Abstract
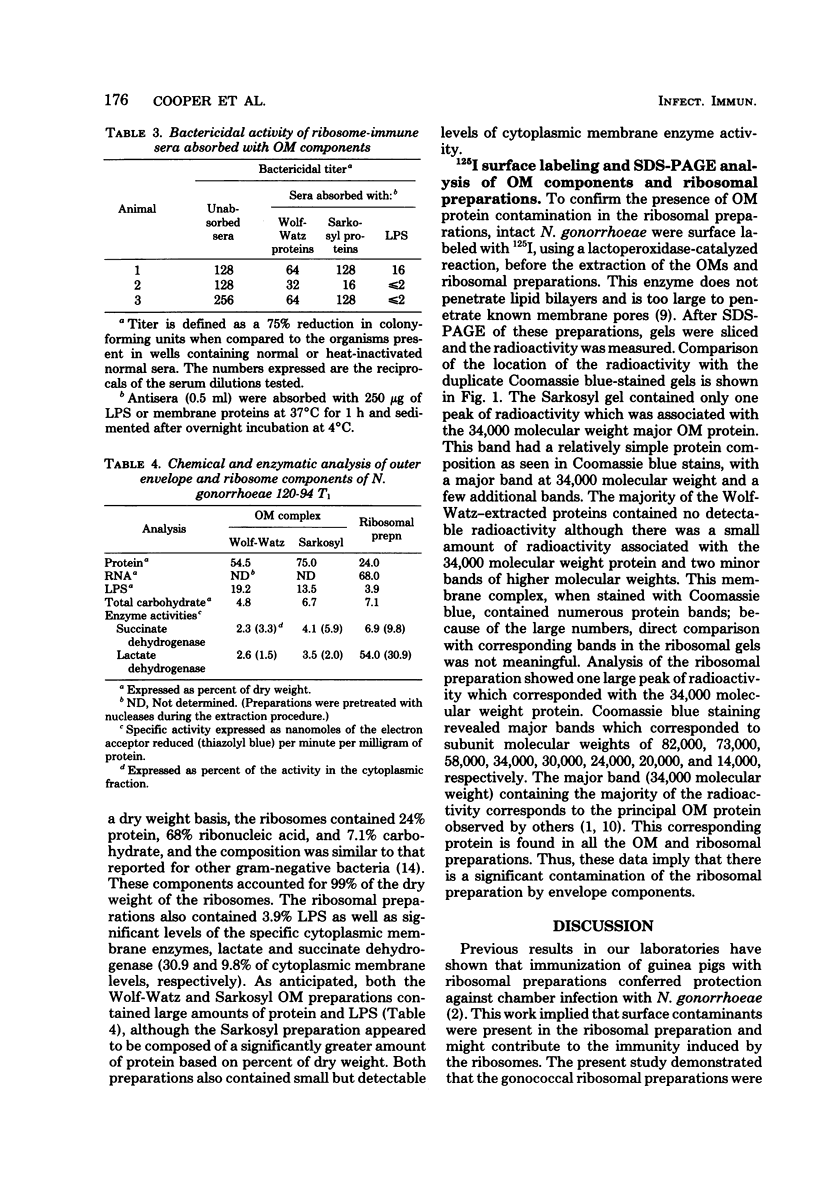
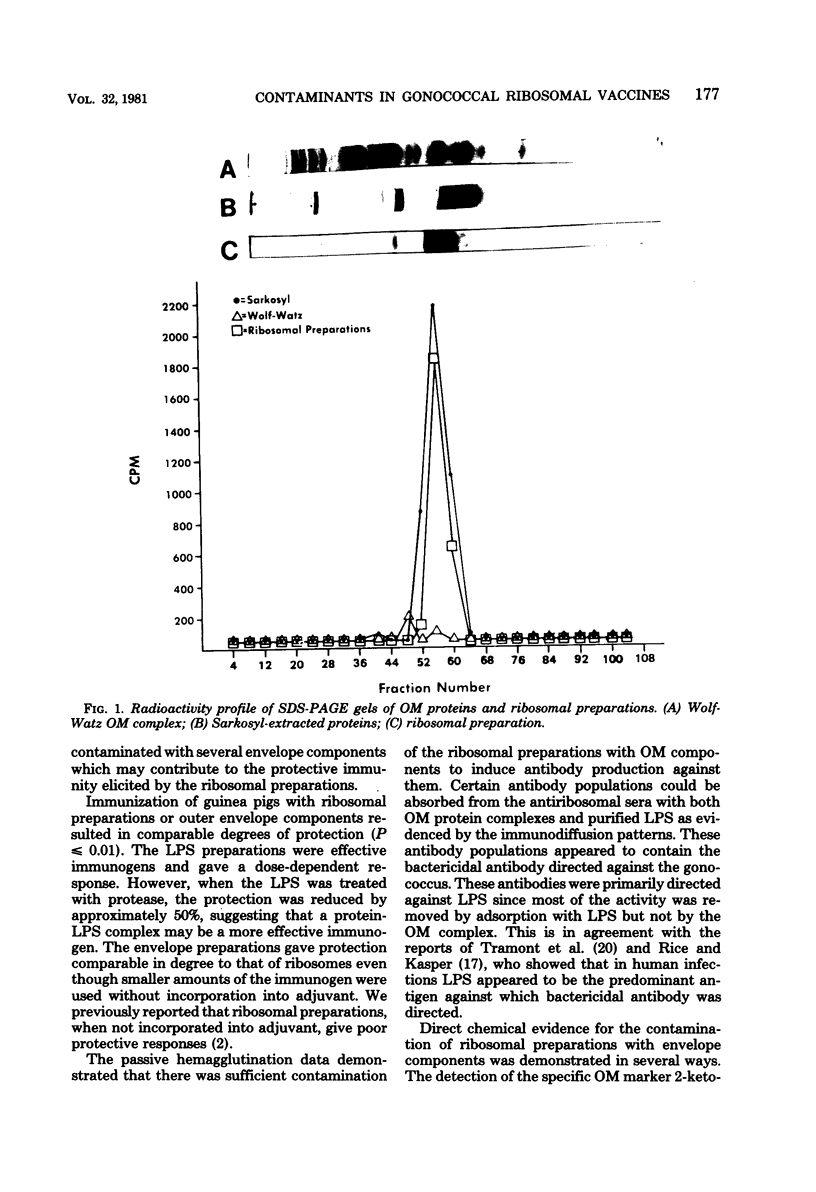
A recent report (Cooper et al., Infect. Immun. 28:92-100, 1980) demonstrated that immunization of guinea pigs with ribosomal preparations was protective (approximately 90%) against chamber infections with Neisseria gonorrhoeae. Similar protection has been demonstrated with other cellular immunogens such as outer membranes (OM) (92%) or purified lipopolysaccharide (LPS) (83%). Protection of LPS (5 to 100 micrograms) was dose dependent (83% with 100 micrograms). Treatment of LPS with pronase reduced the protection by 50%. Ribosomal preparations contained LPS contamination (3.9%) based on dry weight determinations by 2-keto-3-deoxyoctonate analysis. Analysis of ribosomal preparations isolated from cells after lactoperoxidase-mediated 125I labeling indicated a major OM contamination (Protein I). The ribosomal preparation also contained low levels of succinic and lactic dehydrogenase. Passive hemagglutination tests revealed that sera from guinea pigs immunized with ribosomal preparations also demonstrated antibody to OM proteins and LPS. LPS was able to absorb one line of precipitation seen in immunodiffusion reactions as well as the bactericidal activity of such sera. OM preparations were unable to absorb the remaining precipitin line or remove the bactericidal activity. It appears that LPS is the major antigen responsible for the bactericidal activity seen in ribosome-immune sera.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Arko R. J. Immunity to gonococcal infection induced by vaccination with isolated outer membranes of Neisseria gonorrhoeae in guinea pigs. J Infect Dis. 1977 Jun;135(6):879–887. doi: 10.1093/infdis/135.6.879. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Tewari R. P., Bowser D. V. Immunogenicity of ribosomal preparations from Neisseria gonorrhoeae. Infect Immun. 1980 Apr;28(1):92–100. doi: 10.1128/iai.28.1.92-100.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. A., Johnson W. Immunogenicity of ribosomes from enzymatically lysed Streptococcus pyogenes. Infect Immun. 1980 Feb;27(2):424–430. doi: 10.1128/iai.27.2.424-430.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Anderson R. L., Zeltner J. Y., Carlo D. J., Stoudt T. H. Antigenic polypeptide complex from the Melvin strain of Neisseria gonorrhoeae: isolation and properties. Infect Immun. 1979 Aug;25(2):635–644. doi: 10.1128/iai.25.2.635-644.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman M. M. Pseudomonas ribosomal vaccines: preparation, properties, and immunogenicity. Infect Immun. 1978 Jul;21(1):76–86. doi: 10.1128/iai.21.1.76-86.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn M., Tewari R. P., Solotorovsky M. Immunoprotective activity of ribosomes from Haemophilus influenzae. Infect Immun. 1977 Feb;15(2):453–460. doi: 10.1128/iai.15.2.453-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Morse S. A. Binding of progesterone to Neisseria gonorrhoeae and other gram-negative bacteria. Infect Immun. 1977 Apr;16(1):115–123. doi: 10.1128/iai.16.1.115-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Arko R. J., Logan L. C., Bullard J. C. Immunological and serological diversity of Neisseria gonorrhoeae: gonococcal serotypes and their relationship with immunotypes. Infect Immun. 1976 Dec;14(6):1297–1301. doi: 10.1128/iai.14.6.1297-1301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]