Abstract
Regular moderate exercise has been proposed to enhance immune function, but its effects on immunity and their consequences have not been well studied. Mice without (AL) or with access (AL+EX) to voluntary running wheels were vaccinated with a model antigen (ovalbumin (OVA)) via intranasal or subcutaneous routes to target the mucosal and systemic immune compartments, respectively. EX enhanced OVA-specific CD4+ T cell cytokine production and proliferation in all lymphoid organs examined without changes in cell distribution in any organ. These results suggest that coupling moderate exercise with vaccination may enhance vaccine efficacy for the prevention and/or therapy of numerous diseases.
Keywords: adaptive immunity, cytokines, cell proliferation
1. Introduction
The favorable effects of a physically active lifestyle on a number of physiological processes, including cardiovascular function and insulin sensitivity, and the concomitant reduction in disease outcomes such as coronary heart disease, hypertension and diabetes are well documented [1, 2]. However, the effects of physical activity on immune function and the downstream consequences on disease risk have been studied to a lesser extent. The current hypothesis to explain the relationship between exercise and immune function is the Inverted J Hypothesis [3], which proposes that regular, moderate exercise enhances immune function, and in turn, may reduce the incidence of infectious disease and cancer. In contrast, physical inactivity and intense, exhaustive exercise at opposite ends of the curve both suppress immune function and may increase disease risk.
Although it has been proposed that moderate physical activity may provide protection from the incidence of infectious disease via an enhancement of immunity, few studies have addressed this question. Clinical and epidemiological studies demonstrate that the incidence of upper respiratory tract infections (URTI)7 [4–7] and the severity of symptoms [8–12] are significantly lower in moderately active individuals as compared to their sedentary counterparts. In animal studies, the survival rates following infection with Salmonella typhimurium [13] or influenza virus [14] are higher in active mice as compared to sedentary controls. However, immune function was measured in only two of the studies in which the incidence and severity of symptoms of URTI were reduced with moderate activity [6, 12]. Both studies demonstrate an increase in the mucosal antibody response (i.e. salivary IgA concentration) in moderately active individuals [6, 12]. Several other studies report an elevation in mucosal IgA in moderately active young [15, 16] and older adults [17] but did not measure URTI or other clinical endpoints. Finally, we have demonstrated that moderate exercise enhances mucosal T cell proliferation and cytokine production in response to concanavalin A (Con A) stimulation in mice [18]. The limited work in this area suggests that moderate exercise enhances antigen independent measures of immune function, e.g. total IgA and mitogen-induced T cell responses. However, no studies have examined the effect of moderate exercise on antigen-specific mucosal immunity in response to vaccination.
In addition to examining broad-based, mucosal immune endpoints, numerous studies have demonstrated a beneficial effect of moderate exercise on systemic innate immunity, in particular the phagocytic and tumoricidal activities of macrophages and the cytotoxicity of NK cells (reviewed in [19, 20]). A few studies have examined the effect of regular moderate exercise on systemic adaptive immune responses, but in most cases, in the context of an aging model. Several cross-sectional studies demonstrate that active older adults have higher antigen-specific antibody titers [21–23], higher influenza-specific in vitro peripheral blood mononuclear cell proliferation [22] and greater in vivo delayed type hypersensitivity (DTH) responses [23] as compared to sedentary individuals. Furthermore, two prospective studies in older adults reported that a 10-month exercise intervention increased influenza-and KLH-specific antibody titers [24, 25] and granzyme B activity [25]. In contrast to the exercise-induced enhancement of antigen-specific antibody titers in older adults, moderate exercise does not enhance antibody responses in young adults [23, 26, 27] or in rodent models utilizing young animals [23, 28–31]. However, one report demonstrates that DTH responses to KLH are higher in active versus sedentary young adults [23]. The studies that have been done in young animals suggest that moderate exercise may enhance cell-mediated but not humoral responses; however, additional well-designed mechanistic studies are needed to further characterize the effects of moderate activity on adaptive immune responses.
Therefore, the goals of the present study were 1) to establish a reliable model to systematically evaluate the effects of moderate physical activity on adaptive immune responses to vaccination, 2) to characterize the effect of moderate exercise on humoral and cell-mediated immune responses in the mucosal compartment using a vaccine platform that is well-documented to stimulate mucosal immunity [32] and 3) to explore the effect of the same vaccine platform on systemic immunity in an effort to compare and contrast the effect of exercise on adaptive immunity in both compartments.
2. Materials and Methods
2.1. Animals and treatment regimens
Female 6-week-old C57BL/6 mice were obtained from Charles River Breeding Laboratory (Frederick, MD). Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals.” Upon receipt, mice were screened for voluntary running behavior by being placed into individual cages fitted with a mouse running wheel apparatus (MiniMitter Co.; Bend, OR) for 4 days to determine the average level of running activity per mouse. Wheel revolutions of individual mice were recorded and analyzed using the VitalView software (MiniMitter Co.; Bend, OR). Mice with running activity at or above the 50th percentile (approximately 4.0 km/day) were selected for this study and randomized to either the ad libitum food consumption (AL) or AL plus access to voluntary running wheels (AL+EX) treatment groups. Thus, mice in both the AL and AL+EX treatment groups exhibited high running behavior. All mice were housed individually for the duration of the study. Mice were vaccinated via two different routes, intranasal (i.n.) and subcutaneous (s.c.) to target the mucosal and systemic immune compartments, respectively. Mice were assigned to one of the following treatment groups 1) AL plus mucosal vaccination (n=20); 2) AL+EX plus mucosal vaccination (n=20); 3) AL plus s.c. vaccination (n=10); and 4) AL+EX plus s.c. vaccination (n=10). All mice were fed AIN-76A diet (Research Diets, Inc.; New Brunswick, NJ). Mice were maintained on AL or AL+EX regimens for 8 weeks prior to the primary vaccination and were continued on these treatments through 3 successive weeks (weeks 9, 10, and 11 of the study). Mice were sacrificed 1 week following the last vaccination at week 12 for collection of lymphoid organs. Mice were removed from the running wheel cages and placed in standard mouse cages 24 hours prior to sacrifice to standardize the timing of lymphocyte collection with respect to the last exercise bout. Food intake, body weights and distance run were monitored weekly, and mice were observed daily for signs of ill health.
2.2. Vaccinations
Vaccinations consisted of 1 primary and 2 booster vaccinations, each separated by 1 week, with 75 μg ovalbumin, grade VI (OVA) (Sigma-Aldrich; St. Louis, MO) plus 1 μg lymphotactin (LT) (R&D Systems, Inc.; Minneapolis, MN) in PBS. Mucosal vaccinations were given intranasally (i.n.) in 10 μl and systemic vaccinations were given subcutaneously (s.c.) in 100 μl in the lumbar region of the animal.
2.3. Mixed lymphocyte response (MLR)
Single cell suspensions of splenocytes were prepared from individual C57BL/6 mice and BALB/c mice as previously described [18]. Splenocytes from experimental C57BL/6 mice and naive BALB/c mice were irradiated (20 Gy), counted, and serially diluted in triplicate in a 96-well plate and used as antigen presenting cells (APCs). CD4+ T lymphocytes from experimental C57BL/6 mice and naïve BALB/c mice were isolated via Dynal® CD4 negative isolation kits (Invitrogen; Carlsbad, CA) according to the manufacturer’s instructions. APCs or CD4+ T cells from experimental mice were incubated with BALB/c T cells or APCs, respectively, to evaluate allogeneic proliferative responses as previously described [33].
2.4. CD4+ T cell proliferation assay
Single cell suspensions of splenocytes and lymphoid cells from Peyer’s patches and mesenteric and inguinal lymph nodes were prepared from individual mice as previously described [18]. Cells from the Peyer’s patches and inguinal and mesenteric lymph nodes were pooled from 2 animals within a treatment group to generate adequate cell numbers for functional assays. CD4+ T cell lymphoproliferation was assessed as previously described [34].
2.5. Cytokine production assays
CD4+ T cells (1 × 105) from experimental animals were incubated with 5 × 105 irradiated APCs from naïve syngeneic mice in triplicate wells of a 96-well plate. Cells were stimulated with 100 μg/ml of OVA, 2.0 μg/ml of Con A, or media alone. Supernatants were harvested after 48 h of incubation with Con A and 72 h of incubation with OVA. TNF-α, IFN-γ, interleukin-2 (IL-2), IL-4, and IL-5 were measured using the Th1/Th2 Cytokine Cytometric Bead Array kit (BD Biosciences; San Jose, CA) according to the manufacturer’s instructions.
2.6. Serum antibody responses
Antigen-specific serum antibody responses were measured 7 days following the second s.c. or i.n. booster vaccination via ELISA as previously described [33].
2.7. Flow cytometric analyses
Single cell suspensions of cells from the spleen, Peyer’s patches, inguinal and mesenteric lymph nodes were prepared for flow cytometric analyses and analyzed on a Becton Dickinson FACScan flow cytometer (BD Biosciences; San Jose, CA) as previously described [18].
2.8. Serum cytokine measures
Serum was collected at the time of sacrifice, frozen and stored at −80°C until analyzed. LINCOplex mouse cytokine kits (Millipore; St. Charles, MO) were used for the quantification of serum leptin and IL-6. Samples were run in duplicate. The intra-assay coefficients of variation for leptin and IL-6 were <10%.
2.9. Body composition analysis
Mouse carcasses were scanned using a GE Lunar PIXImus Dual-Energy X-ray Absorptiometer (DEXA) to assess lean mass, fat mass, and percent body fat, as previously described [18].
2.10. Statistical analyses
All data are presented as the mean plus or minus the standard error of the mean. Differences in average kilometers run per day, body weight, lean mass, fat mass, percent body fat, leptin, IL-6, cytokine production and flow cytometric analyses were determined using two-tailed, paired t-tests assuming unequal variances. Differences in lymphocyte proliferation were examined using two-way analysis of variance (ANOVA) (treatment X concentration of APC or OVA), followed by Fisher Least Significant Difference post-hoc tests and a Bonferroni correction for multiple comparisons where appropriate. All analyses were conducted using STATA software (version 7.0 Stata Corp; College Station, TX) and statistical significance was accepted at the P ≤ 0.05 level.
3. Results
3.1. Establishment of a reliable model of moderate voluntary running in mice
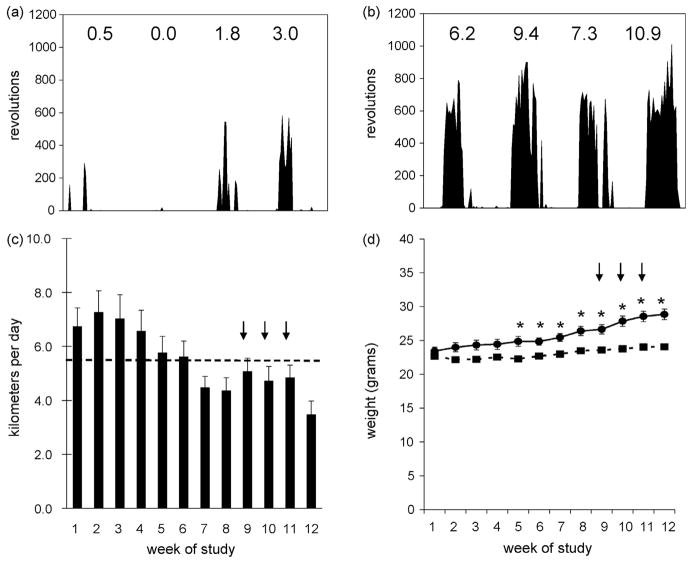
Previous studies in our laboratory demonstrated that running behavior is heterogeneous in C57BL/6 mice given access to voluntary running wheels [18]. Thus, to study the effects of moderate voluntary exercise on immune function we selected animals that would choose to run over the course of the 12-week experiment. In a pilot study, we compared the average running activity of mice (km/day) after 1, 4, 10, and 14 days of access to running wheels to the average distance/day run over 6 weeks. We found a significant correlation between the average km run per day over 4, 10, and 14 days and the average km run per day over 6 weeks (ρ=0.606, ρ=0.696, ρ=0.772, respectively; P<0.05). Thus, we measured running activity in all mice for 4 days prior to the onset of the study to select mice with high running activity for enrollment in the current study. Individual histograms of running activity for animals that exhibited low and high running behavior over a 4-day test period are shown in Fig. 1a and Fig. 1b, respectively. The average distance run per day among mice selected for the study ranged from 3.5–7.3 km/day over the course of the 12-week study with an overall average of 5.5 km/day (Fig. 1c). Using speed to estimate the percent of maximal oxygen consumption (%VO2max) or relative intensity of exercise [35], we determined that mice were exercising at 60–70% of VO2max, which is indicative of moderate exercise training. The average distance run was not altered following the administration of the primary and booster vaccinations (designated by arrows) given in weeks 9, 10, and 11 of the study (Fig. 1c). Access to running wheels in the AL+EX group prevented weight gain over the course of the 12-wk study (Fig. 1d). After 12 weeks of training, AL+EX mice had significantly lower total body weight, fat mass, and percent body fat (Table 1; P<0.05), but had similar lean mass as compared to AL animals. Additionally, AL+EX mice had lower serum leptin and higher serum IL-6 levels than the AL controls (Table 1; P<0.05). The average distance run and the exercise-induced changes in body weight and body composition did not differ between mice that were vaccinated i.n. or s.c.; therefore, data from all vaccinated animals (n=26) are shown in Fig. 1 and Table 1.
Fig. 1.
Individual histograms from 2 representative female C57BL/6 mice demonstrate the heterogeneity in running activity. (a) Running activity in 1 mouse that exhibited (a) low running activity and (b) high running activity over the 4-day test period. The average km/day run by each mouse in (a & b) are shown above the histogram plots. (c) Among mice selected for the study, the average km/day run was 5.5 km/day (dashed line) during the 12-week study (n=26). (d) Average weekly body weights of AL (●) or AL+EX (■) mice (n=26/group). Arrows indicate the administration of primary and booster vaccinations. Asterisks indicate significantly different body weights between AL and AL+EX groups (P<0.05).
Table 1.
Body composition measures and serum cytokine levels (mean ± SEM) of mice on either AL or AL+EX treatments for 12 weeks
| Treatment Group | Body Weighta (grams) | Fat Massa (grams) | Lean Massa (grams) | Percent Body Fata | Leptinb (ng/ml) | IL-6b (pg/ml) |
|---|---|---|---|---|---|---|
| AL | 29.1 ± 0.8 | 9.3 ± 0.7 | 16.6 ± 0.3 | 34.0 ± 1.9 | 2.3 ± 0.4 | 8.9 ± 2.9 |
| AL+EX | 24.8 ± 0.3* | 5.4 ± 0.3* | 16.2 ± 0.3 | 24.7 ± 1.1* | 1.6 ± 0.2* | 30.6 ± 7.9* |
n=26/group (both i.n. and s.c. vaccinated mice)
Serum leptin and IL-6 were measured in a subset of mice that did not receive any vaccinations (n=10/group).
Significantly different from AL group (P<0.05).
3.2. Exercise enhanced CD4+ T cell proliferation in response to allogeneic stimulation
The proliferation of T cells from BALB/c mice following stimulation with irradiated splenocytes from C57BL/6 mice was used as a measure of antigen presenting capability of AL and AL+EX mice. Exercise did not increase the antigen-presenting capabilities of splenocytes (Fig. 2a; F2, 70 = 1.89, P = 0.191). The proliferation of CD4+ T lymphocytes from C57BL/6 mice following stimulation with irradiated splenocytes from allogeneic BALB/c mice was used as a measure of CD4+ T cell proliferation in AL and AL+EX mice. Exercise significantly enhanced CD4+ T cell proliferation in response to stimulation with allogeneic APCs (Fig. 2b; F1,84 = 8.14, P = 0.006). Significant differences between AL and AL+EX groups at each concentration of APCs were determined using Bonferroni’s correction for multiple comparisons.
Fig. 2.
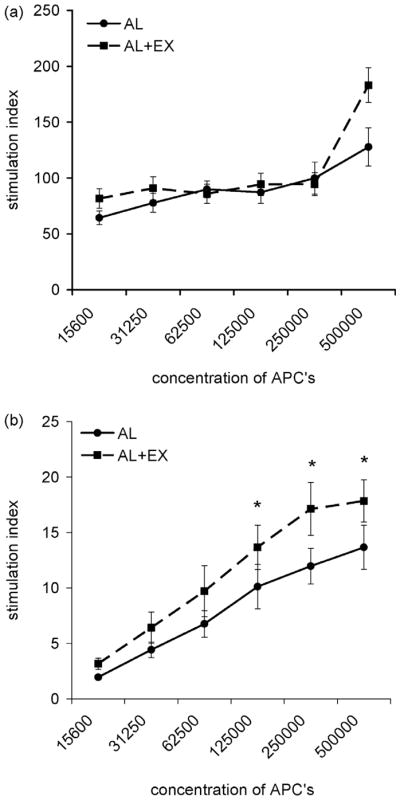
Exercise had no effect on APC function but enhanced CD4+ T cell proliferation assessed via mixed lymphocyte response. (a) BALB/c CD4+ T cell proliferation induced by increasing concentrations of allogeneic APC from AL (●) or AL+EX (■) C57BL/6 mice. Data shown are mean ± SEM (n=8/group). Two-way ANOVA revealed no significant effect of exercise on the antigen presenting capability of splenocytes (F2, 70 = 1.89, P = 0.191) (b) C57BL/6 CD4+ T cell proliferation from AL (●) or AL+EX (■) induced by increasing concentrations of allogeneic BALB/c APCs. Data shown are mean ± SEM (n=8/group). Two-way ANOVA revealed a significant effect of exercise on CD4+ T cell proliferation in response to simulation with allogeneic APC (F 1,84 = 8.14, P = 0.006). *Post-hoc analyses using Bonferroni’s test for multiple comparisons found significant differences between AL and AL+EX groups at the designated concentration of APCs.
3.3. Exercise had no effect on the distribution of cells in any lymphoid compartment
Exercise reduced the total number of cells in the spleen in mice receiving either i.n. or s.c. vaccinations, although this did not reach statistical significance (Table 2; P = 0.08 and P=0.07, respectively). In contrast, the total numbers of cells in the Peyer’s patches, mesenteric and inguinal lymph nodes were not different between AL+EX and AL mice. Additionally, we examined the number of CD3+, CD3+CD4+, CD3+CD8+, CD19+ or B220+, NK1.1+, CD11b+I-Ab+, and CD11c+I-Ab+ cells in all lymphoid organs of mice in both treatment groups in an effort to determine if exercise altered the distribution of cells post-vaccination (Table 2). Exercise did not alter the number of any cell type in the spleen, Peyer’s patches, or mesenteric or inguinal lymph nodes.
Table 2.
Number of cells (mean ± SEM) in each tissue compartment 7 days after the last booster vaccination among AL and AL+EX mice
| Tissue | Treatment group | Total cell number | CD3+ | CD3+CD4+ | CD3+CD8+ | CD19+ or B220+a | NK1.1+ | CD11b+I-Ab+ | CD11c+I-Ab+ |
|---|---|---|---|---|---|---|---|---|---|
| ———(X 106)——— | ———(X 105)——— | ||||||||
| i.n. vaccination | |||||||||
| Peyer’s patchesb | AL | 11.0 ± 0.3 | 2.6 ± 0.2 | 1.3 ± 0.1 | 0.8 ± 0.1 | 8.4 ± 0.2 | 0.3 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.1 |
| AL+EX | 12.0 ± 1.8 | 2.5 ± 0.5 | 1.3 ± 0.2 | 0.8 ± 0.1 | 9.1 ± 1.3 | 0.3 ± 0.2 | 2.1 ± 0.4 | 3.1 ± 0.6 | |
| mesenteric lymph nodesb | AL | 26.7 ± 2.2 | 13.1 ± 1.1 | 7.5 ± 0.7 | 5.9 ± 0.6 | 12.4 ± 1.4 | 11.1 ± 2.8 | 12.2 ± 1.1 | 9.4 ± 0.8 |
| AL+EX | 23.8 ± 6.6 | 15.0 ± 4.7 | 8.3 ± 2.8 | 7.0 ± 2.0 | 7.7 ± 1.8 | 12.7 ± 4.1 | 10.9 ± 2.7 | 7.8 ± 1.1 | |
| spleenc | AL | 106.8 ± 7.7 | 37.3 ± 3.1 | 22.9 ± 2.1 | 16.8 ± 1.5 | 52.8 ± 5.9 | 74.3 ± 6.9 | 32.7 ± 2.5 | 22.1 ± 2.6 |
| AL+EX | 86.3 ± 7.5 | 35.9 ± 3.8 | 20.7 ± 2.2 | 16.1 ± 1.8 | 49.1 ± 5.9 | 62.4 ± 7.6 | 21.3 ± 1.7 | 18.0 ± 1.2 | |
| s.c. vaccination | |||||||||
| inguinal lymph nodesd | AL | 23.0 ± 2.9 | 15.5 ± 1.8 | 8.6 ± 1.1 | 6.9 ± 0.7 | 5.7 ± 0.9 | 3.0 ± 0.3 | 3.6 ± 0.4 | 3.2 ± 0.4 |
| AL+EX | 20.3 ± 2.7 | 14.0 ± 1.9 | 7.6 ± 1.1 | 6.4 ± 0.8 | 4.6 ± 0.7 | 2.8 ± 0.6 | 3.3 ± 0.5 | 3.0 ± 0.5 | |
| spleene | AL | 131.5 ± 9.0 | 49.4 ± 5.2 | 29.1 ± 2.5 | 20.3 ± 2.9 | 64.7 ± 3.2 | 58.4 ± 2.3 | 30.8 ± 5.0 | 25.8 ± 5.0 |
| AL+EX | 100.8 ± 12.5 | 39.9 ± 4.0 | 22.9 ± 2.3 | 16.9 ± 1.7 | 53.4 ± 5.1 | 53.7 ± 4.1 | 41.8 ± 5.7 | 23.2 ± 3.9 | |
Anti-CD19 antibodies were used to identify B cells in the lymph nodes and spleen; anti-B220 antibodies were used to identify B cells in the Peyer’s patches.
n = 5 pooled groups of 2 mice per group.
n = 10 individual mice per group.
n = 3 pooled groups of 2 mice per group.
n = 6 individual mice per group.
3.4. Exercise had no effect on antigen-specific humoral responses
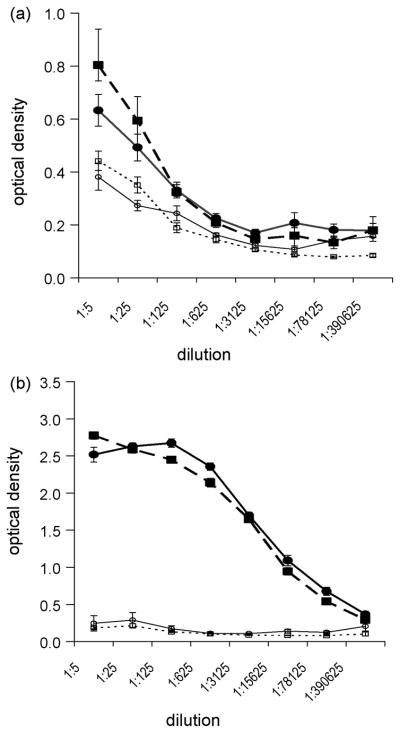
AL and AL+EX mice were vaccinated i.n. or s.c. with a primary and 2 booster vaccinations of OVA+LT. Serum β-galactosidase IgG titers (negative control antigen) in mice that were vaccinated i.n. (Fig. 3a) or s.c. (Fig. 3b) were similar in AL and AL+EX mice. There was over a 3-fold greater humoral response, i.e. OVA-specific total IgG response, to s.c. vaccination with OVA+LT than to i.n. vaccination with OVA+LT (Fig. 3, panel b vs. panel a). However, exercise had no effect on serum IgG titers to OVA in mice that were vaccinated i.n. (Fig. 3a) or s.c. (Fig. 3b).
Fig. 3.
Exercise had no effect on antigen-specific serum IgG levels. Antibody titers from mice vaccinated either (a) i.n. or (b) s.c. are shown. OVA-specific serum IgG titers from AL (●) and AL+EX (■) mice are similar. β-galactosidase (control antigen)-specific serum IgG titers from AL (○) and AL+EX (□) mice are also similar. Antibody titers were measured 7 days after the second booster vaccination via ELISA. Data shown are mean ± SEM. Data are representative of two independent experiments (n=6-10/group per experiment).
3.5. Exercise enhanced antigen-specific cytokine production
Splenic CD4+ T cells were stimulated in vitro with 100 μg/ml of OVA or 2 μg/ml of Con A (as a positive control) 7 days after the last booster vaccination. Exercise significantly enhanced OVA-specific TNF-α production in mice that received either i.n. or s.c. vaccinations and OVA-specific IL-5 production in mice that received s.c. vaccination (Table 3; P < 0.05). AL+EX mice receiving i.n. vaccination had higher OVA-induced IL-5 levels than AL mice, although this did not reach statistical significance. Similarly, IFN-γ and IL-2 levels produced by OVA-stimulated CD4+ T cells from AL+EX were higher than AL animals in response to both i.n. and s.c. vaccination, although these did not reach statistical significance. IL-4 levels were below the limit of detection of the assay in all OVA- and Con A- stimulated T cell cultures.
Table 3.
Cytokine responses (mean ± SEM) from splenic CD4+ T cells stimulated in vitro with OVA (100μg/ml)a 7 days after the administration of either a s.c. or i.n. booster vaccination with 75 μg OVA + 1 μg LT
| Treatment Group | TNF-α | IFN-γ | IL-2 | IL-5 |
|---|---|---|---|---|
| ———(pg/ml/5 × 105 cells)——— | ||||
| s.c. vaccination | ||||
| AL (n=6) | 248.4 ± 32.4 | 8.4 ± 2.1 | 96.8 ± 27.1 | 187.5 ± 72.6 |
| AL+EX (n=6) | 355.6 ± 32.3b | 13.4 ± 1.7 | 139.3 ± 20.6 | 377.9 ± 89.4b |
| i.n. vaccination | ||||
| AL (n=10) | 811.2 ± 107.9 | 25.9 ± 4.9 | 149.5 ± 29.1 | 209.3 ± 79.1 |
| AL+ EX (n=10) | 1418.5 ± 115.7b | 36.1 ± 15.9 | 255.4 ± 57.4 | 453.2 ± 161.6 |
Con A stimulation (2 μg/ml) of isolated CD4+ T cells was used as positive control. In all cultures the amount of each cytokine produced exceeded 0.5 ng/ml/5 × 105 cells.
Significantly different from respective AL group (P<0.05)
3.6. Exercise enhanced antigen-specific CD4+ T cell production
CD4+ T cells collected from the spleen, Peyer’s patches, inguinal and mesenteric lymph nodes were stimulated in vitro with 3.125-100 μg/ml of OVA or 2 μg/ml of Con A 7 days the last booster vaccination. Exercise significantly enhanced in vitro OVA-specific CD4+ T cell proliferation following i.n. vaccination in cells collected from the spleen (Fig. 4a; F 1,108 = 25.95, P < 0.001); mesenteric lymph nodes (Fig. 4b; F 1,56 = 45.43, P < 0.001) and Peyer’s patches (Fig. 4c; F 1,56 = 6.75, P = 0.012) with the exercise-induced enhancement of CD4+ T cell proliferation most pronounced in cells collected from the Peyer’s patches (Fig. 4c). Exercise did not increase Con A-induced CD4+ T cell proliferation in cells collected from the spleen (Fig. 4a insert graph) or mesenteric lymph nodes (Fig. 4b insert graph). However, exercise did increase Con A-induced CD4+ T cell proliferation in cells collected from the Peyer’s patches (Fig. 4c insert graph; P < 0.05).
Fig. 4.
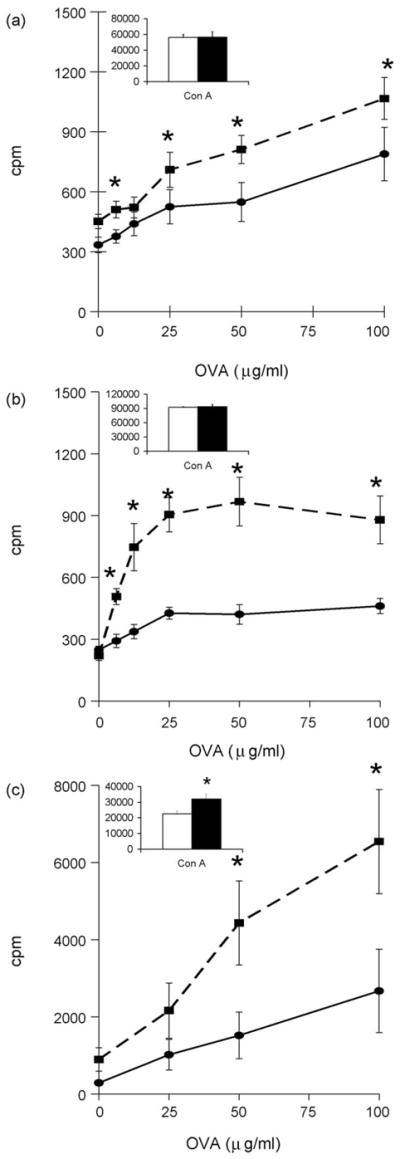
Exercise enhanced antigen-specific CD4+ T cell proliferation following i.n. vaccination. CD4+ T cells were collected from the (a) spleen (n=10/group), (b) mesenteric lymph nodes (n=5 pooled groups of 2 mice/group for each treatment) and (c) Peyer’s patches (n=5 pooled groups of 2 mice/group for each treatment) of AL (●) or AL+EX (■) mice. 1 × 105 experimental CD4+ T cells were co-incubated with 5 ×105 irradiated APCs from unvaccinated syngeneic mice in the presence of increasing concentrations of OVA in vitro. Insert graphs show Con A-induced CD4+ T cell proliferation from each lymphoid organ in AL (white bars) or AL+EX (black bars) mice. Data shown are mean ± SEM. Data are representative of two independent experiments. Two-way ANOVA revealed a significant effect of exercise on CD4+ T cell proliferation in response to simulation with OVA in the spleen (F 1,108 = 25.95, P < 0.001); mesenteric lymph nodes (F 1,56 = 45.43, P < 0.001); and Peyer’s patches (F 1,56 = 6.75, P = 0.012). *Post-hoc analyses using Bonferroni’s test for multiple comparisons found significant differences between AL and AL+EX groups at the designated concentration of OVA.
Exercise also significantly enhanced in vitro OVA-specific CD4+ T cell proliferation following s.c. vaccination in cells collected from the spleen (Fig. 5a; F 1,60 = 12.74, P < 0.001) and inguinal lymph nodes (Fig. 5b; F 1,80 = 10.64, P = 0.002). Exercise did not increase Con A-induced CD4+ T cell proliferation in cells collected from the spleen (Fig. 5a insert graph) or inguinal lymph nodes (Fig. 5b insert graph). Significant differences between AL and AL+EX groups at each concentration of APCs in Figs, 4 & 5 were determined using Bonferroni’s correction for multiple comparisons.
Fig. 5.
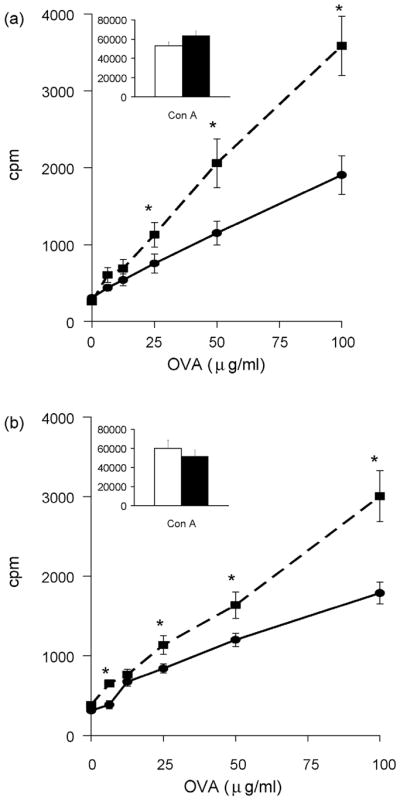
Exercise enhanced antigen-specific CD4+ T cell proliferation following s.c. vaccination. CD4+ T cells were collected from the (a) spleen (n=10/group) and (b) inguinal lymph nodes (n=5 pooled groups of 2 mice/group for each treatment) of AL (●) or AL+EX (■) mice. 1 × 105 experimental CD4+ T cells were co-incubated with 5 ×105 irradiated APCs from unvaccinated syngeneic mice in the presence of increasing concentrations of OVA in vitro. Insert graphs show Con A-induced CD4+ T cell proliferation from each lymphoid organ in AL (white bars) or AL+EX (black bars) mice. Data shown are mean ± SEM (n=6/group). Two-way ANOVA revealed a significant effect of exercise on CD4+ T cell proliferation in response to simulation with OVA in the spleen (F 1,60 = 12.74, P < 0.001) and inguinal lymph nodes (F 1,80 = 10.64, P = 0.002). *Post-hoc analyses using Bonferroni’s test for multiple comparisons found significant differences between AL and AL+EX groups at the designated concentration of OVA.
4. Discussion
To our knowledge, this study is the first to evaluate the effects of moderate exercise on antigen specific mucosal immunity in response to vaccination, and the first to compare and contrast the effect of moderate exercise on antigen specific immune responses in mucosal and systemic compartments. Furthermore, we evaluated both humoral and cell-mediated immune responses to vaccination in young, healthy animals. We have demonstrated that by selecting mice that voluntarily choose to run, all mice maintain running wheel activity (average 5.5 km/day) throughout the duration of the experiment. Mice with access to running wheels for 12 weeks had significantly lower body weights and fat mass as compared to sedentary mice, indicative of a training effect. Mice that were active had significantly higher CD4+ T cell proliferation in response to stimulation with allogeneic APCs but did not have enhanced APC function, suggesting that exercise selectively enhances T cell responsiveness. Furthermore, active mice that received either i.n. or s.c vaccinations with OVA+LT had greater OVA-specific cytokine production from splenic CD4+ T cells and greater OVA-specific CD4+ T cell proliferation in all lymphoid compartments examined. Together, these results suggest that moderate activity enhances cell-mediated immune responses in young, healthy mice after mucosal or systemic vaccination with OVA+ LT.
In the current study our goal was to determine if regular moderate activity (i.e. exercise training) enhances adaptive immune responses following vaccination. Evaluation of the relationship between exercise training and vaccine response requires that mice run consistently for the duration of the experiment. Previous reports that quantified running wheel activity in mice have documented a wide range of running behavior among individual animals [18, 36, 37]. Thus, we evaluated running activity in animals for 4 days prior to the assignment of mice to our study and selected animals that voluntarily chose to run consistently over the 4-day test period. Several studies have documented that when high running behavior is selected in rodents over the course of many genetic crosses (>10 generations), other physiological traits emerge that differ between low and high runners, such as aerobic capacity [38–40], oxidative capabilities of muscle tissue [41, 42], and cardiovascular function [43]. However, none of these studies demonstrated any difference in the physiological traits between low and high runners in the first generation. Furthermore, we have evaluated the immune response to both mucosal and systemic vaccination in animals with low and high running behavior, and animals with low running behavior have no decrements in their immune responses (data not shown). These findings, as well as the fact that we used animals that exhibited high running behavior in both the AL and the AL+EX groups, argue against the possibility that we introduced a selection bias by using only animals that voluntarily chose to run for these immune-based experiments. Additionally, the significantly lower body weight and, in particular, body fat in AL+EX mice (Table 1), in conjunction with reports of exercise-induced changes in skeletal muscle oxidative enzyme activity in other voluntary running wheel studies in mice [44], suggest that exposure to voluntary running wheels for 12 weeks is a valid form of aerobic endurance training. In addition to the changes in body composition, the differences in serum leptin and IL-6 between the AL and the AL+EX mice (Table 1) correspond with the well-documented positive association between leptin and fat mass [45] and the increase in serum IL-6 in response to exercise training [46, 47]. However, while utilizing only animals that exhibited high running behavior in this study is experimentally sound, we recognize that this design feature may narrow the applicability of our findings. Including animals with both high and low running behavior in future studies may broaden the scope of the results and allow us to further examine the relationship between running wheel activity and immune responses.
Although there were no differences in the number of cells in any lymphoid organs examined in AL and AL+EX mice, significant exercise-induced functional changes were observed. We have shown that moderate exercise enhances CD4+ T cell proliferation in C57BL/6 mice in response to allogeneic stimulation with APCs from BALB/c mice; however, splenic antigen presenting capabilities were not enhanced by moderate activity. Furthermore, in animals that received either a mucosal or systemic vaccination, OVA-specific in vitro cytokine production, specifically TNF-α and IL-5, was higher in splenic CD4+ T cells collected from AL+EX as compared to AL mice (Table 3). CD4+ T cell proliferation was also significantly higher in AL+EX mice as compared to AL mice when CD4+ T cells were collected from the spleen, mesenteric and inguinal lymph nodes, and Peyer’s patches (Figs. 4 and 5). In contrast to the stimulatory effect on antigen-specific CD4+ T cell function, exercise had no effect on the generation of OVA-specific serum total IgA or IgG levels (Fig. 3). In prior animal studies, moderate activity enhanced herpes simplex virus-1 (HSV-1)-specific cytokine production (IL-2 and IFN-γ) in older mice [48]. Furthermore, higher DTH responses to KLH were reported in active as compared to sedentary young adults [23]. Taken together, these data suggest that moderate exercise training does not universally activate immune processes, but rather it selectively enhances antigen-specific cell-mediated immunity, e.g. cytokine production and proliferation by CD4+ T cells, in young hosts.
The enhancement of an in vitro recall response found in our study could be due to either quantitative or qualitative difference in the T cells. First, exercise training could increase the number of antigen-specific cells following vaccination, which would result in a greater percentage of the total cells that were antigen specific in both the cytokine and proliferation assays in AL+EX animals. The enhancement of the T cell memory response could also be due to an enhancement of the quality of the antigen-specific T cell, i.e. cells from AL+EX mice were functionally different. Preliminary work from our laboratory suggests that the number of antigen-specific T cells in the spleens of vaccinated animals is not different in AL and AL+EX mice (data not shown), suggesting that a qualitative difference exists in the function of T cells from AL and AL+EX mice.
The exercise-induced enhancement of T cell function could be mediated via a number of cellular mechanisms. First, exercise training reduces the susceptibility of CD4+ T cells to apoptotic stimuli. Splenic [49, 50] and intestinal CD4+ T cells [51] from trained mice are less susceptible to the in vitro administration of apoptosis-inducing agents (e.g. hydrogen peroxide, dexamethasone, TNF-α, or anti-CD3) and in vivo physical stressors (e.g. exhaustive exercise). Perhaps the training effect on anti-apoptotic mechanisms in CD4+ T cells confers protection from environmental stimuli in vivo that may lead to CD4+ T cell death, thus allowing for greater in vitro CD4+ T cell proliferation in AL+EX animals.
Second, we (Table 1) and others [46] demonstrate an exercise-induced increase in serum IL-6. IL-6 has many physiological, metabolic and immunological roles and is produced by a myriad of immune cells [52, 53] and skeletal muscle [46]. Of interest to our study, IL-6 has been shown to enhance the proliferation of CD4+CD25− T cells by inhibiting CD4+CD25+ regulatory T cell (Treg) function in two different autoimmunity models [54, 55], suggesting that IL-6 enables CD4+CD25− T cells to escape from Treg-mediated inhibition. In several reports, dendritic cells are the source of IL-6 which mediates the inhibition of Treg function [55, 56]. These findings suggest that local IL-6 may be important in regulating Treg function and consequently CD4+ T cell proliferation. It is unknown if elevated levels of serum IL-6 mediate a similar inhibition of Treg function. Additionally, since the effect of IL-6 on CD4+ T cell proliferation (via its effect on Treg function) has been studied only in the context of autoimmunity in which pathological dysregulation of CD4+ T cell proliferation has occurred, it is not clear if IL-6 in healthy mice in the absence of other inflammatory cytokines and genetic factors would mediate inhibitory effects on Treg function and subsequent enhancement of CD4+ T cell proliferation. Additional studies are required to determine if the exercise-induced increase in serum IL-6 alters the function of regulatory T cells and CD4+CD25− T cell proliferation.
In summary, we have established a reliable animal model of moderate physical activity that can be used to evaluate the effect of exercise on immune, as well as other physiological endpoints in mice. We have demonstrated that regular moderate exercise selectively enhances antigen-specific cell-mediated responses following vaccination via either a mucosal or systemic route of antigen delivery. These results suggest that regular moderate exercise may enhance vaccine efficacy and thus, may be an important lifestyle intervention in humans to couple with immunization against infectious diseases such as influenza. These findings may prove to have important public health significance. If regular physical activity can enhance cell-mediated adaptive immune responses in young, healthy animals, perhaps participation in regular physical activity would benefit other populations in which the immune system is compromised to a greater extent.
Acknowledgments
We are grateful for the technical assistance of Garland Davis, Eileen Thompson, and Bertina Gibbs. The authors thank Debra Weingarten for her assistance in the preparation of this manuscript.
Footnotes
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by the National Cancer Institute Cancer Prevention Fellowship Program. This work was also supported by funding to S. Hursting from NIEHS # P30 ES007784 and the Breast Cancer Research Foundation.
Author disclosures: C. J. Rogers, D. A. Zaharoff, K. W. Hance, S. N. Perkins, J. Schlom, J. W. Greiner, and S. D. Hursting, no conflicts of interest.
Abbreviations used: AL, consumed food ad libitum; AL+EX, consumed food ad libitum plus given access to voluntary running wheels; APC, antigen-presenting cell; Con A, concanavalin A; DTH, delayed type hypersensitivity; HSV-1, herpes simplex virus-1; IL, interleukin; KLH, keyhole-limpet hemocyanin; km, kilometer; LT, lymphotactin; mAb, monoclonal antibodies; MLR, mixed lymphocyte response; NK, natural killer; OVA, ovalbumin; PBMC, peripheral blood mononuclear cell; PMA, phorbol 12-myristate 13-acetate; SI, stimulation index; TNF-α, tumor necrosis factor alpha; Treg, regulatory T cell; URTI, upper respiratory tract infection.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6 Suppl):S379–99. doi: 10.1097/00005768-200106001-00007. discussion S419–20. [DOI] [PubMed] [Google Scholar]
- 2.Melzer K, Kayser B, Pichard C. Physical activity: the health benefits outweigh the risks. Curr Opin Clin Nutr Metab Care. 2004;7(6):641–7. doi: 10.1097/00075197-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31(1):57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, et al. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25(7):823–31. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34(8):1242–8. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Cieslak TJ, Frost G, Klentrou P. Effects of physical activity, body fat, and salivary cortisol on mucosal immunity in children. J Appl Physiol. 2003;95(6):2315–20. doi: 10.1152/japplphysiol.00400.2003. [DOI] [PubMed] [Google Scholar]
- 7.Chubak J, McTiernan A, Sorensen B, Wener MH, Yasui Y, Velasquez M, et al. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119(11):937–42. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Nieman DC, Nehlsen-Cannarella SL, Markoff PA, Balk-Lamberton AJ, Yang H, Chritton DB, et al. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11(6):467–73. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 9.Nieman DC, Nehlsen-Cannarella SL, Henson DA, Koch AJ, Butterworth DE, Fagoaga OR, et al. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc. 1998;30(5):679–86. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kostka T, Berthouze SE, Lacour J, Bonnefoy M. The symptomatology of upper respiratory tract infections and exercise in elderly people. Med Sci Sports Exerc. 2000;32(1):46–51. doi: 10.1097/00005768-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Strasner A, Barlow CE, JBK, Dunn AL. Impact of physical activity on URTI symptoms in Project PRIME participants. Med Sci Sports Exerc. 2001;33 (Suppl):S304. [Google Scholar]
- 12.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87(2):153–8. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 13.Cannon JG, Kluger MJ. Exercise enhances survival rate in mice infected with Salmonella typhimurium. Proc Soc Exp Biol Med. 1984;175(4):518–21. doi: 10.3181/00379727-175-41830. [DOI] [PubMed] [Google Scholar]
- 14.Ilback NG, Friman G, Beisel WR, Johnson AJ, Berendt RF. Modifying effects of exercise on clinical course and biochemical response of the myocardium in influenza and tularemia in mice. Infect Immun. 1984;45(2):498–504. doi: 10.1128/iai.45.2.498-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schouten WJ, Verschuur R, Kemper HC. Habitual physical activity, strenuous exercise, and salivary immunoglobulin A levels in young adults: the Amsterdam Growth and Health Study. Int J Sports Med. 1988;9(4):289–93. doi: 10.1055/s-2007-1025024. [DOI] [PubMed] [Google Scholar]
- 16.Tharp GD. Basketball exercise and secretory immunoglobulin A. Eur J Appl Physiol Occup Physiol. 1991;63(3–4):312–4. doi: 10.1007/BF00233868. [DOI] [PubMed] [Google Scholar]
- 17.Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, et al. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37(1):76–9. doi: 10.1136/bjsm.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers CJ, Berrigan D, Zaharoff DA, Hance KW, Patel AC, Perkins SN, et al. Energy restriction and exercise differentially enhance components of systemic and mucosal immunity in mice. J Nutr. 2008;138(1):115–22. doi: 10.1093/jn/138.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods J, Lu Q, Ceddia MA, Lowder T. Special feature for the Olympics: effects of exercise on the immune system: exercise-induced modulation of macrophage function. Immunol Cell Biol. 2000;78(5):545–53. doi: 10.1111/j.1440-1711.2000.t01-9-.x. [DOI] [PubMed] [Google Scholar]
- 20.Hance KW, Rogers CJ, Hursting SD, Greiner JW. Combination of physical activity, nutrition, or other metabolic factors and vaccine response. Front Biosci. 2007;12:4997–5029. doi: 10.2741/2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keylock KT, Lowder T, Leifheit KA, Cook M, Mariani RA, Ross K, et al. Higher antibody, but not cell-mediated, responses to vaccination in high physically fit elderly. J Appl Physiol. 2007;102(3):1090–8. doi: 10.1152/japplphysiol.00790.2006. [DOI] [PubMed] [Google Scholar]
- 22.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57(9):M557–62. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 23.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97(2):491–8. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 24.Grant RW, Mariani RA, Vieira VJ, Fleshner M, Smith TP, Keylock KT, et al. Cardiovascular exercise intervention improves the primary antibody response to keyhole limpet hemocyanin (KLH) in previously sedentary older adults. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohut ML, Arntson BA, Lee W, Rozeboom K, Yoon KJ, Cunnick JE, et al. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22(17–18):2298–306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Schuler PB, Lloyd LK, Leblanc PA, Clapp TA, Abadie BR, Collins RK. The effect of physical activity and fitness on specific antibody production in college students. J Sports Med Phys Fitness. 1999;39(3):233–9. [PubMed] [Google Scholar]
- 27.Whitham M, Blannin AK. The effect of exercise training on the kinetics of the antibody response to influenza vaccination. J Sports Sci. 2003;21(12):991–1000. doi: 10.1080/0264041031000140464. [DOI] [PubMed] [Google Scholar]
- 28.Coleman KJ, Rager DR. Effects of voluntary exercise on immune function in rats. Physiol Behav. 1993;54(4):771–4. doi: 10.1016/0031-9384(93)90090-3. [DOI] [PubMed] [Google Scholar]
- 29.Jonsdottir IH, Asea A, Hoffmann P, Dahlgren UI, Andersson B, Hellstrand K, et al. Voluntary chronic exercise augments in vivo natural immunity in rats. J Appl Physiol. 1996;80(5):1799–803. doi: 10.1152/jappl.1996.80.5.1799. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin DR, Wilcox ZC, Zheng G. The effects of voluntary exercise and immobilization on humoral immunity and endocrine responses in rats. Physiol Behav. 1997;61(3):447–53. doi: 10.1016/s0031-9384(96)00459-3. [DOI] [PubMed] [Google Scholar]
- 31.Elphick GF, Greenwood BN, Campisi J, Fleshner M. Increased serum nIgM in voluntarily physically active rats: a potential role for B-1 cells. J Appl Physiol. 2003;94(2):660–7. doi: 10.1152/japplphysiol.00547.2002. [DOI] [PubMed] [Google Scholar]
- 32.Lillard JW, Jr, Boyaka PN, Hedrick JA, Zlotnik A, McGhee JR. Lymphotactin acts as an innate mucosal adjuvant. J Immunol. 1999;162(4):1959–65. [PubMed] [Google Scholar]
- 33.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007;25(52):8673–86. doi: 10.1016/j.vaccine.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernando P, Bonen A, Hoffman-Goetz L. Predicting submaximal oxygen consumption during treadmill running in mice. Can J Physiol Pharmacol. 1993;71(10–11):854–7. doi: 10.1139/y93-128. [DOI] [PubMed] [Google Scholar]
- 36.Dohm MR, Richardson CS, Garland T., Jr Exercise physiology of wild and random-bred laboratory house mice and their reciprocal hybrids. Am J Physiol. 1994;267(4 Pt 2):R1098–108. doi: 10.1152/ajpregu.1994.267.4.R1098. [DOI] [PubMed] [Google Scholar]
- 37.Friedman WA, Garland T, Jr, Dohm MR. Individual variation in locomotor behavior and maximal oxygen consumption in mice. Physiol Behav. 1992;52(1):97–104. doi: 10.1016/0031-9384(92)90438-8. [DOI] [PubMed] [Google Scholar]
- 38.Lambert MI, Noakes TD. Spontaneous running increases VO2max and running performance in rats. J Appl Physiol. 1990;68(1):400–3. doi: 10.1152/jappl.1990.68.1.400. [DOI] [PubMed] [Google Scholar]
- 39.Swallow JG, Garland T, Jr, Carter PA, Zhan WZ, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus) J Appl Physiol. 1998;84(1):69–76. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland T., Jr Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J Exp Biol. 2005;208(Pt 12):2447–58. doi: 10.1242/jeb.01631. [DOI] [PubMed] [Google Scholar]
- 41.Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol. 1989;66(3):1250–7. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- 42.Zhan WZ, Swallow JG, Garland T, Jr, Proctor DN, Carter PA, Sieck GC. Effects of genetic selection and voluntary activity on the medial gastrocnemius muscle in house mice. J Appl Physiol. 1999;87(6):2326–33. doi: 10.1152/jappl.1999.87.6.2326. [DOI] [PubMed] [Google Scholar]
- 43.Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–20. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 44.Davidson SR, Burnett M, Hoffman-Goetz L. Training effects in mice after long-term voluntary exercise. Med Sci Sports Exerc. 2006;38(2):250–5. doi: 10.1249/01.mss.0000183179.86594.4f. [DOI] [PubMed] [Google Scholar]
- 45.Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–88. doi: 10.1111/j.1749-6632.2002.tb04293.x. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Curr Opin Clin Nutr Metab Care. 2007;10(3):265–71. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- 47.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 48.Kohut ML, Boehm GW, Moynihan JA. Moderate exercise is associated with enhanced antigen-specific cytokine, but not IgM antibody production in aged mice. Mech Ageing Dev. 2001;122(11):1135–50. doi: 10.1016/s0047-6374(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 49.Avula CP, Muthukumar AR, Zaman K, McCarter R, Fernandes G. Inhibitory effects of voluntary wheel exercise on apoptosis in splenic lymphocyte subsets of C57BL/6 mice. J Appl Physiol. 2001;91(6):2546–52. doi: 10.1152/jappl.2001.91.6.2546. [DOI] [PubMed] [Google Scholar]
- 50.Fu SC, Qin L, Leung CK, Chan BP, Chan KM. Regular moderate exercise training prevents decrease of CD4+ T-lymphocytes induced by a single bout of strenuous exercise in mice. Can J Appl Physiol. 2003;28(3):370–81. doi: 10.1139/h03-027. [DOI] [PubMed] [Google Scholar]
- 51.Davidson SR, Hoffman-Goetz L. Freewheel running selectively prevents mouse CD4+ intestinal lymphocyte death produced after a bout of acute strenuous exercise. Brain Behav Immun. 2006;20(2):139–43. doi: 10.1016/j.bbi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda T, Hirano T. IL-6. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. New York: Academic Press; 2000. [Google Scholar]
- 53.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195(4):173–83. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 54.Chen X, Howard OM, Oppenheim JJ. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J Immunol. 2007;178(10):6123–9. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 55.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178(1):271–9. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 56.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]