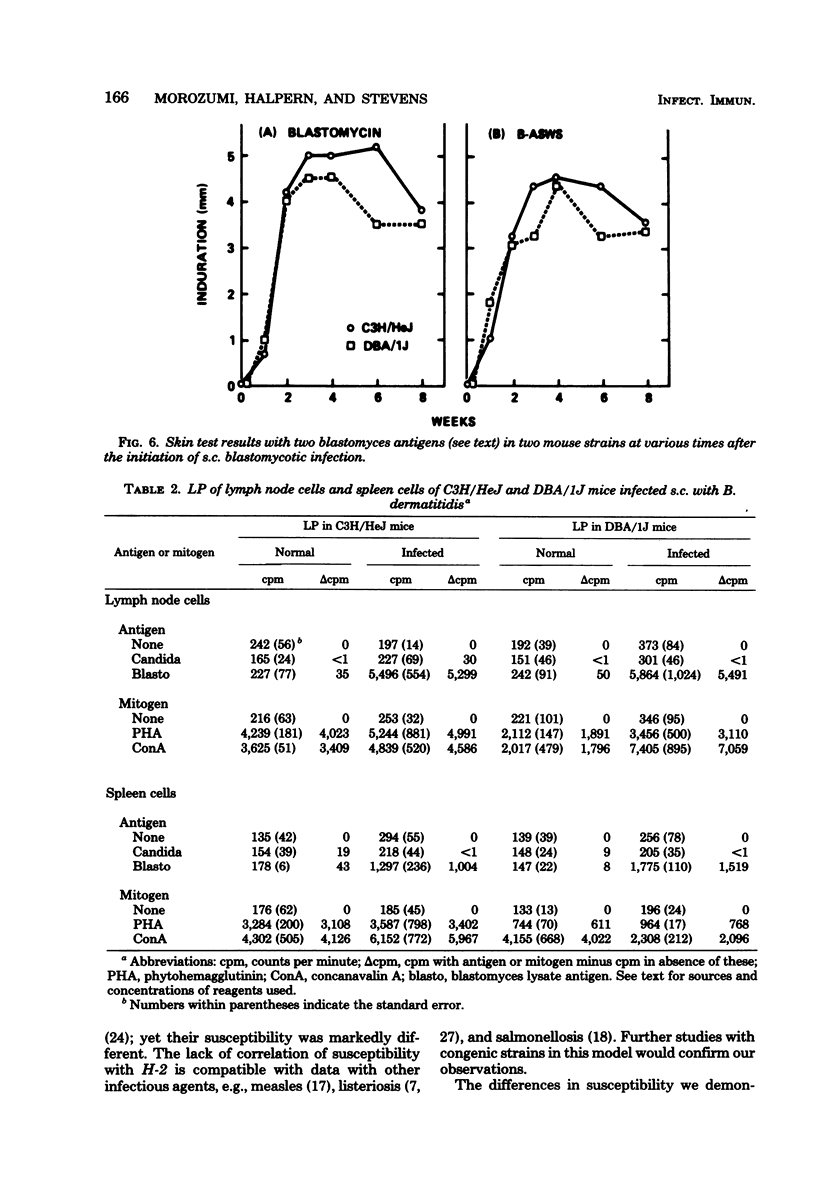
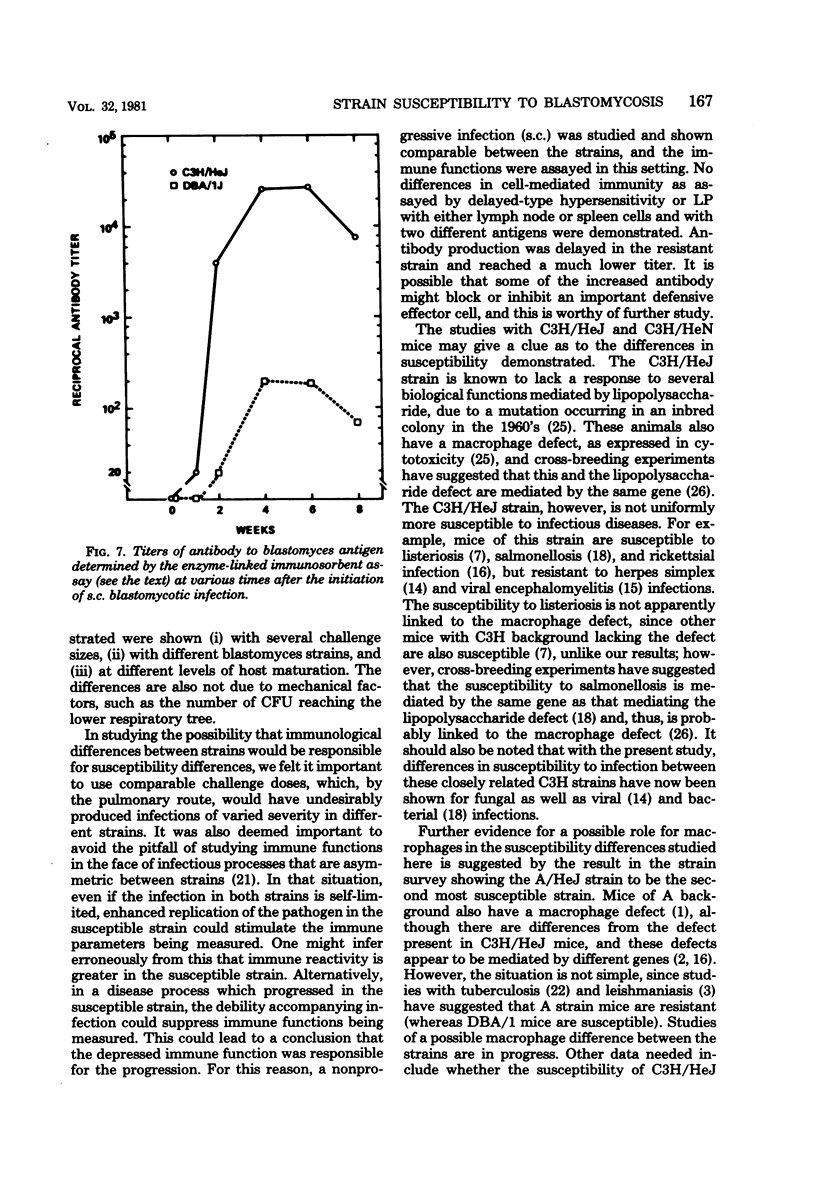
Abstract
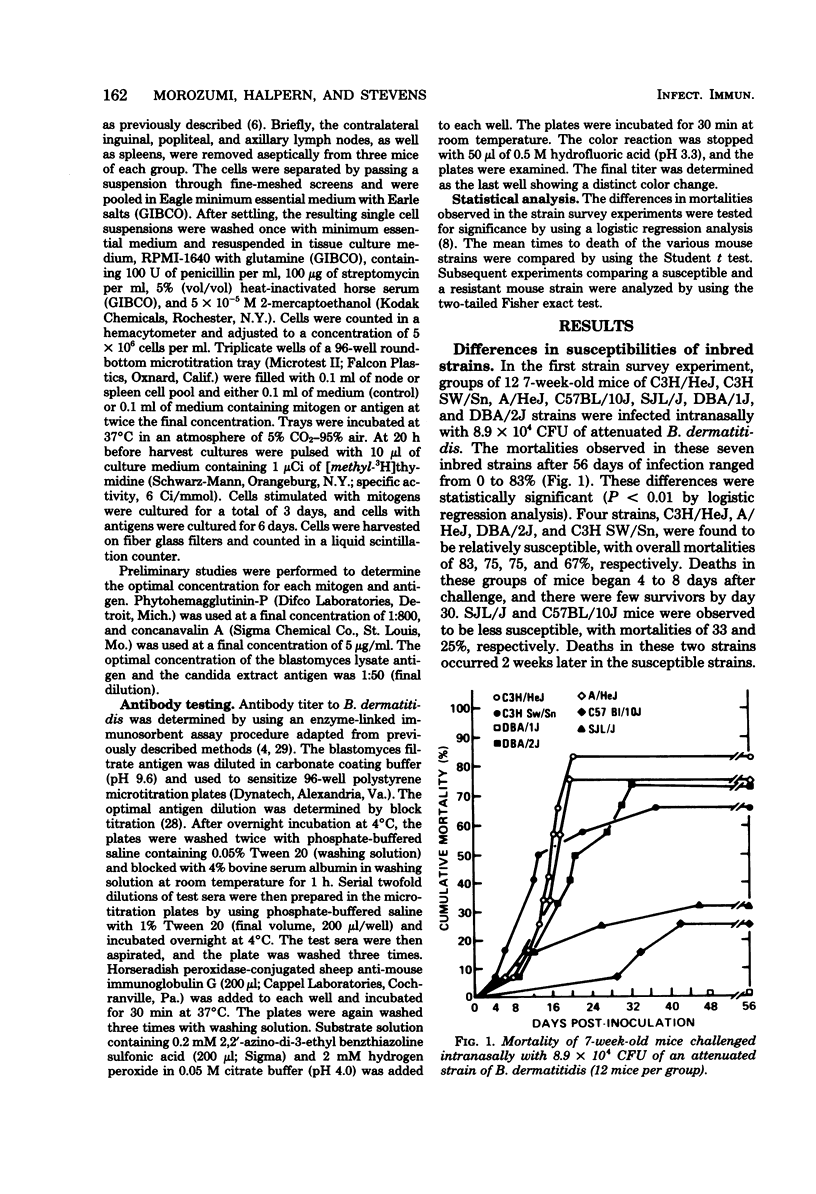
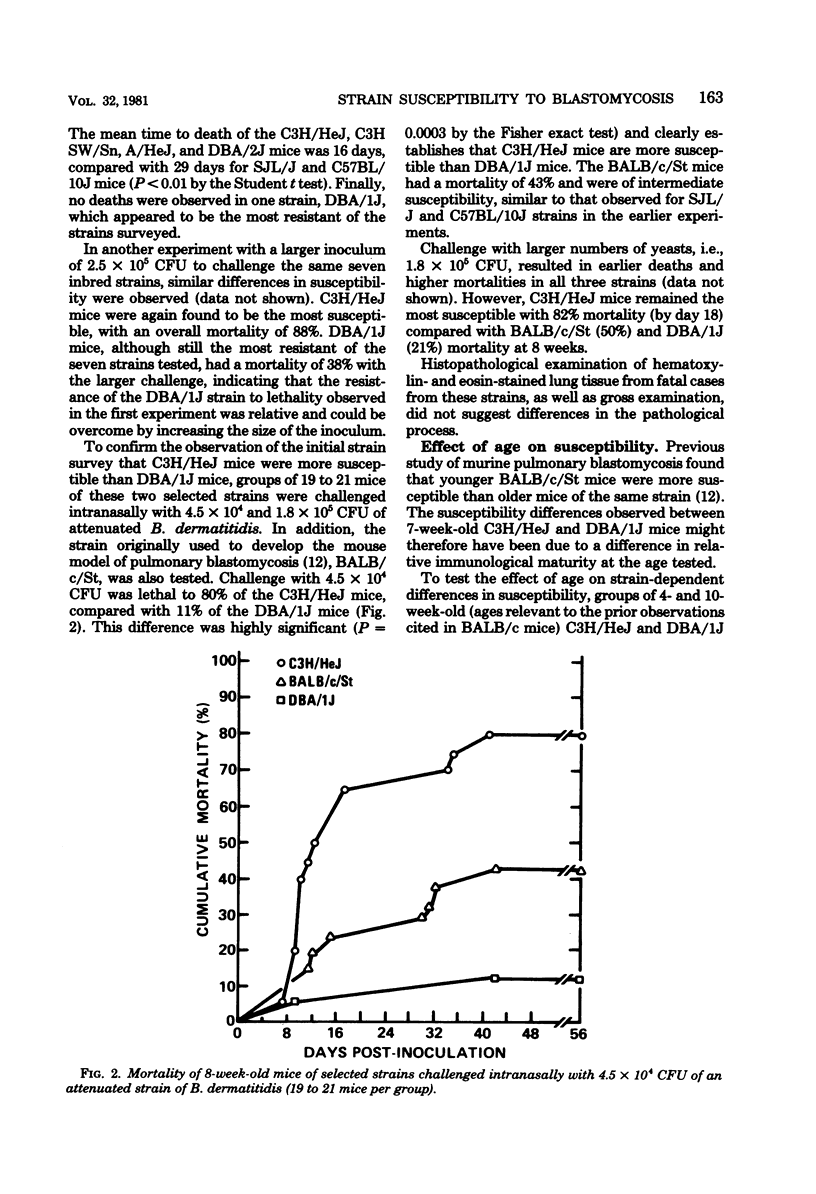
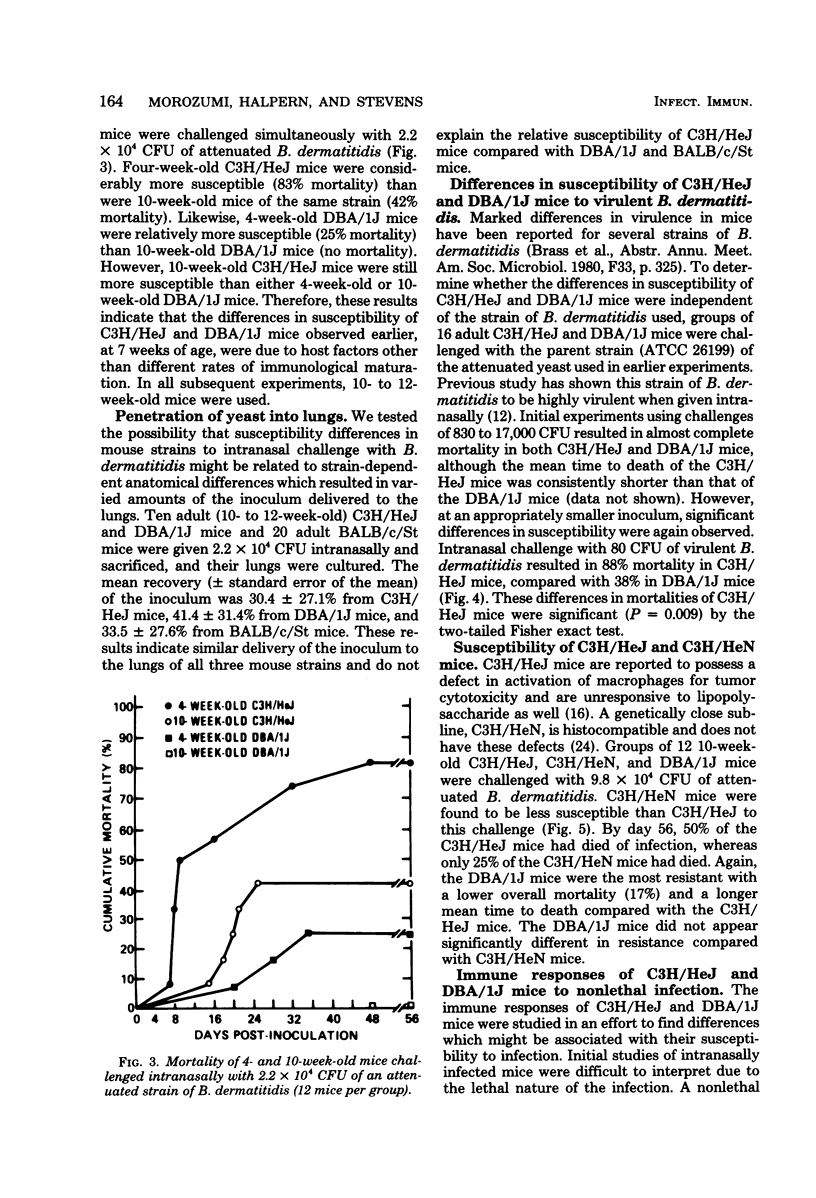
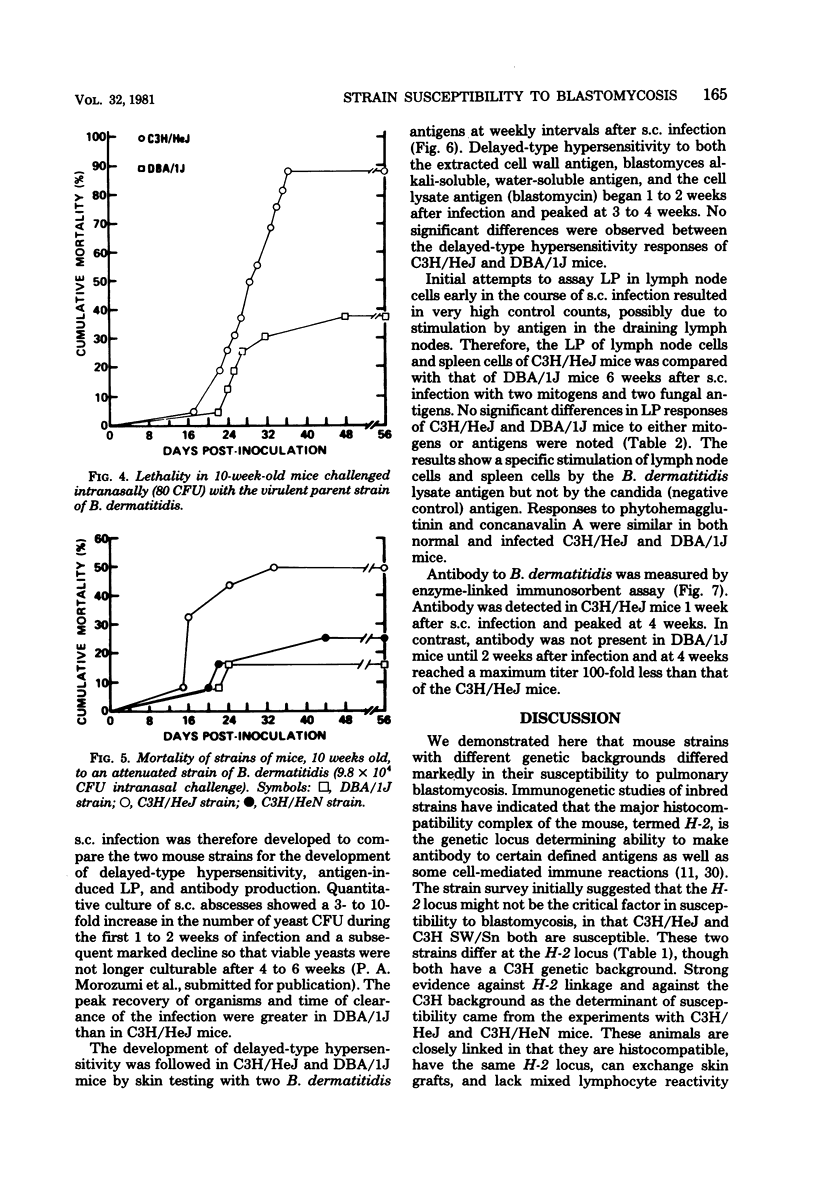
The susceptibility of inbred strains of mice to pulmonary blastomycosis was studied to derive information relevant to host resistance and genetic background. Initial studies with eight strains with various H-2 backgrounds revealed the C3H/HeJ strain to be highly susceptible and DBA/1J mice to be resistant. These observations were confirmed with various challenge inocula. These differences were not dependent on the size of the challenge, the strain of Blastomyces dermatitidis, host age, or ability of the challenge to penetrate to the lower airways. Differences between the susceptible and resistant strains in lymphocyte proliferation in vitro and delayed-type hypersensitivity in vivo after nonlethal subcutaneous infection were not demonstrated; the susceptible strain made a significantly greater antibody response to blastomyces antigens as determined by an enzyme-linked immunosorbent assay. The resistance of the C3H/HeN strain of mice, which differs from the C3H/HeJ in sensitivity to lipopolysaccharide and lacks the macrophage cytotoxicity defect of the latter, suggests that the susceptibility of C3H/HeJ mice is not related to their C3H background or the H-2 locus. As the A/HeJ strain, which also has a macrophage cytotoxicity defect, was found in this study to be the second most susceptible strain, this also suggests macrophages as the subject for further study with respect to the mechanism of genetic resistance to this infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from A/J mice. II. Comparison of the macrophage cytotoxic defect of A/J mice with that of lipid A-unresponsive C3H/HeJ mice. J Immunol. 1979 Apr;122(4):1592–1597. [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Macrophage activation for tumor cytotoxicity: genetic variation in macrophage tumoricidal capacity among mouse strains. Cell Immunol. 1979 Jun;45(1):188–194. doi: 10.1016/0008-8749(79)90375-7. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Regulation of Leishmania populations within the host. II. genetic control of acute susceptibility of mice to Leishmania donovani infection. Clin Exp Immunol. 1977 Oct;30(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Larsh H. W. Isolation of skin test-active preparations from yeast-phase cells of Blastomyces dermatitidis. Infect Immun. 1974 Jul;10(1):42–47. doi: 10.1128/iai.10.1.42-47.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet F. C. Genetic control of the immune response. Am J Clin Pathol. 1975 May;63(5):646–655. doi: 10.1093/ajcp/63.5.646. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Schmid E. S., Carrington C. C., Stevens D. A. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am Rev Respir Dis. 1978 Apr;117(4):695–703. doi: 10.1164/arrd.1978.117.4.695. [DOI] [PubMed] [Google Scholar]
- Hoeprich P. D., Finn P. D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972 Oct;126(4):353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Hirt H. M., Rosenstreich D. L., Mergenhagen S. E. Resistance of C3H/HeJ mice to lethal challenge with herpes simplex virus. Proc Soc Exp Biol Med. 1978 Jan;157(1):29–32. doi: 10.3181/00379727-157-39983. [DOI] [PubMed] [Google Scholar]
- Martinez D. Histocompatibility-linked genetic control of susceptibility to age-dependent polioencephalomyelitis in mice. Infect Immun. 1979 Jan;23(1):45–48. doi: 10.1128/iai.23.1.45-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Ruco L. P., Boraschi D., Nacy C. A. Macrophage activation for tumor cytotoxicity: analysis of intermediary reactions. J Reticuloendothel Soc. 1979 Oct;26(4):403–415. [PubMed] [Google Scholar]
- Neighbour P. A., Rager-Zisman B., Bloom B. R. Susceptibility of mice to acute and persistent measles infection. Infect Immun. 1978 Sep;21(3):764–770. doi: 10.1128/iai.21.3.764-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- Pappagianis D. Epidemiological aspects of respiratory mycotic infections. Bacteriol Rev. 1967 Mar;31(1):25–34. doi: 10.1128/br.31.1.25-34.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Arredondo B., González M. Comparative study of American cutaneous leishmaniasis and diffuse cutaneous leishmaniasis in two strains of inbred mice. Infect Immun. 1978 Nov;22(2):301–307. doi: 10.1128/iai.22.2.301-307.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Wicker L. S., Urba W. J. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980 Aug;29(2):494–499. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Glode L. M. Difference in B cell mitogen responsiveness between closely related strains of mice. J Immunol. 1975 Sep;115(3):777–780. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S., Rosenstreich D. L. Macrophage activation for tumor cytotoxicity: control of macrophage tumoricidal capacity by the LPS gene. J Immunol. 1978 Aug;121(2):543–548. [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Role of the H-2 gene complex in cell-mediated immunity to infectious disease. Transplant Proc. 1977 Dec;9(4):1835–1838. [PubMed] [Google Scholar]


