Vaccine. 2005;23:5798–5806. doi: 10.1016/j.vaccine.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 23.Nainan OV, Armstrong GL, Han XH, Williams I, Bell BP, Margolis HS. Hepatitis A molecular epidemiology in the United States, 1996-1997: Sources of infection and implications of vaccination policy. J Infect Dis. 2005;191:957–963. doi: 10.1086/427992. [DOI] [PubMed] [Google Scholar]
- 24.Sjogren M. Immunization and the decline of viral hepatitis as a cause of acute liver failure [Editorial] Hepatology. 2003;38:554–556. doi: 10.1053/jhep.2003.50401. [DOI] [PubMed] [Google Scholar]
- 25.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–1022. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advisory Committee on Immunization Practices (ACIP) Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 27.Wai CT, Fontana RJ, Polson J, Hussain M, Shakil AO, Han SH, et al. Clinical outcome and virological characteristics of hepatitis B-related acute liver failure in the United States. J Viral Hepatitis. 2005;12:192–198. doi: 10.1111/j.1365-2893.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 28.Masada CT, Shaw BW, Jr, Zetterman RK, Kaufman SS, Markin RS. Fulminant hepatic failure with massive necrosis as a result of hepatitis A infection. J Clin Gastroenterol. 1993;17:158–162. doi: 10.1097/00004836-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Pitre J, Soubrane O, Dousset B, Ozier Y, Baudin F, Devictor D, et al. How valid is emergency liver transplantation for acute liver necrosis in patients with multiple-organ failure? Liver Transpl Surg. 1996;2:1–7. doi: 10.1002/lt.500020102. [DOI] [PubMed] [Google Scholar]
- 30.Ring-Larsen H, Palazzo U. Renal failure in fulminant hepatic failure and terminal cirrhosis: a comparison between incidence, types, and prognosis. Gut. 1981;22:585–591. doi: 10.1136/gut.22.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: Clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 32.Polson J, James LP, Davern TJ, Hynan L, Rossaro L, Larson AM, et al. Acetaminophen as a co-factor in acute liver failure due to viral hepatitis determined by measurement of acetaminophen-protein adducts [Abstract] Gastroenterology. 2006;130(Suppl 2):S1002. [Google Scholar]