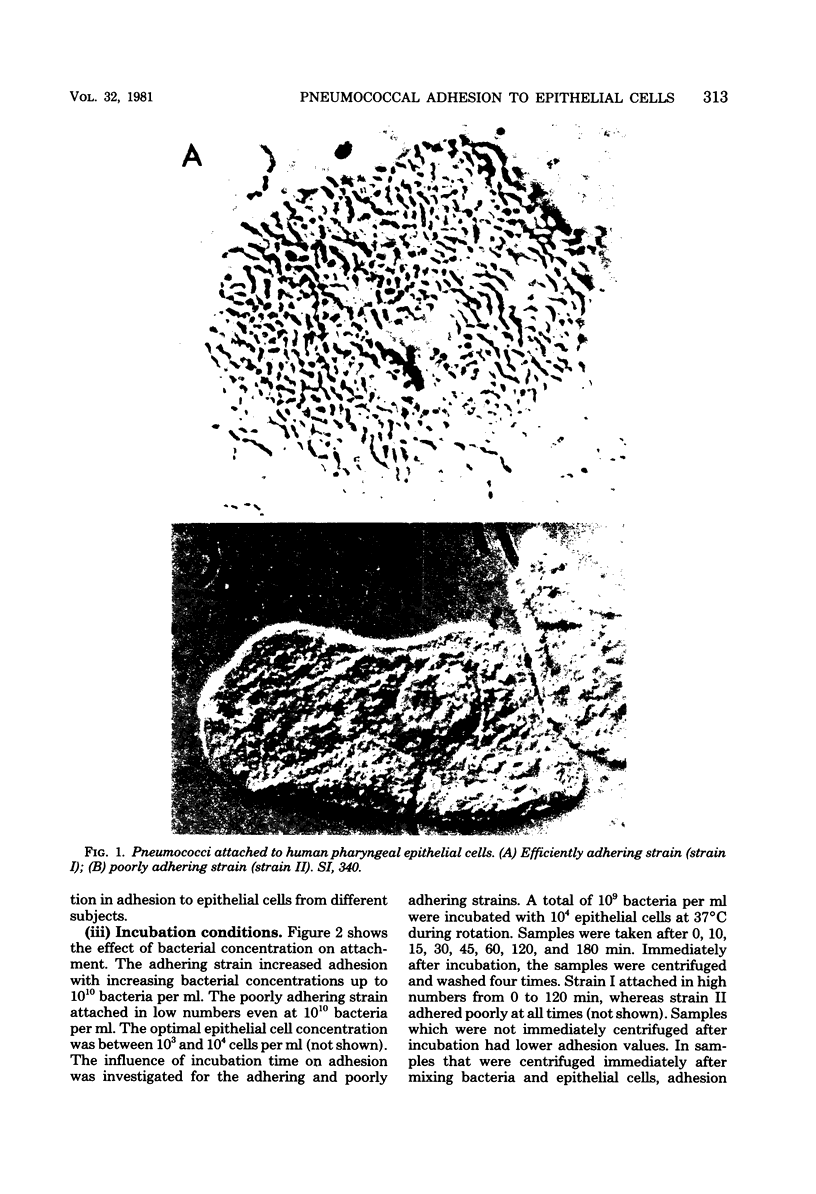
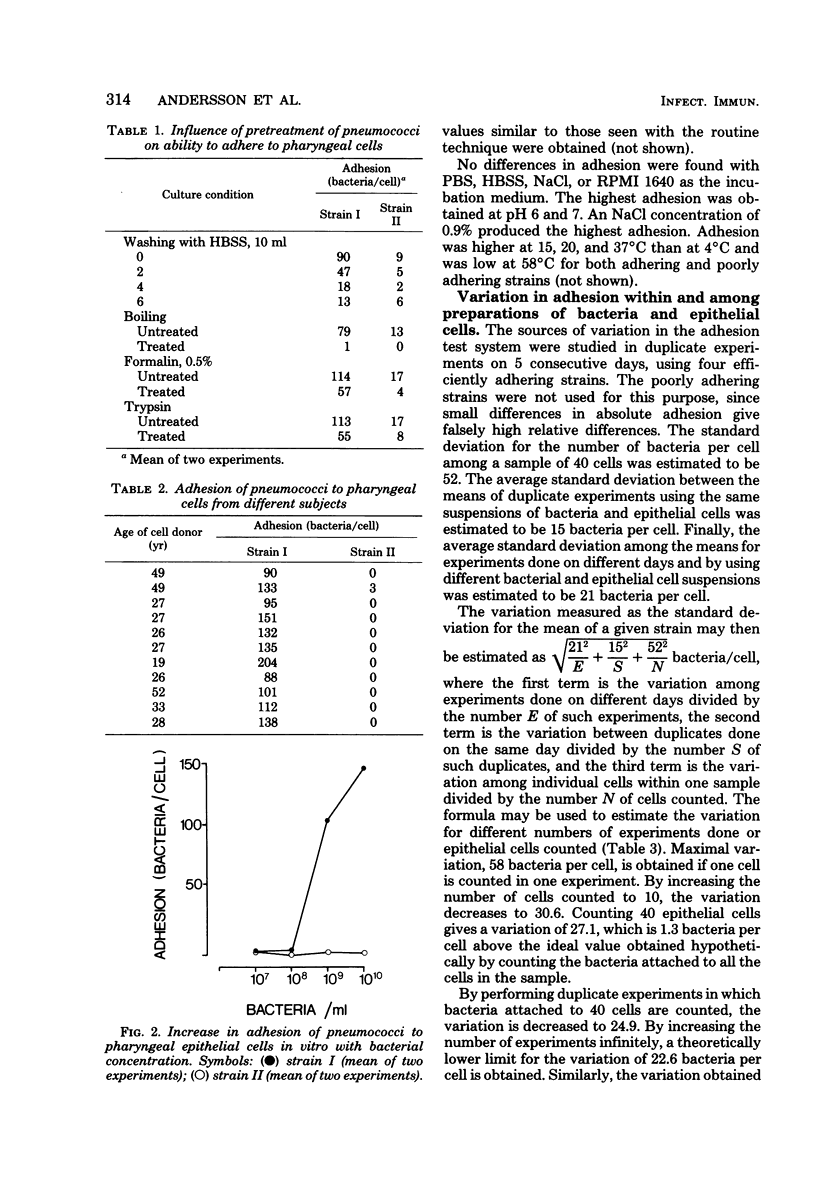
Abstract
A method was developed to study the adhesion of Streptococcus pneumoniae to human pharyngeal epithelial cells. Epithelial cells from healthy persons, pneumococcal strains from patients with otitis media, meningitis, or septicemia, and pneumococcal cells from the nasopharynx of healthy carriers were used. Adhesion was found to be influenced by changes in the bacterial incubation medium and growth phase, the concentration of bacteria and epithelial cells, the epithelial cell donor, the incubation time and temperature, and the pH and osmolarity of the incubation medium. Pretreatment of bacteria with heat, Formalin, or trypsin decreased adhesion. The highest adhesion was obtained when 10(9) bacteria cultivated for 18 h in streptococcus cultivation broth were added to 10(4) pharyngeal cells and incubated at 37 degrees C for 30 min. S. pneumoniae strains from patients with frequent episodes of otitis media and strains from healthy carriers had the highest adhesion values; septicemia and meningitis strains had the lowest. The capsular polysaccharide type did not determine the adhesive capacity of the strains, but otitis strains belonging to the capsular types often associated with otitis media adhered in high numbers. Adhesion may be important for pneumococci colonizing the nasopharynx or inducing otitis media.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Nylén O., Peterson C. M., Svanborg-Edén C. Attachment of Streptococcus pneumoniae to human pharyngeal epithelial cells in vitro. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):115–116. doi: 10.1177/00034894800890s330. [DOI] [PubMed] [Google Scholar]
- Austrian R., Howie V. M., Ploussard J. H. The bacteriology of pneumococcal otitis media. Johns Hopkins Med J. 1977 Sep;141(3):104–111. [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. E., Jr, Stamey T. A. Studies of introital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J Urol. 1977 Apr;117(4):472–476. doi: 10.1016/s0022-5347(17)58501-8. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie V. M., Ploussard J. H. Bacterial etiology and antimicrobial treatment of exudative otitis media: relation of antibiotic therapy to relapses. South Med J. 1971 Feb;64(2):233–239. doi: 10.1097/00007611-197102000-00022. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Julie N., Reed W. P., Williams R. C., Jr Adherence of group A streptococci to pharyngeal cells: a role in the pathogenesis of rheumatic fever. Science. 1978 Aug 4;201(4354):455–457. doi: 10.1126/science.351810. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg-Edén C., Jodal U. Attachment of Escherichia coli to urinary sediment epithelial cells from urinary tract infection-prone and healthy children. Infect Immun. 1979 Dec;26(3):837–840. doi: 10.1128/iai.26.3.837-840.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]