Abstract
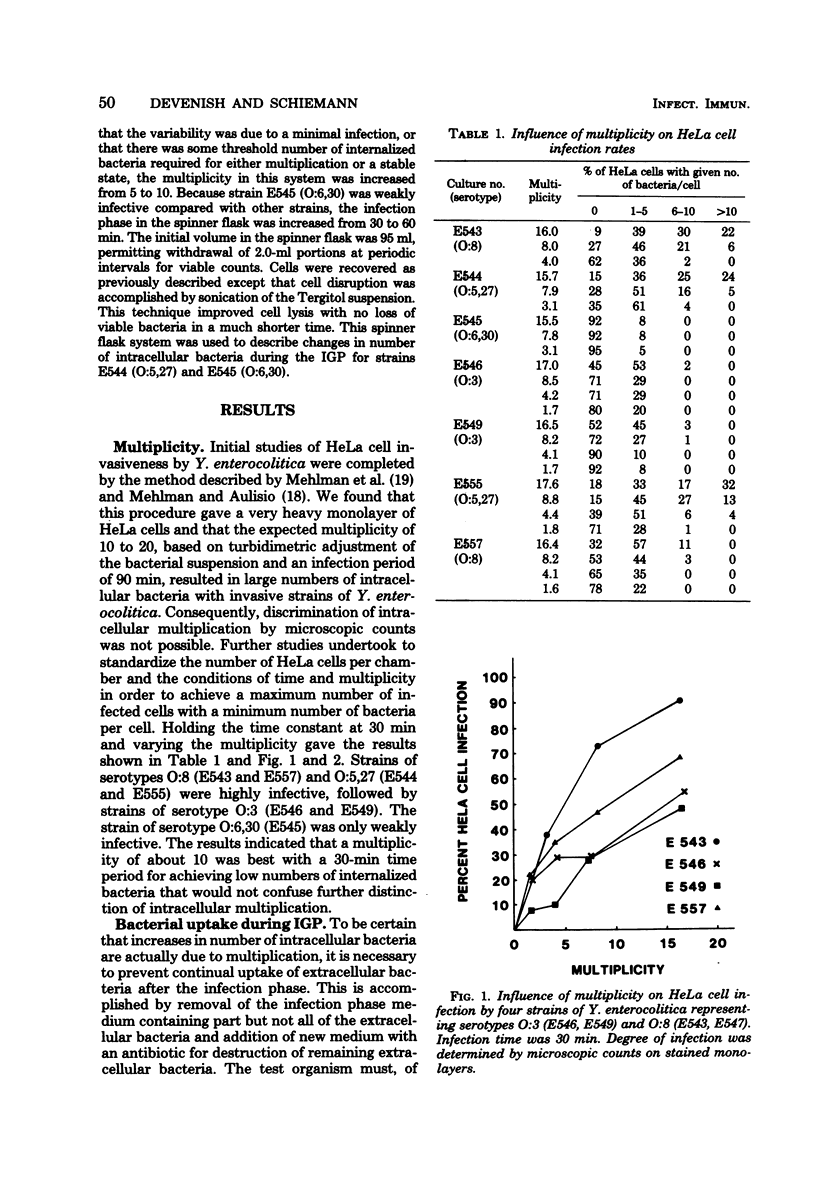
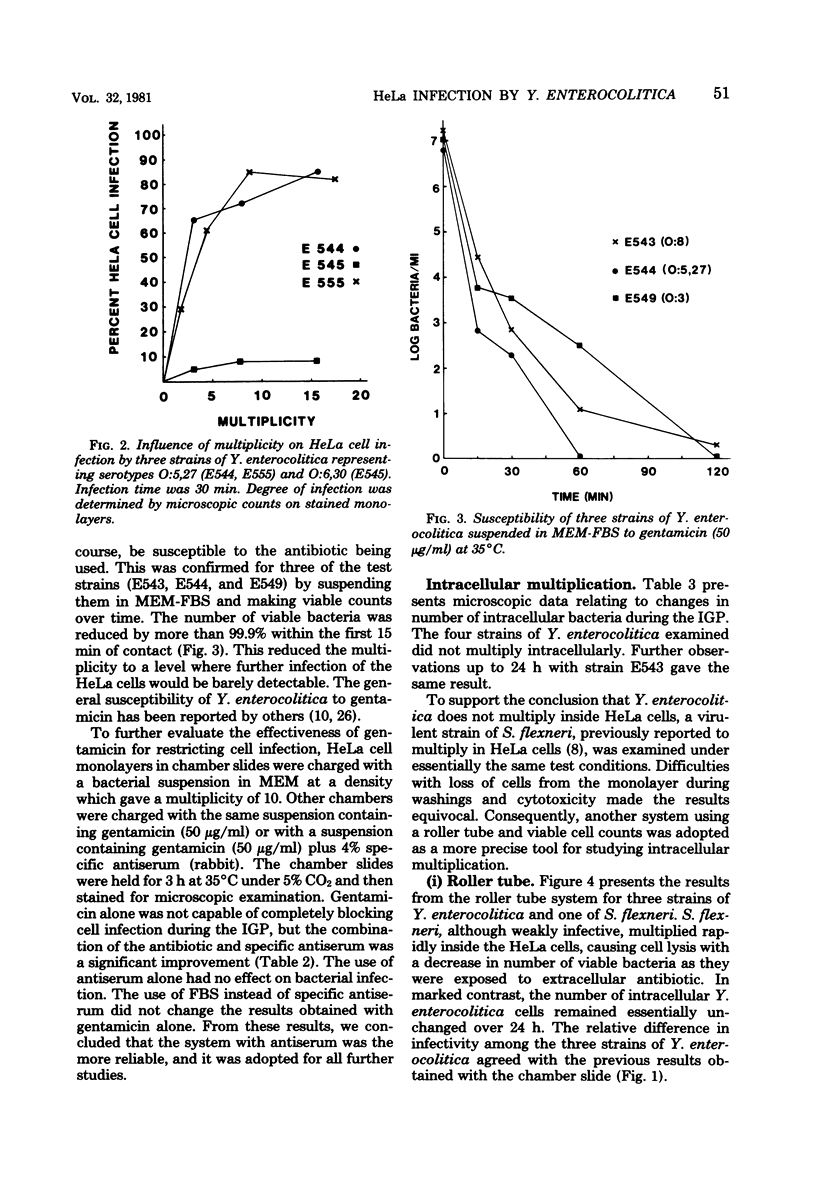
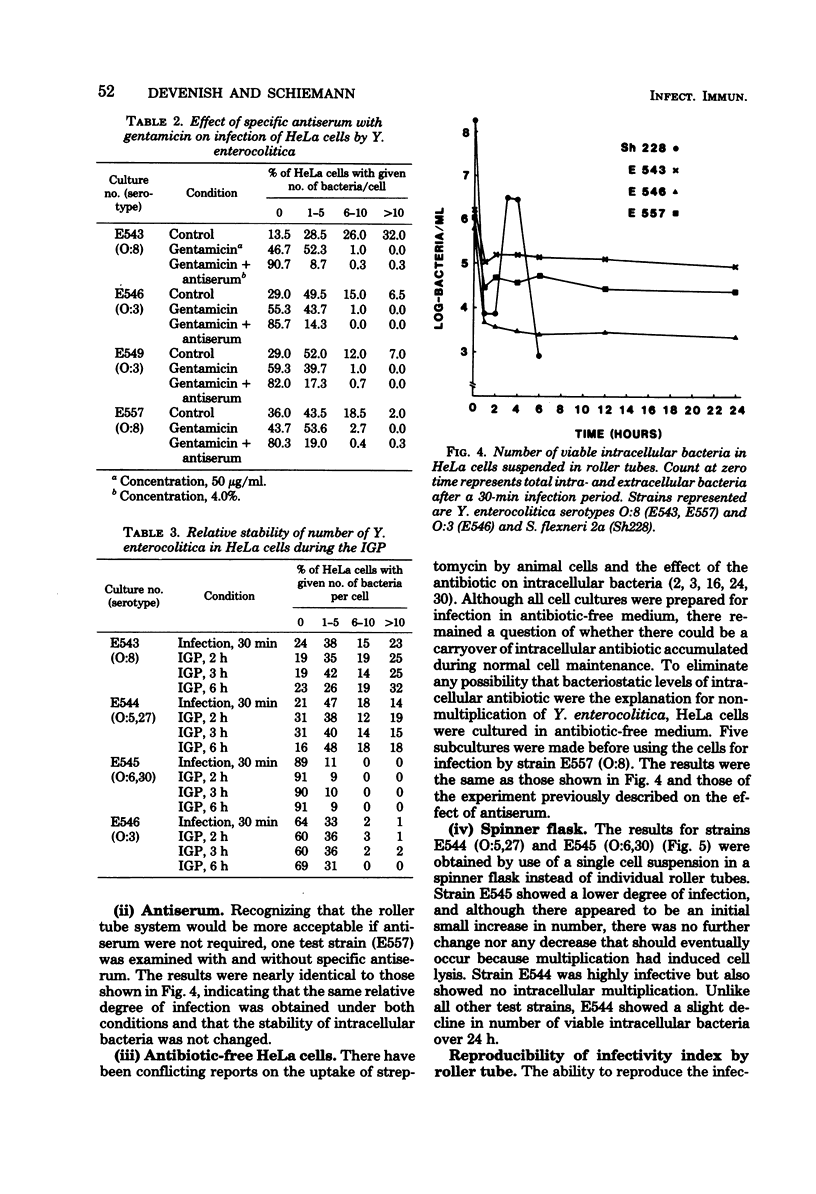
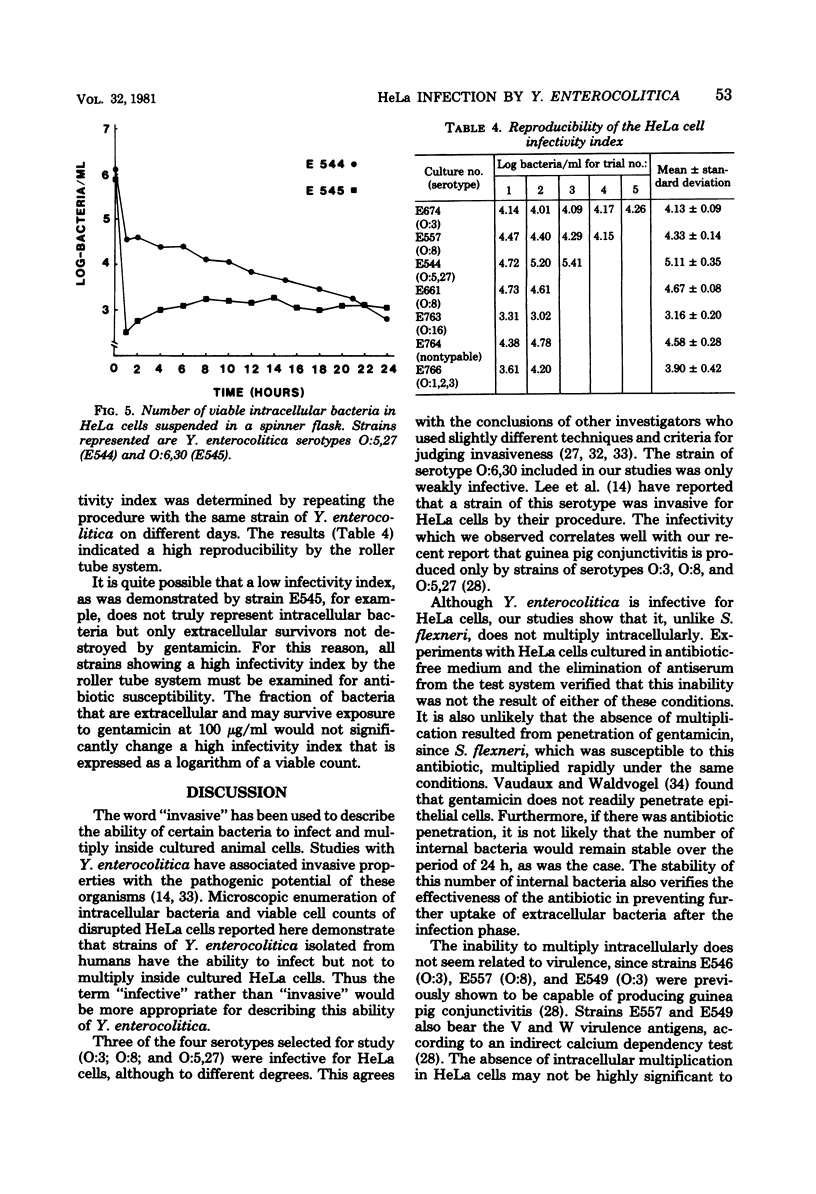
The in vitro invasive properties of bacteria have frequently been studied by the use of HeLa cell cultures in chamber slides, using microscopic examination to enumerate intracellular bacteria. When this system was used to examine invasive properties of Yersinia enterocolitica, it resulted in rapid internalization of high numbers of bacteria during the infection phase which prevented subsequent discrimination of intracellular multiplication. A modified procedure was developed which standardized the ratio of bacteria to HeLa cells (i.e., multiplicity), the time for the infection phase, and the addition of specific antiserum with gentamicin for restricting bacterial uptake during the intracellular growth phase. Studies with this modified chamber slide system found that strains of human isolates of Y. enterocolitica (serotypes O:3, O:8, O:5,27, and O:6,30) exhibited different degrees of cell infection but did not multiply intracellularly. A second test system was developed that used roller tubes and viable cell counts for the enumeration of intracellular bacteria. This roller tube system confirmed that internalized bacteria did not multiply inside HeLa cells over a 24-h period. The roller tube system with viable cell counts for enumeration is a simplified technique for quantitative comparison of in vitro infectivity of HeLa cells by Y. enterocolitica.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissett M. L. Yersinia enterocolitica isolates from humans in California, 1968-1975. J Clin Microbiol. 1976 Aug;4(2):137–144. doi: 10.1128/jcm.4.2.137-144.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Hayes R., Imhoff J. Autoradiographic evidence for the impermeability of mouse peritoneal macrophages to tritiated streptomycin. J Bacteriol. 1967 Jan;93(1):445–450. doi: 10.1128/jb.93.1.445-450.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabi O. In-vitro interaction of Shigella flexneri with leukocytes and HeLa cells. J Infect Dis. 1970 Jul-Aug;122(1):1–9. doi: 10.1093/infdis/122.1-2.1. [DOI] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER D. F., WATKINS H. M. Growth of shigellae in monolayer tissue cultures. J Bacteriol. 1961 Dec;82:815–822. doi: 10.1128/jb.82.6.815-822.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerberg S., Sorger S., Marks M. I. Antimicrobial susceptibilities of Yersinia enterocolitica biotype 4, serotype O:3. Antimicrob Agents Chemother. 1977 Mar;11(3):566–568. doi: 10.1128/aac.11.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihlström E., Edebo L. Association of viable and inactivated Salmonella typhimurium 395 MS and MR 10 with HeLa cells. Infect Immun. 1976 Oct;14(4):851–857. doi: 10.1128/iai.14.4.851-857.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., McGrath P. P., Carter P. H., Eide E. L. The ability of some Yersinia enterocolitica strains to invade HeLa cells. Can J Microbiol. 1977 Dec;23(12):1714–1722. doi: 10.1139/m77-247. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973 Jul;52(7):1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Une T., Zen-Yoji H. Observations on the correlation between pathogenicity and serovars of Yersinia enterocolitica by the assay applying cell culture system and experimental mouse infection. Contrib Microbiol Immunol. 1979;5:317–323. [PubMed] [Google Scholar]
- Mehlman I. J., Aulisio C. C., Sanders A. C. Microbiological methods. Problems in the recovery and identification of Yersinia from food. J Assoc Off Anal Chem. 1978 Jul;61(4):761–771. [PubMed] [Google Scholar]
- Mehlman I. J., Eide E. L., Sanders A. C., Fishbein M., Aulisio C. C. Methodology for recognition of invasive potential of Escherichia coli. J Assoc Off Anal Chem. 1977 May;60(3):546–562. [PubMed] [Google Scholar]
- Mäki M., Grönroos P., Vesikari T. In vitro invasiveness of Yersinia enterocolitica isolated from children with diarrhea. J Infect Dis. 1978 Nov;138(5):677–680. doi: 10.1093/infdis/138.5.677. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Nakaya R. Cinemicrographic study of tissue cell cultures infected with Shigella flexneri. Jpn J Med Sci Biol. 1968 Aug;21(4):259–273. doi: 10.7883/yoken1952.21.259. [DOI] [PubMed] [Google Scholar]
- Osada Y., Nakajo M., Une T., Ogawa H., Oshima Y. Application of cell culture in studying antibacterial activity of rifampicin to Shigella and enteropathogenic Escherichia coli. Jpn J Microbiol. 1972 Nov;16(6):525–533. doi: 10.1111/j.1348-0421.1972.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Winblad S., Bitsch V. Studies on the interaction between different O-serotypes of Yersinia enterocolitica and HeLa cells. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):141–145. doi: 10.1111/j.1699-0463.1979.tb02417.x. [DOI] [PubMed] [Google Scholar]
- Raevuori M., Harvey S. M., Pickett M. J., Martin W. J. Yersinia enterocolitica: in vitro antimicrobial susceptibility. Antimicrob Agents Chemother. 1978 May;13(5):888–890. doi: 10.1128/aac.13.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Harkness T. K. Intracellular Pasteurella pseudotuberculosis: Multiplication in Cultured Spleen and Kidney Cells. Infect Immun. 1970 Nov;2(5):631–639. doi: 10.1128/iai.2.5.631-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J Exp Med. 1957 Jan 1;105(1):39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Devenish J. A. Virulence of Yersinia enterocolitica determined by lethality in Mongolian gerbils and by the Serény test. Infect Immun. 1980 Aug;29(2):500–506. doi: 10.1128/iai.29.2.500-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma S., Lafleur L., Deidrick V. R. Canadian experience with Yersinia enterocolitica (1966--1977). Contrib Microbiol Immunol. 1979;5:144–149. [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. II. Interaction with cultured cells in vitro. Microbiol Immunol. 1977;21(7):365–377. doi: 10.1111/j.1348-0421.1977.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Une T., Zen-Yoji H., Maruyama T., Yanagawa Y. Correlation between epithelial cell infectivity in vitro and O-antigen groups of Yersinia enterocolitica. Microbiol Immunol. 1977;21(12):727–729. doi: 10.1111/j.1348-0421.1977.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979 Dec;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS H. M. Some attributes of virulence in Shigella. Ann N Y Acad Sci. 1960 Nov 21;88:1167–1186. doi: 10.1111/j.1749-6632.1960.tb20107.x. [DOI] [PubMed] [Google Scholar]
- Zen-Yoji H., Maruyama T. The first successful isolations and identification of Yersinia enterocolitica from human cases in Japan. Jpn J Microbiol. 1972 Nov;16(6):493–500. doi: 10.1111/j.1348-0421.1972.tb00689.x. [DOI] [PubMed] [Google Scholar]


