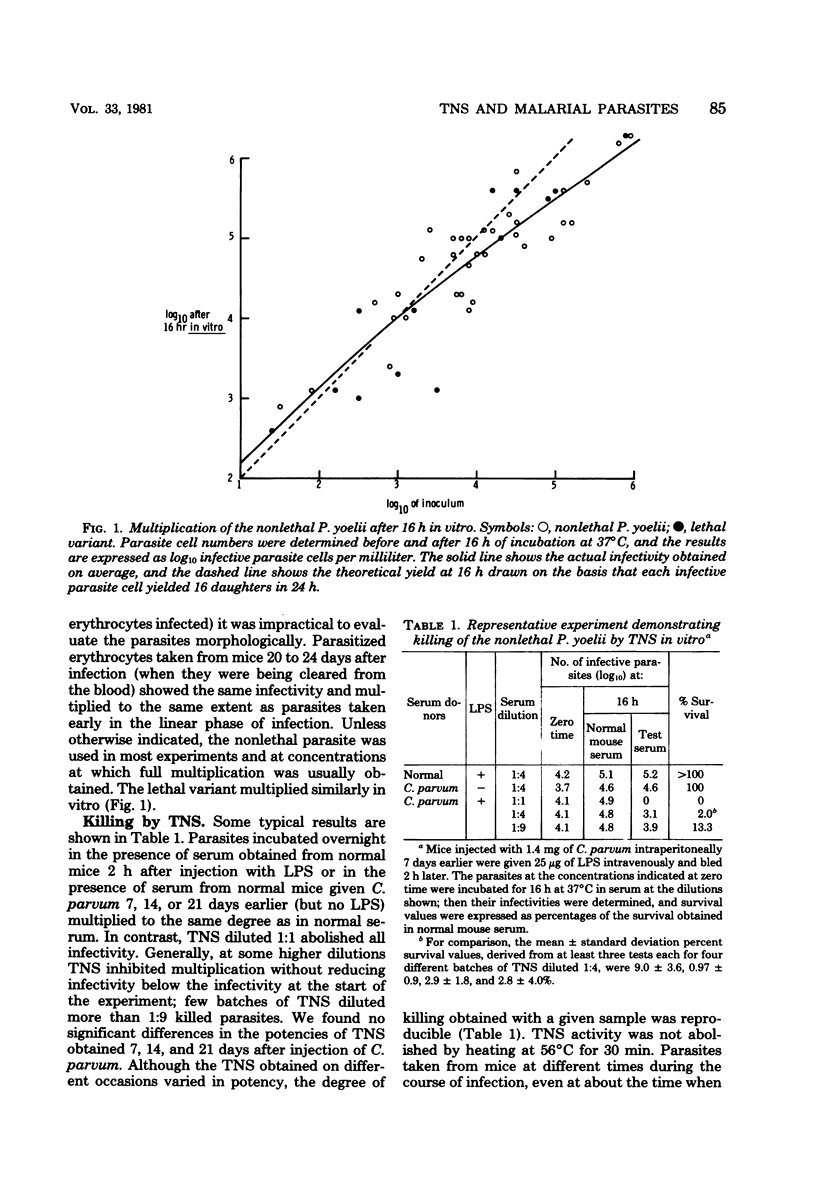
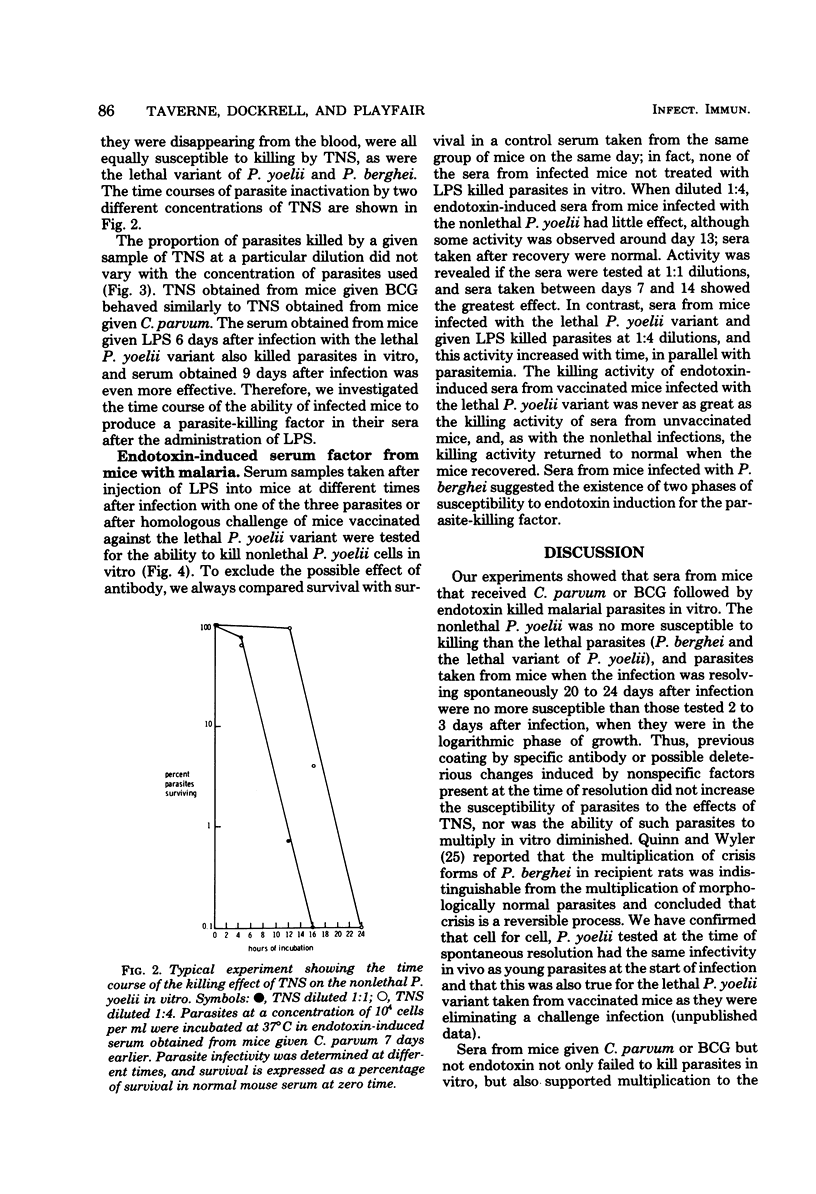
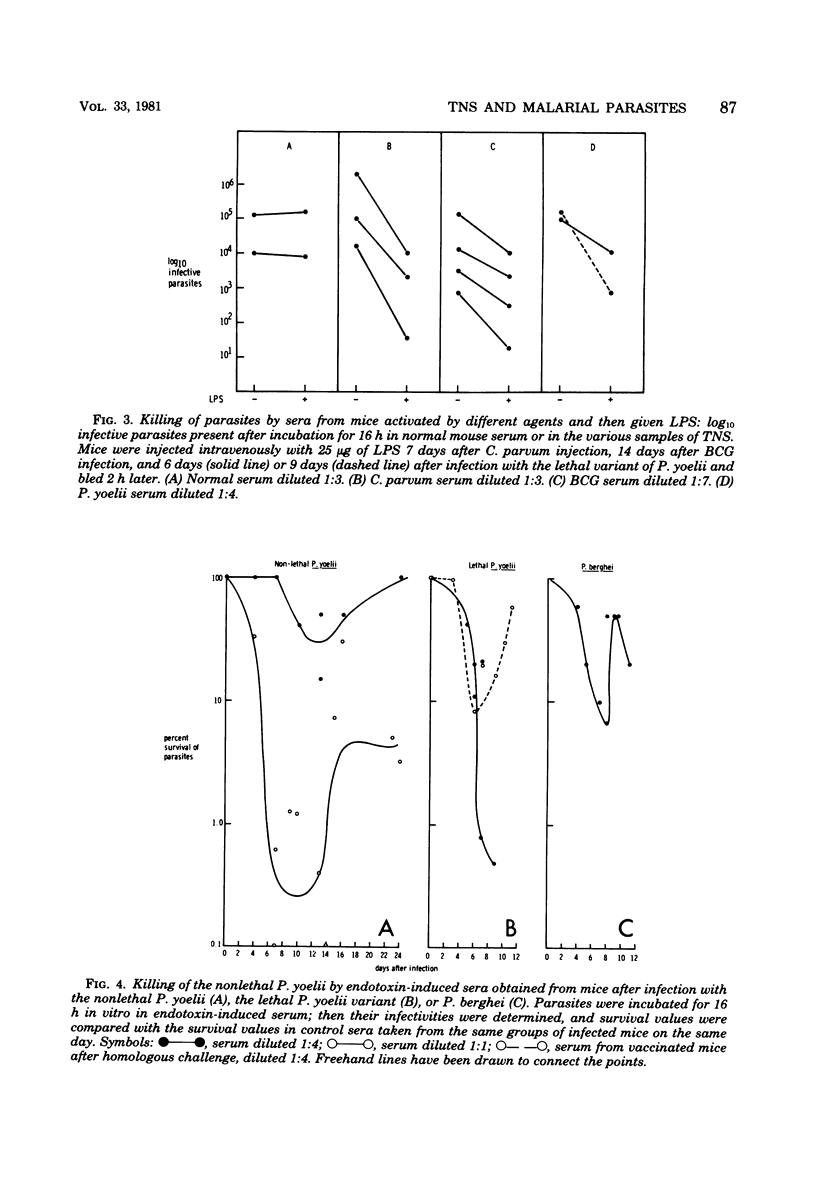
Abstract
We investigated the possibility that malarial parasites may be killed by nonspecific soluble mediators, such as those in tumor necrosis serum, that are obtained from mice given macrophage-activating agents like Corynebacterium parvum or Mycobacterium bovis BCG, followed by endotoxin. Such sera killed parasites in vitro after overnight incubation; killing was measured directly by using an in vivo infectivity assay. Parasite infectivity was not decreased by incubation in sera from mice given C. parvum or BCG alone (no endotoxin) or by incubation in sera from normal mice given endotoxin. Plasmodium yoelii, its lethal variant, and Plasmodium berghei were equally susceptible to inactivation. Sera obtained from mice given endotoxin during the course of infection with these parasites also contained parasite-killing factor. The activity of this factor appeared to be proportional to parasitemia in that it was higher in the sera from mice infected with the lethal parasites than in the sera from mice with infections which resolved either spontaneously or after vaccination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt M. J., Newborg M. F., North R. J. Increased toxicity of endotoxin for tumor-bearing mice and mice responding to bacterial pathogens: macrophage activation as a common denominator. Infect Immun. 1980 May;28(2):645–647. doi: 10.1128/iai.28.2.645-647.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX F. E., BILBEY D. L., NICOL T. RETICULO-ENDOTHELIAL ACTIVITY IN MICE INFECTED WITH PLASMODIUM VINCKEI. J Protozool. 1964 May;11:229–230. doi: 10.1111/j.1550-7408.1964.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Cantrell W., Elko E. E., Hopff B. M. Plasmodium berghei: phagocytic hyperactivity of infected rats. Exp Parasitol. 1970 Oct;28(2):291–297. doi: 10.1016/0014-4894(70)90099-8. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Wills E. J., Richmond J. E., Allison A. C. Suppression of babesiosis in BCG-infected mice and its correlation with tumor inhibition. Infect Immun. 1977 Aug;17(2):430–438. doi: 10.1128/iai.17.2.430-438.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell B. J., Playfair J. H., de Sousa B. Plasmodium yoelii and Plasmodium vinckei: the effects of nonspecific immunostimulation on murine malaria. Exp Parasitol. 1977 Oct;43(1):45–53. doi: 10.1016/0014-4894(77)90006-6. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., de Souza J. B., Playfair J. H. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980 Oct;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M. K., Oettgen H. F., Old L. J., Mittler R. S., Hammerling U. Induction and immunological properties of tumor necrosis factor. J Reticuloendothel Soc. 1978 Apr;23(4):307–319. [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lelchuk R., Taverne J., Agomo P. U., Playfair J. H. Development and suppression of a population of late-adhering macrophages in mouse malaria. Parasite Immunol. 1979 Spring;1(1):61–78. doi: 10.1111/j.1365-3024.1979.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Lucia H. L., Nussenzweig R. S. Plasmodium chabaudi and Plasmodium vinckei: phagocytic activity of mouse reticuloendothelial system. Exp Parasitol. 1969 Aug;25(1):319–323. doi: 10.1016/0014-4894(69)90077-0. [DOI] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. N., Goodrum K. J., Couch R., Jr, Berry L. J. Factors affecting macrophage function: glucocorticoid antagonizing factor. J Reticuloendothel Soc. 1978 Apr;23(4):321–332. [PubMed] [Google Scholar]
- Männel D. N., Meltzer M. S., Mergenhagen S. E. Generation and characterization of a lipopolysaccharide-induced and serum-derived cytotoxic factor for tumor cells. Infect Immun. 1980 Apr;28(1):204–211. doi: 10.1128/iai.28.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M. A., Parant F. J., Chedid L. A. Enhancement of resistance to infections by endotoxin-induced serum factor from Mycobacterium bovis BCG-infected mice. Infect Immun. 1980 Jun;28(3):654–659. doi: 10.1128/iai.28.3.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M., Musher D. M. Effects of BCG infection on the susceptibility of mouse macrophages to endotoxin. Infect Immun. 1979 Apr;24(1):59–64. doi: 10.1128/iai.24.1.59-64.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Resolution of acute malaria (Plasmodium berghei in the rat): reversibility and spleen dependence. Am J Trop Med Hyg. 1980 Jan;29(1):1–4. doi: 10.4269/ajtmh.1980.29.1. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. W., Huang K. Y., Gordon F. B. Role of interferon in experimental mouse malaria. Nature. 1968 Nov 16;220(5168):709–710. doi: 10.1038/220709a0. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Senterfitt V. C. Endotoxin-induced hepatic damage in BCG-infected mice. Am J Pathol. 1972 Apr;67(1):23–40. [PMC free article] [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst D. C., Folwell R. O. Measurement of the growth rate of the erythrocytic stages of Plasmodium berghei and comparisons of the potency of inocula after various treatments. Ann Trop Med Parasitol. 1968 Sep;62(3):349–360. doi: 10.1080/00034983.1968.11686570. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Wyler D. J., Gallin J. I. Spleen-derived mononuclear cell chemotactic factor in malaria infections: a possible mechanism for splenic macrophage accumulation. J Immunol. 1977 Feb;118(2):478–484. [PubMed] [Google Scholar]
- Wyler D. J., Oppenheim J. J., Koontz L. C. Influence of malaria infection on the elaboration of soluble mediators by adherent mononuclear cells. Infect Immun. 1979 Apr;24(1):151–159. doi: 10.1128/iai.24.1.151-159.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]


