Abstract
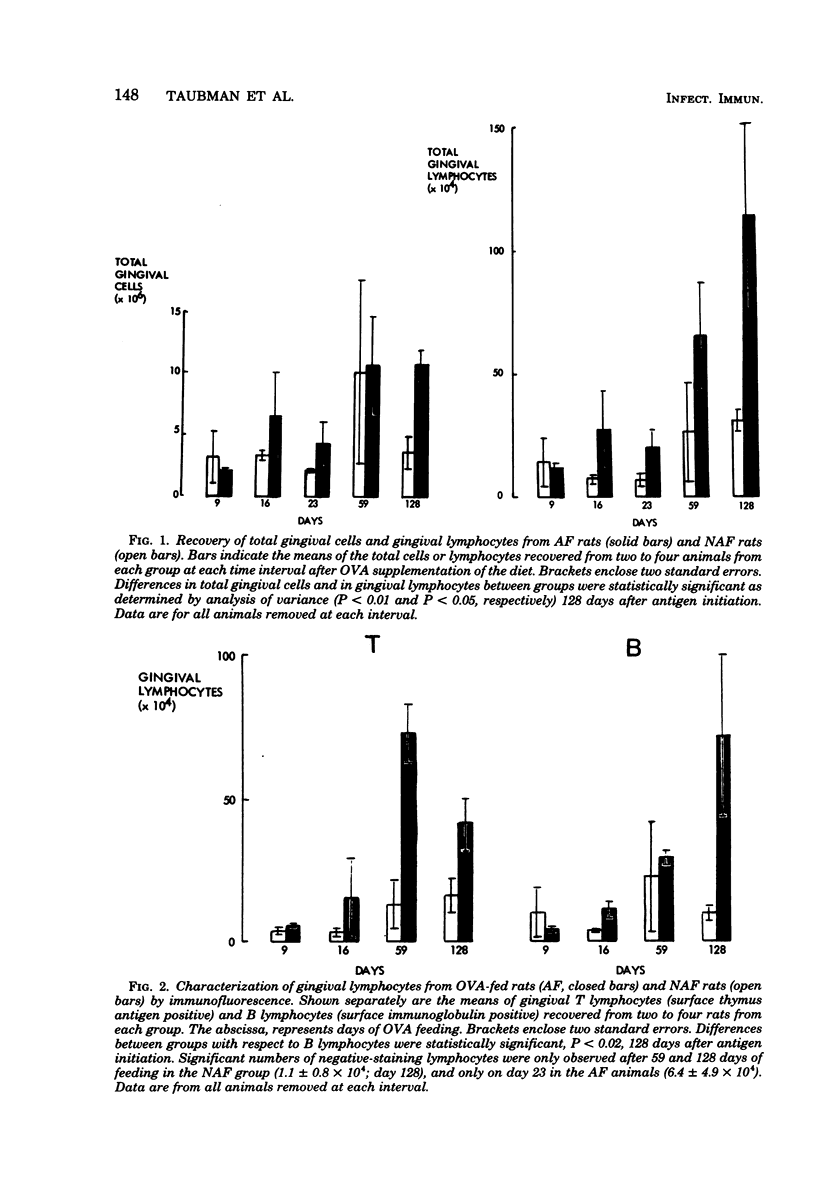
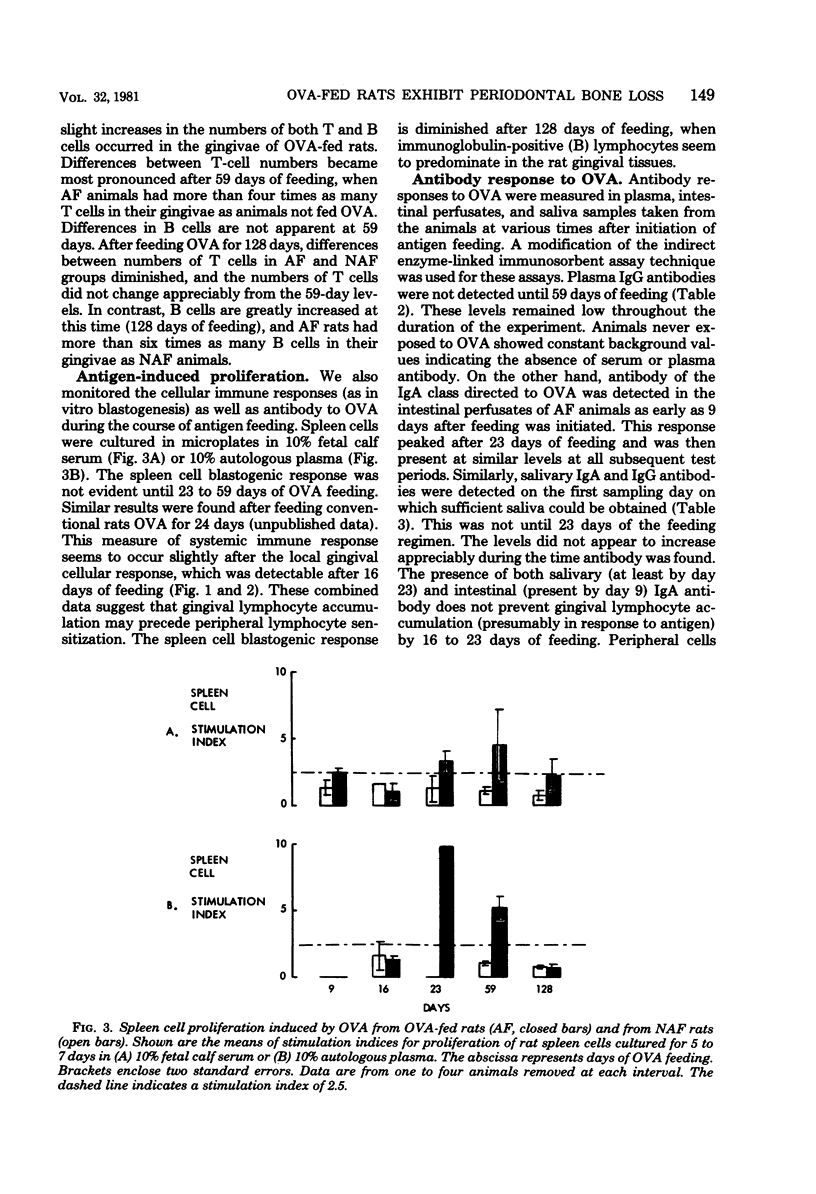
A technique for the characterization of rat gingival lymphocytes has been described. The technique was used to obtain gingival cells from rats maintained on antigen-free diets or such diets with ovalbumin (OVA) added. Increases in gingival lymphocyte numbers in the antigen-fed (AF) animals occurred by 16 to 23 days of OVA feeding. The elevated gingival lymphocyte numbers were predominantly T lymphocytes at the initial intervals of the experiment (to 59 days of OVA feeding). At 128 days of OVA feeding T-lymphocyte numbers diminished but B-lymphocyte numbers increased, and AF animals had more than six times as many gingival B lymphocytes as animals not fed antigen. Also, AF animals showed immunoglobulin A antibody in intestinal perfusates (after 9 days of OVA feeding) and in saliva (within 23 days of OVA initiation). Plasma immunoglobulin G antibodies were not detected until 59 days of feeding. Spleen cells from AF rats showed in vitro blastogenic responses to OVA at 23 to 59 days of feeding. Periodontal bone loss was greater in AF animals after 59 and 128 days of OVA. Germfree animals fed only one antigen experienced more periodontal bone loss than animals fed the same diet not containing antigen. Therefore, immune phenomena can contribute to experimental bone loss in germfree rats.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstad-Jossi M., Schroeder H. E. Age-related alterations of periodontal structures around the cemento-enamel junction and of the gingival connective tissue composition in germ-free rats. J Periodontal Res. 1978 Jan;13(1):76–90. doi: 10.1111/j.1600-0765.1978.tb00156.x. [DOI] [PubMed] [Google Scholar]
- Armerding D. Two allotypic specifities of rat immunoglobulin. Eur J Immunol. 1971 Jan;1(1):39–45. doi: 10.1002/eji.1830010108. [DOI] [PubMed] [Google Scholar]
- Baer N., Fitzgerald R. J. Periodontal disease in the 18-month-old germfree rat. J Dent Res. 1966 Mar-Apr;45(2):406–406. doi: 10.1177/00220345660450023401. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Feldman J. D. Thymus-dependent (T) lymphocytes in the rat. J Immunol. 1974 Jan;112(1):79–86. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Crawford J. M., Taubman M. A., Smith D. J. The natural history of periodontal bone loss in germfree and gnotobiotic rats infected with periodontopathic microorganisms. J Periodontal Res. 1978 Jul;13(4):316–325. doi: 10.1111/j.1600-0765.1978.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Cypess R. H., Ebersole J. L., Molinari J. A. Specific antibody levels in the intestinal perfusates of Heligosomoides polygyrus-infected mice. Int Arch Allergy Appl Immunol. 1977;55(1-6):496–503. doi: 10.1159/000231963. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J. The effect of neonatal thymectomy on the level of salivary and serum immunoglobulins in rats. Immunology. 1979 Apr;36(4):649–657. [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J. Thymic control of secretory antibody responses in the rat. J Immunol. 1979 Jul;123(1):19–24. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Genco R. J., Mashimo P. A., Krygier G., Ellison S. A. Antibody-mediated effects on the periodontium. J Periodontol. 1974 May;45(5):330–337. doi: 10.1902/jop.1974.45.5.330. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Anatomical distribution of T and B lymphocytes in the rat. Development of lymphocyte-specific antisera. J Exp Med. 1973 Dec 1;138(6):1443–1465. doi: 10.1084/jem.138.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad T. Antibodies reacting with lipopolysaccharides from Bacteroides melaninogenicus, in serum from normal human subjects. J Infect Dis. 1974 Mar;129(3):349–352. doi: 10.1093/infdis/129.3.349. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Leikin S., Oppenheim J. J. Human lymphoproliferative reaction to saliva and dental plaque-deposits: an in vitro correlation with periodontal disease. J Periodontol. 1972 Sep;43(9):522–527. doi: 10.1902/jop.1972.43.9.522. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Mergenhagen S. E. A role for cell-mediated immunity in the pathogenesis of periodontal disease. J Periodontol. 1974 May;45(5):351–360. doi: 10.1902/jop.1974.45.5.351. [DOI] [PubMed] [Google Scholar]
- Hunt S. V., Williams A. F. The origin of cell surface immunoglobulin of marrow-derived and thymus-derived lymphocytes of the rat. J Exp Med. 1974 Mar 1;139(3):479–496. doi: 10.1084/jem.139.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970 Nov;15(11):1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Abo T., Sekizawa T., Sasaki M. Studies of surface immunoglobulins on human B lymphocytes. I. Dissociation of cell-bound immunoglobulins with acid pH or at 37 degrees C. J Immunol. 1975 Oct;115(4):982–987. [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Mackler B. F., Frostad K. B., Robertson P. B., Levy B. M. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodontal Res. 1977 Jan;12(1):37–45. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Mackler B. F., Waldrop T. C., Schur P., Robertson P. B., Levy B. M. IgG subclasses in human periodontal disease. I. Distribution and incidence of IgG subclass bearing lymphocytes and plasma cells. J Periodontal Res. 1978 Mar;13(2):109–119. doi: 10.1111/j.1600-0765.1978.tb00159.x. [DOI] [PubMed] [Google Scholar]
- McDougall W. A. Penetration pathways of a topically applied foreign protein into rat gingiva. J Periodontal Res. 1971;6(2):89–99. doi: 10.1111/j.1600-0765.1971.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Patters M. R., Genco R. J., Reed M. J., Mashimo P. A. Blastogenic response of human lymphocytes to oral bacterial antigens: comparison of individuals with periodontal disease to normal and edentulous subjects. Infect Immun. 1976 Nov;14(5):1213–1220. doi: 10.1128/iai.14.5.1213-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. A., Page R. C., Ogilvie A. L., Hall W. B. Histopathologic features of the initial and early stages of experimental gingivitis in man. J Periodontal Res. 1975 May;10(2):51–64. doi: 10.1111/j.1600-0765.1975.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Pleasants J. R., Reddy B. S., Wostmann B. S. Qualitative adequacy of a chemically defined liquid diet for reproducing germfree mice. J Nutr. 1970 May;100(5):498–508. doi: 10.1093/jn/100.5.498. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. II. Correlation of the in vitro response with skin reactivity. J Exp Med. 1968 Dec 1;128(6):1255–1265. doi: 10.1084/jem.128.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossberg A., Ferrigno P. D. A histological study of the incidence of plasma cells and lymphocytes in human gingival tissues. J Oral Med. 1971 Dec;26(3):99–105. [PubMed] [Google Scholar]
- Schroeder H. E. Quantitative parameters of early human gingival inflammation. Arch Oral Biol. 1970 May;15(5):383–400. doi: 10.1016/0003-9969(70)90066-x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Greenspan J. S. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodontal Res. 1979 Jan;14(1):39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Powell R. N., Davies W. I. Conversion of a stable T-cell lesion to a progressive B-cell lesion in the pathogenesis of chronic inflammatory periodontal disease: an hypothesis. J Clin Periodontol. 1979 Oct;6(5):267–277. doi: 10.1111/j.1600-051x.1979.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Sharawy A. M., Socransky S. S. Effect of human Streptococcus strain GS-5 on caries and alveolar bone loss in conventional mice and rats. J Dent Res. 1967 Nov-Dec;46(6):1385–1391. doi: 10.1177/00220345670460064101. [DOI] [PubMed] [Google Scholar]
- Sinden P. R., Walker D. M. Inflammatory cells extracted from chronically inflamed gingiva. J Periodontal Res. 1979 Nov;14(6):467–474. doi: 10.1111/j.1600-0765.1979.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Talbman M. A., Smith D. J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974 Jun;9(6):1079–1091. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Williams A. F., Gowans J. L. The presence of IgA on the surface of rat thoractic duct lymphocytes which contain internal IgA. J Exp Med. 1975 Feb 1;141(2):335–345. doi: 10.1084/jem.141.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]
- Wostmann B. S., Pleasants J. R., Bealmear P. Dietary stimulation of immune mechanisms. Fed Proc. 1971 Nov-Dec;30(6):1779–1784. [PubMed] [Google Scholar]


