Abstract
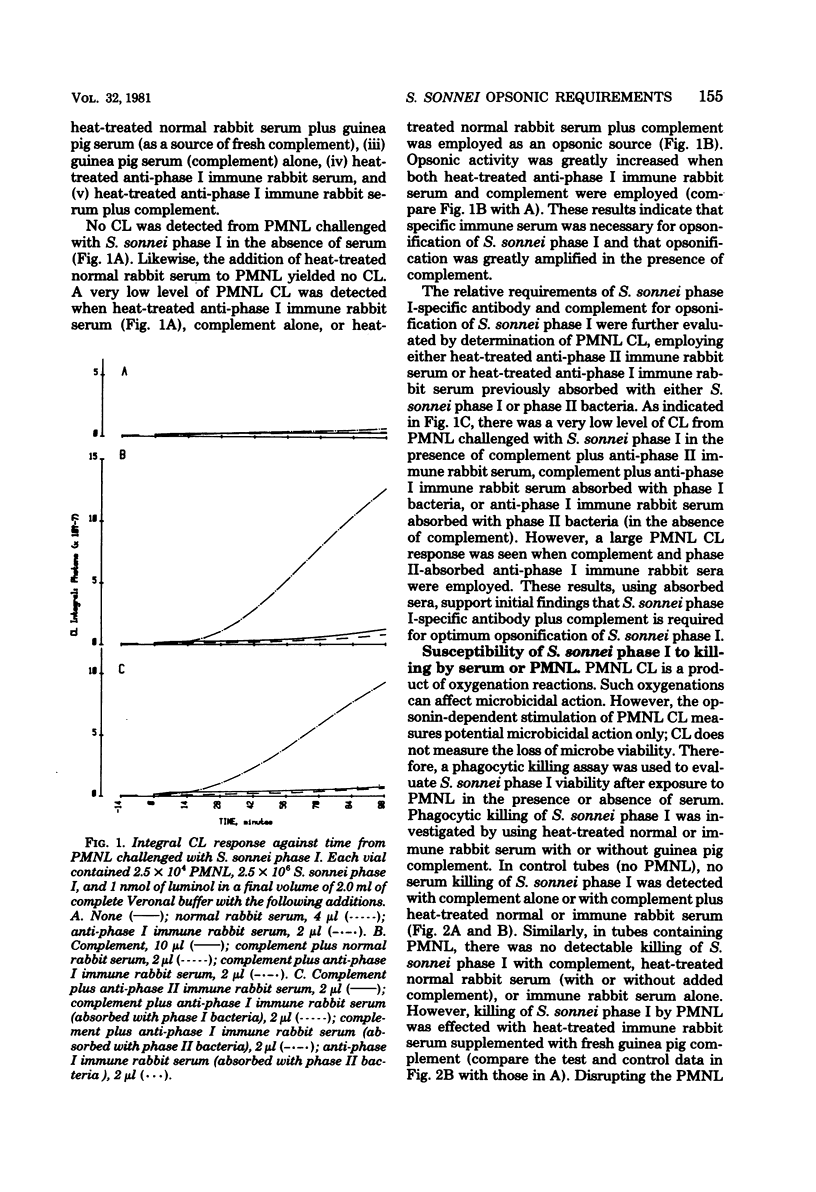
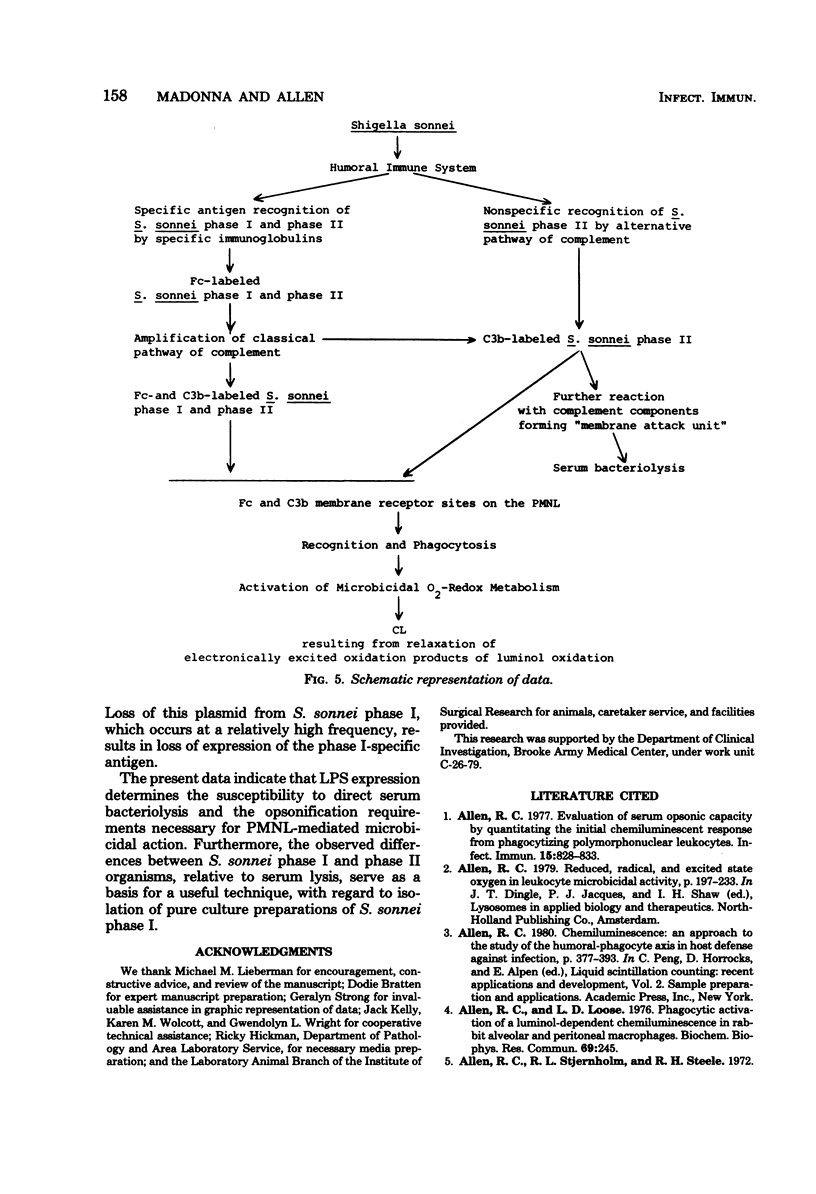
The synthesis of the lipopolysaccharide O-specific repeat polymer by Shigella sonnei phase I is a clearly defined bacterial virulence factor necessary for penetrating epithelial cells; S. sonnei phase II does not synthesize this antigen and is uniformly avirulent. The serum opsonic requirements, relative to differences in gross lipopolysaccharide structure, were investigated by quantification and comparison of polymorphonuclear leukocyte (PMNL) metabolism and PMNL-mediated microbicidal action to phase I and phase II organisms, using normal and immune serum. The stimulation of PMNL O2-redox metabolism, as required for oxidative killing, was quantified by a chemiluminescent technique, using luminol as a chemilumigenic substrate. Susceptibility to direct serum or serum PMNL-mediated killing was evaluated by serum and serum-phagocytic killing assays. Stimulation of PMNL metabolism and phagocytic killing of S. sonnei phase I required opsonification by specific phase I antibody plus the classical pathway of complement. S. sonnei phase II was susceptible to direct complement-mediated serum killing. Likewise, opsonification of the phase II microbe, as measured by PMNL-associated chemiluminescence, was effected by complement in the absence of immune antibody. These data demonstrate the importance of the O-specific repeat polymer in protecting the microbe from the microbicidal action of PMNL and the bacteriolytic action of serum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C. Reduced, radical, and excited state oxygen in leukocyte microbicidal activity. Front Biol. 1979;48:197–233. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Sheahan D. G., Washington O., Formal S. B. Virulence of Shigella flexneri hybrids expressing Escherichia coli somatic antigens. Infect Immun. 1972 Aug;6(2):104–111. doi: 10.1128/iai.6.2.104-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Kontrohr T. The identification of 2-amino-2-deoxy-L-altruronic acid as a constituent of Shigella sonnei phase I lipopolysaccharide. Carbohydr Res. 1977 Oct;58(2):498–500. doi: 10.1016/s0008-6215(00)84379-7. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Seliger H. H. Absolute spectral sensitivity of phototubes and the application to the measurement of the absolute quantum yields of chemiluminescence and bioluminescence. Photochem Photobiol. 1965 Dec;4(6):1015–1048. doi: 10.1111/j.1751-1097.1965.tb09293.x. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Marucci A. A., Fuller T. C. Quantitative micro-complement fixation test. Appl Microbiol. 1971 Feb;21(2):260–264. doi: 10.1128/am.21.2.260-264.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P. Serum factors capable of opsonizing Shigella for phagocytosis by polymorphonuclear neutrophils. Immunology. 1975 Jun;28(6):1051–1059. [PMC free article] [PubMed] [Google Scholar]
- SERENY B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung. 1955;2(3):293–296. [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


