Abstract
The management of ovarian teratomas in normal conditions is well established, but in rare giant cases (tumor diameter over 15 cm), the choice of management, such as laparotomic or laparoscopic approaches, are controversial and may be therapeutically challenging for surgeons. The aims of the current study were to analyze the clinical features of giant ovarian teratoma and to discuss its management. The clinical data of 330 patients with giant ovarian teratoma (of whom 1 patient was treated by the authors and 329 were admitted to the Second Affiliated Hospital of Zhejiang University Medical College between January 1st 2000 and December 31st 2010) were reviewed and analyzed. The patients had an age range of 6 to 83 years and a mean tumor size of 24.9±7.1 cm. Of the 330 patients, 102 (30.9%) were asymptomatic and the majority (69.1%, 228/330) reported symptoms. There were more patients in the laparotomic group than the laparoscopic group, especially for the emergency cases (5.5 vs. 0%, P<0.05). Accidental cyst rupture was more frequent when a laparoscopic approach was used (31.5 vs. 19.6%, P<0.05). These results suggest that laparotomic resection may be preferred for the en bloc mass removal, adequate abdominal cavity irrigation and avoidance of accidental mass rupture in the management of giant ovarian teratomas. Familiarity with the imaging features of giant ovarian teratomas effectively aids preoperative diagnosis and differentiation.
Keywords: giant, teratomas, laparotomy, laparoscopy
Introduction
Teratomas are germ cell tumors commonly composed of multiple cell types derived from one or more of the three germ layers. Pathologically, teratomas are classified into three groups: mature (cystic/solid, benign), immature (malignant) and monodermal (highly specialized, e.g., struma ovarii, carcinoid tumors, neural tumors). Teratomas most commonly arise in the gonads but have also been found in the anterior mediastinum, retroperitoneum and gastrointestinal tract (1). Several studies have reported that laparoscopic approaches are generally accepted in normal cases for their minimal invasiveness, fewer complications and quicker recovery (2–4). The evident cosmetic advantage of this technique for young women is also noted. However, it is debatable whether this technique may be applied to giant ovarian teratomas. Therefore, a study to evaluate the intraperitoneal spillage and oncological safety of laparascopic and laparotomic procedures for giant ovarian teratomas is essential.
In the present study, we report a young woman (20 years old) with a massive ovarian neoplasm, histopathologically diagnosed as a mature teratoma derived from three germ layers which was successfully removed by laparotomy, and review 329 giant ovarian teratomas treated at our hospital.
Materials and methods
Identification of patients with giant teratoma
A total of 329 cases of giant ovarian teratoma (tumor diameter over 15 cm) were admitted to the Second Affiliated Hospital of Zhejiang University School of Medicine (Zhejiang, China) between January 1st 2000 and December 31st 2010. The diagnosis of giant ovarian teratoma was based on clinical examination, imaging evaluation and finally confirmed by pathological results. The patients were divided into two groups according to whether laparoscopic (group 1) or laparotomic (group 2) surgery was employed. The data were analyzed using the Student’s t-test and Fisher’s exact test. Statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA).
Case report
Local ethical committee approval was received and the informed consent of the patients was obtained.
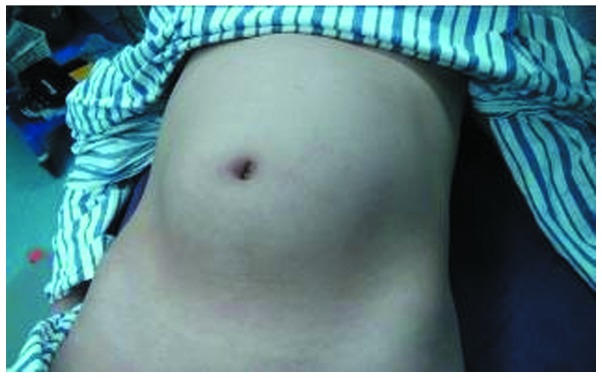
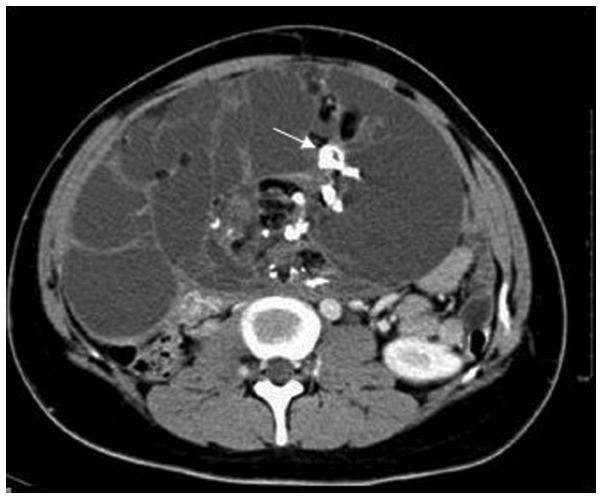
A 20-year-old female presented with a complaint of irregular menstruations with an abdominal mass of increasing size, denying any severe abdominal pain, vomiting, constipation or melena. The woman was otherwise healthy and denied any previous medical problems. Physical examination at admission demonstrated a bulged belly with a huge convex deformation approximately 30×20×30 cm in size in the periumbilical region (Fig. 1). On palpation, the mass was hard with mild tenderness, smooth on its surface and not fixed. No other abnormalities were noted during the physical examination. Serum tumor markers showed high levels of CA199 and CA125. α-fetoprotein (AFP) and β-hCG levels were normal. Abdominal contrast CT revealed a multilocular cystic mass containing tissues of different densities, including bone and fat with calcified nuclei, and an area of heterogeneous enhancement, located from the lower edge of the liver to the pelvis (Fig. 2). The patient was primarily diagnosed with teratoma and underwent a median incisional exploratory laparotomy. We closely inspected the pelvic and abdominal organs and found that no organ was infiltrated and that the mass originated from the right ovary. The Fallopian tube was elongated by traction of the tumor. In view of the patient’s reproductive requirement and the results of the frozen section analysis, we dissected the neoplasm entirely and ovary was spared. The patient recovered without complications and was discharged 6 days after surgery.
Figure 1.
The patient showed abdominal swelling. A huge convex deformation bulged around the umbilicus.
Figure 2.
Image from CT scan with contrast. A heterogeneous enhancement giant mass filled the abdominal cavity, with a multilocular cyst and calcified nuclei (white arrow).
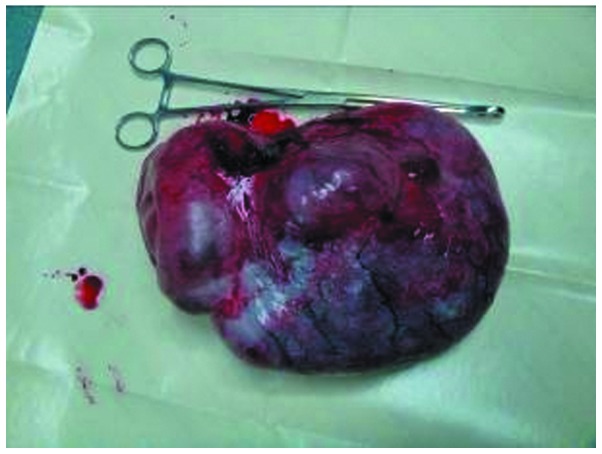
Pathological examination confirmed that the mass was a benign teratoma coated with a smooth grey membrane and was 30×20×30 cm in size (Fig. 3). It was a multilocular cystic mass with hypertension, containing some transparent sticky liquid and lots of yellowish pasty sebaceous material. Areas of calcifications compatible with bone were noted. Brain tissue was also present microscopically. A 12-month follow-up was conducted and no evidence of recurrence was found.
Figure 3.
Image of the gross specimen. The teratoma was excised entirely and was coated with a smooth grey membrane.
Results
Presentation
The median age of the 330 cases at presentation was 26 years, with a range from 6 to 83 years (Table I). The mean tumor size was 24.9±7.1 cm. The largest tumor was a 45×25-cm teratoma removed from a 74-year-old woman. A total of 228 (69.1%) patients reported symptoms. The chief presenting complaint was abdominal distention in 104 women (31.5%), abdominal pain in 103 (31.2%) and other symptoms, including menoxenia and progressive dyspnea, in 21 (6.36%). Of the 330 patients, 102 were asymptomatic (30.9%) and had their cysts discovered incidentally either on ultrasound or at the time of surgery for another indication.
Table I.
Clinical data of the 330 giant teratoma cases.
| Characteristic | Group 1 (n=111) | Group 2 (n=219)a | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 20.5±6.4 | 27.7±11.2 | 0.819 |
| Diameter of tumor (cm), mean ± SD | 19.7±3.8 | 24.5±9.5 | 0.789 |
| Symptoms, positive/negative | 78/33 | 150/69 | 0.741 |
| Abdominal distention, positive/negative | 36/75 | 68/151 | 0.798 |
| Abdominal pain, positive/negative | 38/73 | 65/154 | 0.399 |
| Menoxenia, positive/negative | 3/108 | 12/207 | 0.253 |
| Dyspnea, positive/negative | 1/110 | 5/214 | 0.375 |
| Asymptomatic, positive/negative | 33/78 | 69/150 | 0.741 |
| Bilateral, positive/negative | 9/102 | 20/199 | 0.756 |
| Pregnancy, positive/negative | 5/106 | 13/206 | 0.588 |
| Emergency surgery, positive/negative | 0/111 | 12/207 | 0.028b |
| Surgical approach, positive/negative | |||
| Cystectomy | 45/66 | 96/123 | 0.568 |
| Adnexectomy | 66/45 | 114/105 | 0.235 |
| Radical pelvic dissectionc | 0/111 | 3/216 | 0.554 |
| Spillage, positive/negative | 35/76 | 43/176 | 0.016b |
| Malignancy, positive/negative | 0/111 | 5/214 | 0.172 |
| Chemotherapy, positive/negative | 0/111 | 5/214 | 0.172 |
Including 204 patients who underwent primary laparotomy and 15 cases of conversion to laparotomy.
Significant difference (P<0.05).
A total abdominal hysterectomy with bilateral adnexectomy was performed for melanoma and squamous cell carcinoma. Group 1 underwent laparoscopic surgery; group 2 underwent laparotomic surgery.
A total of 29 patients (8.8%) had teratomas present in both ovaries and 18 (5.5%) were diagnosed during pregnancy [of whom 2 (11.1%, 2/18) had obstructive dystocia and 6 (33.3%, 6/18) had dyspnea]. Clinical data revealed no difference between the laparoscopy and laparotomy groups (Table I).
Treatment
There was no difference in surgical procedures, occurrence of malignancy or chemotherapy treatment between the two groups. There were more emergency cases due to ovarian torsion in group 2 compared with group 1 (5.5 vs. 0%, P<0.05). The overall spillage rate was 23.6% (78/330) and was significantly lower in group 2 compared with group 1 (19.6 vs. 31.5%, P<0.05), reflecting the greater use of laparotomy in group 2.
Malignancy was found in 5 patients (1.52%). Two patients with stage I immature teratoma had unilateral adnexectomy and received three BEP cycles (bleomycin, etoposide and cisplatin) following surgery. Malignant transformation was found in three patients who underwent a total abdominal hysterectomy with bilateral adnexectomy, including one case of melanoma in an 83-year-old woman and two cases of squamous cell carcinoma. The three patients received a cisplatin/5-FU chemotherapy regimen.
Follow-up
There was no difference in the follow-up data between the two groups. One case of recurrence occurred in melanoma arising from mature teratoma. Granulomatous peritonitis was not observed (Table II).
Table II.
Follow-up data of the 330 giant teratoma cases.
| Characteristic | Group 1 (n=111) positive/negative | Group 2 (n=219) positive/negative | P-value |
|---|---|---|---|
| Recurrence | 0/111 | 1/218 | 1.000 |
| Granulomatous peritonitis | 0/111 | 0/219 | 1.000 |
| Surgical site hematoma | 3/108 | 3/216 | 0.674 |
| Surgical site infection | 0/111 | 3/216 | 0.532 |
Group 1 underwent laparoscopic surgery; group 2 underwent laparotomic surgery.
Survival data were collected from 5 patients with malignant teratomas who underwent open surgery. Two patients with immature teratomas who received chemotherapy for between 3 months and 2 years following surgery were disease-free after a period of 3 to 5 years without recurrence or metastasis. The 83-year-old woman with melanoma arising from mature teratoma succumbed to multiple organ failure 7 months after bilateral adnexectomy and hysterectomy. The other two patients with squamous cell carcinoma were well during the 1- to 2-year follow-up.
Discussion
Cystic ovarian teratomas, especially mature cases (dermoid cysts), constitute 10–13% of all ovarian tumors and are the most common benign ovarian germ cell tumors (5). They typically occur during reproductive age (mean age, 27 years) (6). Ovarian cysts are traditionally labeled as large when they are over 5 cm in diameter and giant or voluminous when they are over 15 cm; a more suitable designation for giant cysts may, however, relate the size of the cyst to the size of the peritoneal cavity in these growing young patients (7). The majority of teratomas may be symptom free. With the tolerance of the abdomen, there is no clear symptom at the early stage of ovary teratomas. The tumors tend to enlarge until specific organs are functionally influenced or incidental discovery by ultrasound. Mature cystic teratomas grow slowly at an average rate of 1.8 mm each year, prompting conservative management of smaller (diameter less than 6 cm) tumors (8).
Giant ovarian teratomas commonly present with acute abdominal pain caused by adnexal torsion (9) and abdominal distention due to the rapid growth of a large, unilateral tumor undergoing capsular distention, hemorrhage or necrosis (10). The patients may also have certain non-specific abdominal complaints indicating mass effect, including menoxenia, dyspnea and the symptoms of other organs becoming influenced by the tumor. However, in the present study, the tumors were so voluminous that they tended to be symptomatic. The rate of symptomatic teratomas (69.1%, 228/330) was higher than reported previously (29.4%) (2). Therefore, the aim of treatment is to reduce the severity of teratoma-related symptoms, especially to reduce the mass effect due to the raised abdominal pressure, and to prevent the potential malignancy.
Following the primary assessment of the clinical presentation, CA125, or other tumor markers as clinically indicated, and imaging evaluation are recommended. At ultrasound, mature teratomas are characterized by echogenic sebaceous material and calcification (11). At CT, fat attenuation within a cyst is diagnostic. CT and MRI are straightforward as these modalities are more sensitive for fat within the cyst, which is diagnostic for mature cystic teratomas (12). Immature teratomas, consisting of elements with only partial somatic differentiation, usually have a large, irregular solid component containing coarse calcifications and small foci of fat apparant on CT and MRI scans (13). However, the appearance of the tumor at ultrasound is non-specific. In certain instances, it is difficult to differentiate other germ cell tumors of ovarian origin, including dysgerminoma and yolk sac tumors, from teratomas. Compared with these other germ cell tumors, teratomas tend to exhibit a more heterogeneous appearance with a mixture of fluid, fat and calcifications, as observed in our patient.
Surgical options to treat teratomas are individualized by the possibility of chemical peritonitis and malignancy (3). The existence of giant mature ovarian teratomas, as suggested by the preoperative examination and operative inspection, advises the excision of the tumor. The decision as to whether the whole ovary or only the cyst was to be removed was made according to the desire to retain fertility. Shalev et al (2) suggested that taking a close inspection of pelvic and abdominal organs with cytological sampling on entering the abdomen aimed at ruling out possible malignancy. Hysterectomy and bilateral adnexectomy should be performed with every effort made to keep an encapsulated mass intact during removal. Adnexectomy was also performed in patients with adnexal torsion during emergengy surgery (group 1 vs. group 2, 0 vs. 5.5%, P<0.05), which mostly ocurred in young females lacking routine gynecological examination.
Although laparoscopic surgery has replaced laparotomy as the preferred surgical approach for teratomas of normal size, Teng (14) et al reported spillage rates of 44–100% during laparoscopic management and 0–13% during laparotomy. For the laparoscopic techniques applied to the management of cystic giant teratomas, prelaparoscopic decompression is necessary to allow for room to establish pneumoperitoneum and manipulate the tumors, which is not possible in solid teratomas. Salem (15) reported 15 cases of large ovarian cysts removed following puncture of their walls. Dolan et al presented a patient with a giant ovarian cyst, over 40 cm in diameter, who underwent minilaparotomy drainage followed by complete laparoscopic extirpation (7). Despite drainage of these cystic tumors via a minilaparotomy or percutaneous techniques, inadvertent perforation and spillage must be prevented. In our experience, few studies concerning the spillage of giant ovarian teratomas over 15 cm in diameter have been published. The spillage rate in the laparoscopic group was higher than that of the laparotomic group in the present study (31.5 vs. 19.6%, P<0.05). Inadvertent rupture may result in granulomatous peritonitis. A retrospective study covering 20 years and including 26 cases of intraperitoneal spillage in 314 patients identified 2 patients with postoperative granulomatous peritonitis, giving an incidence of chemical peritonitis of 8% in this group of patients (4). However, the data from another retrospective study of 324 patients who underwent laparoscopic cystectomy and suffered spillage with irrigation of the abdominal cavity document a lower incidence (0.3%) (11). Although irrigation of the peritoneal cavity was also performed in both studies, the difference in incidence of granulomatous peritonitis may be due to the greater volumes of irrigant required to render the washings clear as suggested by Teng et al (14).
A detailed review of previous studies has revealed that the laparoscopic procedure has rarely been performed on masses with a diameter larger than 10 cm (2). Howard, in 1995, suggested that it should not be performed on tumors with a diameter greater than 15 cm (16). By contrast, Shalev and Peleg (17) recommended laparoscopic surgery as a routine treatment for the ovarian teratomas as large as 15 cm in diameter. Decompression of the cyst followed by laparoscopy may cause rupture in certain conditions. On the other hand, the variability of manifestations means that giant ovarian teratomas are easily misdiagnosed as giant abdominal leiomyosarcoma or liposarcoma. Therefore 219 (66.4%) cases chose the median laparotomic exploratory incision in the present study and intraoperative frozen section examination of integrated tumor delivery has become routine. Suspicion of malignancy based on preoperative imaging and inspection should preclude the laparoscopic approach.
The pathological appearance of mature cystic teratomas is characteristic. Squamous epithelium lines the wall of the cyst and mesodermal (fat, bone, cartilage, muscle) and endodermal tissues (e.g., gastrointestinal and bronchial epithelium, thyroid tissue) are present in the cyst cavity in the majority of cases (18). The tumors are unilocular in 88% of cases and filled with sebaceous material, although the patient had a multilocular tumor in our case.
Immature teratomas differ from mature tumors in that they demonstrate malignant biological behavior, are much less common (1% of ovarian teratomas) and affect a younger age group (usually during the first 2 decades of life) (18). The malignant transformation of mature cystic teratomas consists of differentiated tissues giving rise to carcinoma or sarcoma, which is characterized by the malignant transformation of the squamous epithelium in less than 1% of cases (19). Sarcomas, carcinoids and adenocarcinomas have also been reported (20). Slow-growing cysts that reach this giant size are almost always benign. Therefore, the malignancy rate was lower in this series, with two (0.61%, 2/330) squamous malignant transformation histopathologically diagnosed.
Gobel et al (21) wrote in 1998 that surgical therapy alone is an adequate treatment for patients affected by a mature teratoma. The risk of recurrence of immature teratomas is strictly correlated with the histological grading based on the amount of embryo tissue present, according to WHO’s classification (22). A follow-up strategy is suggested for immature teratomas at stage I, but is not sufficient for those at stages II and III with malignant foci (23). Chemotherapy may increase the disease-free rate of these patients and cause the maturation of immature tissues. These retroconverted masses may remain stable for a long period of time (24). The essential regimen has been BEP for immature teratomas from three to four cycles following surgery. However, the treatment for the malignant transformation of mature teratomas is controversial. Platinum-based chemotherapy may be a reasonable adjuvant therapy for squamous cell carcinoma arising from mature teratomas (20).
In conclusion, the cornerstone of the study of giant ovarian teratomas is that the evaluation of possible malignancy, accidental rupture, rapid growth, doubtful infiltration, large size, origin and adjacent structures is based on detailed preparation. We found the prevalence rates of symptomatic tumors, ovarian torsion and accidental rupture to be higher than those previously reported in giant ovarian teratomas. Laparotomic resection may be considered a necessary alternative to laparoscopy in the management of the giant ovarian teratoma.
However, we cannot confirm that laparotomy could replace laparoscopy in all instances. With the development of complicated surgical techniques to meet the avoidance of spillage and oncological safety, giant ovarian teratomas may be managed laparoscopically regardless of the size of the tumor.
References
- 1.Choi DJ, Wallace EC, Fraire AE, Baiyee D. Best cases from the AFIP: intrarenal teratoma. Radiographics. 2005;25:481–485. doi: 10.1148/rg.252045153. [DOI] [PubMed] [Google Scholar]
- 2.Comerci JT, Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994;84:22–28. [PubMed] [Google Scholar]
- 3.Shalev E, Bustan M, Romano S, Goldberg Y, Ben-Shlomo I. Laparoscopic resection of ovarian benign cystic teratomas: experience with 84 cases. Hum Reprod. 1998;13:1810–1812. doi: 10.1093/humrep/13.7.1810. [DOI] [PubMed] [Google Scholar]
- 4.Kondo W, Bourdel N, Cotte B, Tran X, Botchorishvili R, Jardon K, Rabischong B, Pouly JL, Mage G, Canis M. Does prevention of intraperitoneal spillage when removing a dermoid cyst prevent granulomatous peritonitis? BJOG. 2010;117:1027–1030. doi: 10.1111/j.1471-0528.2010.02580.x. [DOI] [PubMed] [Google Scholar]
- 5.Koonings PP, Campbell K, Mishell DR, Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74:921–926. [PubMed] [Google Scholar]
- 6.Stella F, Davoli F. Giant mediastinal mature teratoma with increased exocrine pancreatic activity presenting in a young woman: a case report. J Med Case Reports. 2011;5:238. doi: 10.1186/1752-1947-5-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan MS, Boulanger SC, Salameh JR. Laparoscopic management of giant ovarian cyst. JSLS. 2006;10:254–256. [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi B, Appelman Z, Rabinerson D, Zalel Y, Tulandi T, Shoham Z. The growth pattern of ovarian dermoid cysts: a prospective study in premenopausal and postmenopausal women. Fertil Steril. 1997;68:501–505. doi: 10.1016/s0015-0282(97)00228-8. [DOI] [PubMed] [Google Scholar]
- 9.Hibbard L. Adnexal torsion. Am J Obstet Gynecol. 1985;152:456–461. doi: 10.1016/s0002-9378(85)80157-5. [DOI] [PubMed] [Google Scholar]
- 10.Ghaemmaghami F, Abbasi F, Abadi AG. A favorable maternal and neonatal outcome following chemotherapy with etoposide, bleomycin, and cisplatin for management of grade 3 immature teratoma of the ovary. J Gynecol Oncol. 2009;20:257–259. doi: 10.3802/jgo.2009.20.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldas C, Sitzmann J, Trimble CL, McGuire WP., III Synchronous mature teratomas of the ovary and liver: a case presenting 11 years following chemotherapy for immature teratoma. Gynecol Oncol. 1992;47:385–390. doi: 10.1016/0090-8258(92)90145-9. [DOI] [PubMed] [Google Scholar]
- 12.Guerriero S, Mallarini G, Ajossa S, Risalvato A, Satta R, Mais V, Angiolucci M, Melis GB. Transvaginal ultrasound and computed tomography combined with clinical parameters and CA-125 determinations in the differential diagnosis of persistent ovarian cysts in premenopausal women. Ultrasound Obstet Gynecol. 1997;9:339–343. doi: 10.1046/j.1469-0705.1997.09050339.x. [DOI] [PubMed] [Google Scholar]
- 13.Buy JN, Ghossain MA, Moss AA, Bazot M, Doucet M, Hugol D, Truc JB, Poitout P, Ecoiffier J. Cystic teratoma of the ovary: CT detection. Radiology. 1989;171:697–701. doi: 10.1148/radiology.171.3.2717741. [DOI] [PubMed] [Google Scholar]
- 14.Teng FY, Muzsnai D, Perez R, Mazdisnian F, Ross A, Sayre JW. A comparative study of laparoscopy and colpotomy for the removal of ovarian dermoid cysts. Obstet Gynecol. 1996;87:1009–1013. doi: 10.1016/0029-7844(96)00061-0. [DOI] [PubMed] [Google Scholar]
- 15.Salem HA. Laparoscopic excision of large ovarian cysts. J Obstet Gynecol Res. 2002;28:290–294. doi: 10.1046/j.1341-8076.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 16.Howard FM. Surgical management of benign cystic teratoma. Laparoscopy vs. laparotomy. J Reprod Med. 1995;40:495–499. [PubMed] [Google Scholar]
- 17.Shalev E, Peleg D. Laparoscopic treatment of adnexal torsion. Surg Gynecol Obstet. 1993;176:448–450. [PubMed] [Google Scholar]
- 18.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21:475–490. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 19.Dulmet EM, Macchiarini P, Suc B, Verley JM. Germ cell tumors of the mediastinum. A 30-year experience. Cancer. 1993;72:1894–1901. doi: 10.1002/1097-0142(19930915)72:6<1894::aid-cncr2820720617>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Dos Santos L, Mok E, Iasonos A, Park K, Soslow RA, Aghajanian C, Alektiar K, Barakat RR, Abu-Rustum NR. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol. 2007;105:321–324. doi: 10.1016/j.ygyno.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Göbel U, Calaminus G, Engert J, et al. Teratomas in infancy and childhood. Med Pediatr Oncol. 1998;31:8–15. doi: 10.1002/(sici)1096-911x(199807)31:1<8::aid-mpo2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Candela G, Di Libero L, Varriale S, Manetta F, Napolitano S, Scetta G, Esposito D, Sciascia V, Santini L. Hemoperitoneum caused by the rupture of a giant ovarian teratoma in a 9-year-old female. Case report and literature review. Ann Ital Chir. 2009;80:141–144. [PubMed] [Google Scholar]
- 23.Lo Curto M, D’Angelo P, Cecchetto G, et al. Mature and immature teratomas: results of the first paediatric Italian study. Pediatr Surg Int. 2007;23:315–322. doi: 10.1007/s00383-007-1890-1. [DOI] [PubMed] [Google Scholar]
- 24.Moskovic E, Jobling T, Fisher C, Wiltshaw E, Parsons C. Retroconversion of immature teratoma of the ovary: CT appearances. Clin Radiol. 1991;43:402–408. doi: 10.1016/s0009-9260(05)80570-7. [DOI] [PubMed] [Google Scholar]