Abstract
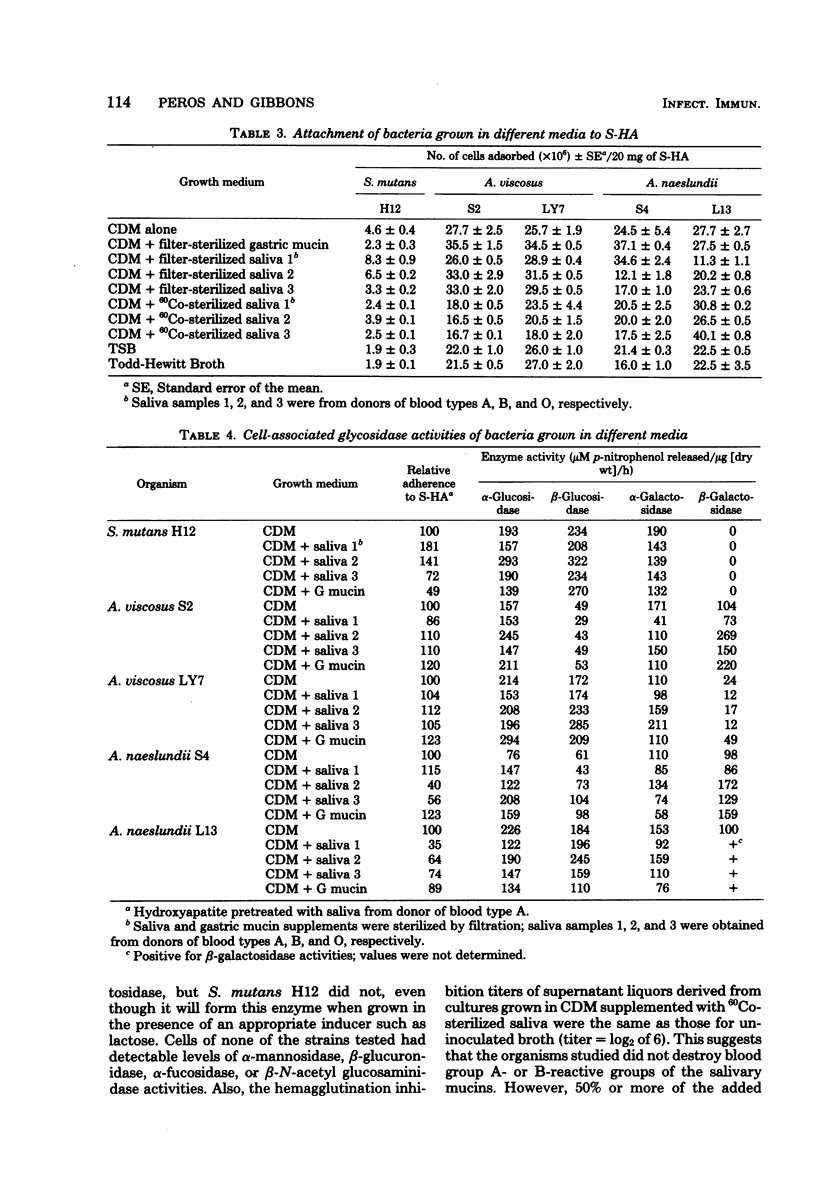
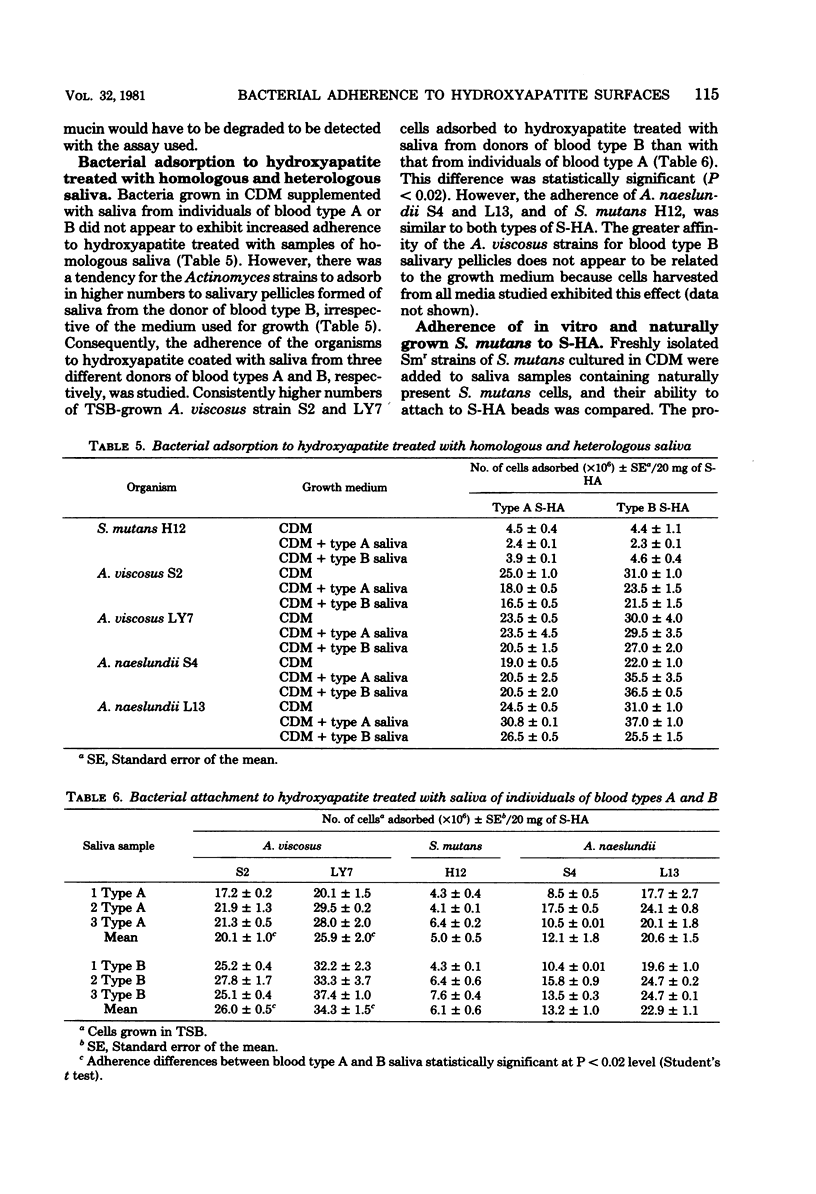
The influence of the growth medium on the ability of strains of Streptococcus mutans, Actinomyces viscosus and A. naeslundii to attach to saliva-treated hydroxyapatite (S-HA) surfaces was studied. Preliminary experiments indicated that cells of each species harvested in lag, log, and early stationary phases of growth adsorbed comparably to S-HA; thus, early stationary phase cells were used in all subsequent assays. Strains were grown in chemically defined medium (CDM), in CDM supplemented with gastric mucin or with filter-sterilized or 60Co-irradiated saliva from human donors of blood types A, B, or O, and in Trypticase soy broth (BBL Microbiology Systems) and Todd-Hewitt broth. Adherence of S. mutans H12 to S-HA tended to vary when the streptococci were grown in saliva-supplemented CDM, but the number of cells which attached was generally within twofold of that of CDM-grown cells. Attachment of A. viscosus S2 and LY7 and of A. naeslundii S4 and L13 was generally similar when grown in CDM or in CDM supplemented with saliva, but it tended to increase for organisms grown in CDM supplemented with gastric mucin. None of the strains studied appeared to destroy the blood group reactivity of the added salivary components, and they attached equally well to HA treated with homologous or heterogous saliva from that present in the medium in which they were grown. The A. viscosus strains adsorbed in 25 to 40% higher numbers to HA treated with blood type B saliva than with type A saliva, irrespective of the medium used for growth. S. mutans H12 cells displayed α- and β-glucosidase and α-galactosidase activity; the Actinomyces strains exhibited these activities plus β-galactosidase when grown in all media. However, the levels of these glycoside hydrolases did not correlate with cell adsorption to S-HA. The apparent weak influence of the growth medium on attachment of S. mutans was studied further. Strains of S. mutans isolated from the saliva of five human donors were made resistant to streptomycin, grown in CDM, and then added to new saliva samples from the respective donors from which they were obtained. The in vitro-grown cells were found to attach to S-HA comparably to S. mutans cells present naturally in the saliva.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. I. In vitro production of ABH blood group-degrading enzymes by enteric bacteria. J Clin Invest. 1976 Jan;57(1):63–73. doi: 10.1172/JCI108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Degradation of blood group antigens in human colon ecosystems. II. A gene interaction in man that affects the fecal population density of certain enteric bacteria. J Clin Invest. 1976 Jan;57(1):74–82. doi: 10.1172/JCI108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. Rapid identification of Actinomycetaceae and related bacteria. J Clin Microbiol. 1978 Aug;8(2):127–133. doi: 10.1128/jcm.8.2.127-133.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Ability of Veillonella and Neisseria species to attach to oral surfaces and their proportions present indigenously. Infect Immun. 1971 Sep;4(3):264–268. doi: 10.1128/iai.4.3.264-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangsri P., Orstavik D. Effect of the acquired pellicle and of dental plaque on the implantation of Streptococcus mutans on tooth surfaces in man. Caries Res. 1977;11(4):204–210. doi: 10.1159/000260269. [DOI] [PubMed] [Google Scholar]
- Rölla G., Robrish S. A., Bowen W. H. Interaction of hydroxyapatite and protein-coated hydroxyapatite with Streptococcus mutans and Streptococcus sanguis. Acta Pathol Microbiol Scand B. 1977 Oct;85B(5):341–346. doi: 10.1111/j.1699-0463.1977.tb01985.x. [DOI] [PubMed] [Google Scholar]


